Abstract
Cardiovascular features in rheumatoid arthritis (RA) are common. However, RA associated with acute myocarditis is seldom described. Here, we report the case of a 58-year-old woman with rheumatoid arthritis and end stage renal disease who suffered chest tightness and diaphoresis during hemodialysis. The electrocardiogram showed ST elevations and the echocardiographic study revealed abnormalities of regional wall motion with moderate left ventricle dysfunction. Acute ST-elevation myocardial infarction was impressed according to clinical presentations and elevated cardiac enzymes. However, emergent coronary angiography revealed no significant coronary stenosis. Magnetic resonance imaging ultimately made the diagnosis of myocarditis. The patient improved gradually without immunosuppressive therapy. This case shows that conservative treatment is a feasible strategy for acute myocarditis in a patient with rheumatoid arthritis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Besides articular symptoms, rheumatoid arthritis (RA) can be associated with extra-articular features. Among those extra-articular features, cardiovascular diseases are common, including pericarditis, cardiomyopathy, cardiac amyloidosis, coronary vasculitis, arrhythmia, valve diseases, congestive heart failure, and ischemic heart disease. Cardiomyopathy was found by echocardiography in 37% of RA patients in a small series study [1]. The RA-associated cardiomyopathy may be the result of focal nonspecific diffuse necrotizing or granulomatous myocarditis. These entities are histological diagnoses, which may be found in 3–30% of RA patients in postmortem studies [2]. The recent introduction of cardiovascular magnetic resonance imaging (MRI) provides an opportunity to evaluate cardiac morphology and function, assess myocardial perfusion reserve, and differentiate ischemic cardiomyopathy more clearly from non-ischemic cardiomyopathy. Here, we report the case of a 58-year-old woman with RA and end-stage renal disease who suffered chest tightness and diaphoresis during hemodialysis. Initial diagnosis was acute ST-segment elevation myocardial infarction and myocarditis was ultimately confirmed by MRI.
Case report
A 58-year-old woman, with a 20-year history of RA and a 1-year history of end-stage renal disease, presented with sudden dyspnea, chest tightness, and diaphoresis during hemodialysis in the morning. No drop of blood pressure was noted during hemodialysis. Seven hours later, she was referred to our emergency room (ER) from a local clinic due to persistent chest pain, general weakness, and an abnormal electrocardiographic pattern. At the ER, hypotension with blood pressure of 86/65 mm Hg and body temperature of 36.2°C were noted. The electrocardiogram (ECG) showed atrial fibrillation with rapid ventricular response and ST elevation over leads I, aVL, and V6 with reciprocal ST depression at the inferior leads. Blood analysis revealed creatine kinase (CK) = 211 U/L (normal range from 24 to 120 U/L), CKMB = 57 U/L, troponin I = 31.42 ng/ml and lactate dehydrogenase = 294 U/L (normal range from 95 to 213 U/L). Elevated C-reactive protein = 22.48 mg/dl (normal range from 0 to 0.5 mg/dl) was also noted. A chest radiograph disclosed cardiomegaly. Also, an echocardiographic study revealed dyskinesis of the mid and apical lateral wall of the left ventricle (LV), abnormal ventricular septal motion, and moderate LV dysfunction with estimated ejection fraction of 32%. There was no evidence of pericardial effusion or intra-cardiac thrombo-embolism. She was prescribed intravenous dopamine 10 μg/kg/min and the patient’s blood pressure increased to 130/74 mm Hg.
After a detailed interrogation, she complained of poor appetite for 2 days followed by a chilly sensation and morning fever (39.8°C). She denied a recent travel history and had no productive cough, abdominal pain, or arthralgia. Because of the typical ECG pattern and elevated cardiac enzymes, acute ST-elevation myocardial infarction with cardiogenic shock was suspected.
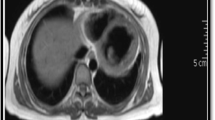
We performed cardiac catheterization immediately. The coronary angiography showed patent coronary arteries with minimal stenosis at the proximal left descending coronary artery and a myocardial bridge at the distal left circumflex coronary artery. Transient embolic occlusion of the coronary artery or prolonged coronary spasm was excluded due to persistent elevated ST segments even with patent coronary arteries. Studies evaluating vasculitis, including antinuclear antibodies, anti-neutrophil cytoplasmic antibodies (c-ANCA and p-ANCA), and rheumatoid factor (RF < 20 IU/ml), were negative and so were viral markers. Under the impression of myocarditis, we arranged magnetic resonance imaging (MRI), which disclosed moderate cardiomegaly, focal bulging with wall thinning at the lateral wall of the LV myocardium and enhancement on post-contrast enhanced images of the LV apex compatible with myocarditis (Fig. 1).
