Abstract
Polyarteritis nodosa (PAN) is a necrotising vasculitis of medium-sized vessels of unknown origin. This type of vasculitis is usually systemic, but restriction to a single organ, for example the testis, the appendix or the gall bladder, can occur. Testicular pain or tenderness are frequent clinical features. In this report, we present three cases of PAN. In every patient, testicular pain was the main symptom or first sign of systemic disease. We state that a thorough history taking, clinical examination and biochemical analyses are obligatory in patients presenting with acute or chronic scrotal pain. Polyarteritis nodosa should always be taken into account, and a search for systemic spread is mandatory. We emphasize that before initiation of systemic therapy with corticosteroids and/or cyclophosphamide, a Five Factor Score should be obtained, which also gives crucial prognostic information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1866, Kussmaul and Maier published the first case of what they called “periarteritis nodosa”. Due to the fact that arterial inflammation is transmural and not restricted to the outer layers, this name was later changed into polyarteritis nodosa (PAN). PAN is not a common disease. The prevalence in a north-eastern suburb of Paris was estimated to be 30.7 per million [1]. There is no sex preference and people in their fourth to sixth decade are the most vulnerable. PAN is a necrotising inflammation restricted to medium-sized arteries without glomerulonephritis or vasculitis in arterioles, capillaries or venules [2]. Anti-neutrophil cytoplasmic antibodies (ANCAs) are not present in ‘classical’ PAN, in contrast to microscopic polyangiitis, Wegener’s disease and Churg–Strauss syndrome. Guillevin et al. established a prognostic score called the Five Factor Score (FFS: serum creatinine level of >1.58 mg/dL, proteinuria of >1 g/day, cardiomyopathy, gastrointestinal tract involvement, central nervous system involvement) which reflects the severity of organ involvement. A patient with a FFS of 0 has a mortality risk at 5 years of 11.9% and a patient with a FFS of 2 has a mortality risk at 5 years of 46% [3].
Organ-limited forms of PAN do exist, although the cutaneous form of PAN probably is a separate disease, as is primary angiitis of the central nervous system. Cases with medium-sized vessel vasculitis restricted to the uterus, the gall bladder, the pancreas, the appendix or the testes [4–9] have been described. Matsumoto et al. showed that the vasculitic process in these isolated necrotic vasculitides is a mildly wall destructive form of PAN-type arteritis without severe wall destruction or aneurysm formation [10].
We describe here a patient with testicular involvement by PAN, give the data of two other patients in table format and discuss the possible treatment options in these patients.
Case presentation
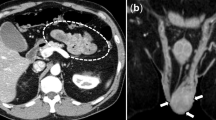
A 72-year-old man presented at the emergency ward with a 1-day history of right-sided testicular pain. This pain worsened while moving. He had received a treatment for acute myeloid leukaemia in 2004 and was in complete remission. Due to this treatment, he developed myelodysplasia. On regular basis, he received transfusions of packed cells and platelets. Clinical examination showed a patient in good condition. The examination of heart, lungs and abdomen was unremarkable. Scrotal examination showed a manifestly swollen and painful right testis.
Doppler sonography showed a heterogenic, enlarged right testis. The testis was hypo-vascular and only venous flow could be detected. Biochemical analysis revealed haemoglobin-level 7.8 g/dl (14.0–18.0), platelets 36 10**9/L (150–450), C-reactive protein 21 mg/L (<5.0) and sedimentation rate 14 mm/h (1–10). Renal and hepatic functions were normal. Urine analysis was normal. ANCA and anti-nuclear factor were negative. Hepatitis B testing was negative.
Scrotal exploration was planned after transfusion of histocompatibility antigen-matched platelets. An oedematous, blue coloured testis was found, and multiple necrotic areas were detected. A right orchidectomy was performed. Pathological examination showed segmental destruction of the vessel wall in small- and medium-sized arteries by a mononuclear inflammatory infiltrate (Fig. 1). Also, some fibrinoid necrosis and organised thrombi were found. In the parenchyma of the testis, there were areas of bleeding and ischemic necrosis. No malignancy was detected.
Overview of an active arteritis within the testes. Residual tubuli seminiferi are seen in the right lower corner (black arrow). The arterial wall is almost completely blurred by a transmural infiltration (black star). At the luminal side, an area of fibrinoid necrosis is present (white arrows). Focally, medial smooth muscle cells are recognizable (white star; H & E, original magnification ×50)
Six months after this initial presentation, myalgia developed. Clinical examination was unremarkable. Biochemical analysis showed a rise in C-reactive protein to 50 mg/L (<5.0). Further investigations were not performed because the patient refused.
We made a diagnosis of polyarteritis nodosa. The patient fulfilled three out of ten classification criteria (myalgia, testicular pain and a positive biopsy). The FFS was 0. Treatment with methylprednisolon 32 mg a day, orally, was initiated and slowly tapered down. The complaints of myalgia disappeared. Until now, this patient is uneventful.
Clinical, biochemical and technical data of two other patients with testicular pain in whom a diagnosis of PAN could be made are given in Table 1.