Conservative treatment with intravenous inotropic agents was initiated. Her blood pressure returned to normal. Because of poor LV systolic function, we prescribed an ACE inhibitor (ramipril) and a β-blocker (carvedilol). The subsequent ECG revealed some resolution of elevated ST segments without Q-wave formation and resumption of sinus rhythm. The patient was finally discharged 10 days later in stable condition. After 3 months, a repeated echocardiography showed moderate hypokinesis of the basal to mid-anterior septum wall and apex of the LV with mild LV dysfunction and estimated LV ejection fraction of 46%. The patient tolerated the medications well.
Discussion
Acute myocarditis, an inflammatory process involving the myocardium, shares similar clinical manifestations with acute myocardial infarction, including chest pain, hemodynamic instability, regional wall motion abnormalities, an ischemic ECG pattern, and elevated cardiac enzymes. Coxsackie B viruses, echo viruses, and other members of the picorna virus group are the main pathogens responsible for acute myocarditis. Autoimmune myocarditis has been described in systemic lupus erythematosus, mixed connective tissue disease, primary Sjögren’s syndrome, and RA. The typical ECG patterns of myocarditis include (1) ST-T wave abnormalities (100%), (2) abnormal Q wave (64%), (3) low voltage (18%), (4) tachyarrhythmia (45%), and (5) conduction disturbance (55%) [3]. According to the committee of the European Society of Cardiology and the American College of Cardiology (ESC/ACC) in 2000, we may sometimes confuse between acute myocardial infarction and acute myocarditis because of similar clinical features. And treatment using thrombolytic agents due to a mistaken diagnosis of acute myocardial infarction may exacerbate the underlying disease and cause a fatal result.
As for our patient who had typical anginal symptoms, a myocardial infarction ECG pattern and elevated cardiac enzymes, it was better to perform coronary angiography instead of thrombolytic therapy. However, it was still difficult to distinguish myocarditis from acute myocardial infarction in patients with normal coronary angiography (due to transient occluded coronary artery by emboli or prolonged coronary spasm). Although endomyocardial biopsy is the accepted standard method for diagnosis of myocarditis worldwide, its limited sensitivity and possibilities of lethal complications (cardiac perforation) are the main concerns before intervention [4]. Also, endomyocardial biopsy provides no advantage in treating such patients.
Cardiac MRI is a useful alternative method for distinguishing myocardial infarction from myocarditis. Cardiac MRI can non-invasively detect myocardial edema and myocyte damage. In myocardial infarction, the cardiac MRI typically shows subendocardial enhancement. In contrast, myocarditis shows a characteristic MRI pattern of contrast enhancement, which originates primarily from the epicardium, sparing the subendocardial layer [5]. Our patient had a classic cardiac MRI pattern of myocarditis corresponding with an ECG presentation and wall-motion abnormality. Other possible diagnoses for this patient included transient emboli or prolonged coronary spasm, but these were improbable due to persistent ST elevation on follow-up ECGs for more than 1 week but normal coronary angiograph.
Although autoimmune myocarditis can be the cause of cardiomyopathy, a possible cardiovascular feature of RA, the diagnosis of autoimmune myocarditis must be made by excluding infectious myocarditis. In our patient, we had no evidence linking RA and myocarditis, and viral infection is the most common cause of acute myocarditis. The recovery from autoimmune myocarditis may depend on immunosuppressive therapy, whereas it has been shown that no survival benefit from immunosuppressive therapy can be expected in infectious myocarditis. We chose conservative treatment for this patient and she improved gradually. This made the diagnosis of autoimmune myocarditis less likely. However, if further deterioration occurs, immunosuppressive therapy should be considered for such conditions.
In conclusion, this case shows that cardiac MRI is a useful method for distinguishing myocardial infarction from focal myocarditis and conservative treatment without immunosuppressive therapy is a feasible strategy for acute myocarditis in a patient with rheumatoid arthritis.
References
Guedes C, Bianchi-Fior P, Cormier B et al (2001) Cardiac manifestations of rheumatoid arthritis: a case-control transesophageal echocardiography study in 30 patients. Arthritis Rheum 45:129–135
Hurd ER (1979) Extraarticular manifestations of rheumatoid arthritis. Sem Arthritis Rheum 8:151–176
Nakashima H, Honda Y, Katayama T (1994) Serial electrocardiographic findings in acute myocarditis. Intern Med 33:659–666
Hrobon P, Kuntz KM, Hare JM (1998) Should endomyocardial biopsy be performed for detection of myocarditis? A decision analytic approach. J Heart Lung Transplant 17:479–486
Laissy JP, Hyafil F, Feldman LJ et al (2005) Differentiating Acute Myocardial Infarction from Myocarditis: Diagnostic Value of Early- and Delayed-Perfusion Cardiac MR Imaging. Radiology 237:75–82
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, CH., Sung, SH. & Wu, TC. Focal myocarditis mimicking myocardial infarction in a patient with rheumatoid arthritis. Clin Rheumatol 28, 479–481 (2009). https://doi.org/10.1007/s10067-009-1088-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-009-1088-0