Discussion
We describe here three patients in whom testicular involvement was a prominent sign of PAN. In patients 2 and 3, general symptoms and scrotal pain developed almost simultaneously. In patient 1, testicular vasculitis remained an isolated finding for 6 months; but then, also in this patient, systemic symptoms emerged. The differential diagnosis of scrotal pain should include torsion of the testis, appendicular torsion, epididymitis, trauma, infectious orchitis or mumps. PAN should also be taken into account. A diagnosis of PAN, limited to a testis, most frequently comes by surprise as an unexpected histological finding at surgery. In two of our patients (cases 1 and 3), we expected to find a tumour, in view of the medical history of the patients. In patient 2, testicular pain was the leading symptom which made us to suspect PAN as an explanation for the general symptoms of the patient.
In the case series that was used for the development of the American College of Rheumatology (ACR) criteria for PAN, testicular pain or tenderness was found in 28.9% of 90 PAN patients and only in 2.6% of 510 patients with other forms of vasculitis [11]. Testicular pain or tenderness was reported in 24% of 17 male PAN patients in a Korean series [12].
If testicular pain is only one of several manifestations of PAN, it is clear that systemic therapy with steroids, and possibly cyclophosphamide, is needed. The question is how patients with isolated vasculitis of a single organ should be managed. There is a growing consensus in the literature that even if the vasculitic mass was entirely removed and when there are no clinical signs of other organ involvement, systemic treatment is needed, due to the systemic nature of PAN [5]. Other authors, however, state that immunosuppressive treatment after surgical removal of the affected organ is not needed [4, 7]. If the patient is completely asymptomatic after the surgical procedure and if there are no abnormal biochemical findings, a “wait-and-see” approach can probably be adopted, with close follow-up of the patient and prompt start of treatment when symptoms of systemic spread become apparent. This approach was followed in case 1. It is clear from case 1 that statements that say that isolated PAN never evolves in systemic disease are false. If there are clues of systemic vasculitis such as fever, myalgia or weight loss, like in cases 2 and 3 and eventually also in case 1, additional examinations (arteriography, electromyography, muscle biopsy, etc.) should be ordered and a treatment with steroids started. In case of bioptic proof of vasculitis, a diagnosis of PAN can be confirmed when the patient fulfils the ACR classification criteria.
Which immune suppressive drugs should be started can be decided after a search for negative prognostic factors. If the FFS is zero, steroids alone will be sufficient. If the FFS is higher, association of cyclophosphamide is needed [13].
In conclusion, PAN should be included in the differential diagnosis of acute scrotal pain or a testicular mass. When bioptic proof of vasculitis is obtained, additional exploration for systemic spread of vasculitis is needed, especially when there are systemic complaints. Classification criteria for PAN should be checked for and the FFS calculated in order to start the appropriate treatment.
References
Mahr A, Guillevin L, Poissonnet M et al (2004) Prevalence of polyarteritis nodosa, microscopic polangiitis, Wegener’s granulomatosis, and Churg–Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum 51:92–99
Jennette JC, Falk RJ, Andrassy K et al (1994) Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 37:187–192
Guillevin L, Lhote F, Gayraud M et al (1996) Prognostic factors in polyarteritis nodosa and Churg-strauss syndrome. A prospective study in 324 patients. Medicine (Baltimore) 75:17–28
Fraenkel-Rubin M, Ergas D, Sthoeger ZM (2002) Limited polyarteritis nodosa of the male and female reproductive systems: diagnostic and therapeutic approach. Ann Rheum Dis 61:362–364
Revital K, Yechezkel S, Hanan G (2000) Systemic vasculitis presenting as a tumorlike lesion: four case reports and an analysis of 79 reported cases. Medicine 79:349–359
Warfield AT, Lee SJ, Phillips SMA et al (1994) Isolated testicular vasculitis mimicking a testicular neoplasm. J Clin Pathol 47:1121–1123
Dotan Z, Laufer M, Heldenberg E et al (2003) Isolated testicular polyarteritis nodosa mimicking testicular neoplasm - long term follow-up. Urology 62:352xxvii–352xxix
Raj GV, Ellington KS, Polascik TJ (2003) Autoimmune testicular vasculitis. Urology 61:1035x–1035xi
Kolar P, Schneider U, Filimonow S, Burmester GR, Buttgereit F (2007) Polyarteritis nodosa and testicular pain: ultrasonography reveals vasculitis of the testicular artery (Letter to the Editor, Case report). Rheumatology 46:1377–1378
Matusomoto T, Kobayashi S, Ogishima D et al (2007) Isolated necrotizing vasculitis (localized polyarteritis nodosa): examination of the histological process and disease entity based on the histological classification of stage and histological differences from polyarteritis nodosa. Cardiovasc Pathol 16:92–97
Lightfoot RW Jr, Michel BA, Bloch DA et al (1990) The American college of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum 33:1088–1093
Bae YD, Choi HJ, Lee JC et al (2006) Clinical features of Polyarteritis Nodosa in Korea. J Koean Med Sci 21:591–595
Gayraud M, Guillevin L, le Toumelin P et al (2001) Long-term follow up of Polyarteritis Nodosa, Microscopic Polyangiitis and Churg–Strauss syndrome. Analysis of four prospective trials including 278 patients. Arthritis Rheum 44:666–675
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meeuwissen, J., Maertens, J., Verbeken, E. et al. Case reports: testicular pain as a manifestation of polyarteritis nodosa. Clin Rheumatol 27, 1463–1466 (2008). https://doi.org/10.1007/s10067-008-0970-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-008-0970-5