Abstract
Tumor necrosis factor (TNF)-alpha antagonists successfully modulate the pathogenesis of rheumatoid arthritis (RA). However, little is known about the effect of TNF-alpha blockade on the histology of chronic viral hepatitis. We describe the cases of two patients with RA, one with concurrent chronic hepatitis B virus and the other with hepatitis C virus infection who, as part of their evaluation, underwent liver biopsies while undergoing treatment with a TNF-alpha antagonist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
TNF-alpha plays a part in the normal host defense as well as in inflammatory processes. Therefore, it is implicated in the pathogenesis of rheumatoid arthritis and is activated in the liver of patients with chronic hepatitis B [1] and C [2, 3] infections.
Rheumatoid arthritis (RA) patients with concurrent chronic viral hepatitis pose unique treatment challenges because many disease-modifying anti-rheumatic drugs such as methotrexate and leflunimide are hepatotoxic and because corticosteroids and immunosuppressive agents can worsen viremia [4]. Tumor necrosis factor (TNF)-alpha antagonists are not directly hepatotoxic, so they are good alternatives in individuals with liver disease. However, their effect on viral infectious hepatitis is not clear.
We report the first histological data of the effect of TNF-alpha blockade on the liver in two rheumatoid arthritis patients one of whom was infected with chronic hepatitis B and the other with hepatitis C.
Case 1
A 51-year-old African American male with rheumatoid factor and anti-cyclic citrullinated peptide positive, erosive RA is referred for management. He was initially treated with prednisone 20 mg and hydroxychloroquine 200 mg bid, and asymptomatic hepatitis C virus (HCV) infection was found upon routine screening prior to methotrexate therapy. His HCV AB was reactive by enzyme-linked immunosorbent assay, and HCV polymerase chain reaction (PCR) revealed 242,122 IU/ml (ref range 600–850,000 IU/ml) (Temple University Hospital, Philadelphia, PA). The genotype was 1b. Clinical assessment of hepatic function revealed mild ALT elevation at 44 (ref 15–40 U/l). Other function assessment was normal.
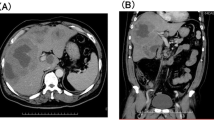
Etanercept 25 mg SC twice weekly was started and approximately 2 months into the therapy, HCV PCR quantitative had increased to 508,675 IU/ml, ALT 49 U/l, and AST 29 U/l. A liver biopsy was performed which demonstrated minimal inflammatory activity with a histologic stage 1–2/6 and an activity grade 6/16. The modified histology activity index (HAI) revealed: (a) interface hepatitis, 2; (b) confluent necrosis, 0; (c) lobular inflammation, 2: and (d) portal inflammation, 2 (Fig. 1). The patient declined treatment for hepatitis C and continued on etanercept therapy.
Case 2
A 28-year-old African American female with rheumatoid factor positive, erosive, rheumatoid arthritis for 4 years is found to have asymptomatic hepatitis B infection. On routine testing, HBsAg and HBcore Ab (total) were found to be positive, while HBe Ag and HBs AB were negative. Prior treatment history included NSAIDS, hydroxychloroquine, prednisone, and methotrexate, with inadequate control of symptoms over the 3 years of treatment at our institution.
Adalimumab 40 mg sc every 2 weeks was initiated and prednisone 10 mg daily was continued. Prior to adalimumab therapy, there were no stigmata of chronic liver disease (jaundice or ascites), and liver function tests and coagulation parameters were normal. After 2 months of adalimumab treatment, the frequency of injection was increased to weekly administration because of continued disease activity.
Three months into adalimumab therapy, hepatitis B virus (HBV) DNA quantification revealed 2.4 × 106 copies/ml (LabCorp Raritan, NJ, USA using National Genetics Institute’s validated, proprietary methodology). Therefore, a liver biopsy was performed at 4 months into treatment and it demonstrated minimally active chronic hepatitis. The histologic stage and grade were: portal 0/4, lobular 0/4, and fibrosis 0/4 (Fig. 2).
No additional antiviral therapy was instituted and after 9 months of adalimumab therapy, the HBV DNA viral load was 26,633 copies/ml (Temple University Hospital, PA, USA). Patient’s symptoms were well controlled on adalimumab and viral load and liver function tests remained stable. Two years later, because of loss of efficacy to adalimumab, the patient was switched to etanercept and also started on lamivudine prophylaxis 100 mg/day. Since then, her HBV DNA viral load has dropped to <300 copies/ml.
Discussion
There is only limited clinical and no histological data on the effect of anti-TNF alpha agents on the liver of patients who suffer from rheumatic diseases and have concurrent HBV or HCV infection.
Case 1 describes the first histological features of the liver of an RA patient with chronic HCV being treated with a TNF-alpha antagonist. The histological absence of acute liver injury, with a rise in HCV PCR levels, is noteworthy, although the significance of a liver biopsy 2 months into TNF-alpha antagonist therapy is not known. Nonetheless, the current literature suggests that TNF-alpha antagonist therapy appears to be safe in patients with chronic HCV [8] who require this treatment for RA.
Case 2 is the first report of liver biopsy results in a rheumatoid arthritis patient with concurrent chronic hepatitis B infection (HBsAg and HBcAB positive and detectable HBV DNA viral load by PCR) being treated with a TNF-alpha antagonist. The absence of histological evidence of hepatic reactivation or acute injury is again noteworthy, although the significance of a liver biopsy 4 months into TNF-alpha therapy is not known. The initiation of lamivudine prophylaxis significantly dropped her viral load and her liver functions and viral load has been stable on TNF-alpha antagonist therapy for over 3 years.
Case 2 contrasts to the two reported cases of acute HBV activation occurring in patients with rheumatic diseases being treated with infliximab [5, 6] and provides additional data to the recent reports of the long-term safety of the use of TNF-alpha antagonist agents for rheumatoid arthritis in patients with chronic HBV infection [7, 9–10].
We had planned to follow both the patients with serial biopsies, but case 1 transferred his care to another institution and in case 2, the HBV viral loads have dropped and are stable with lamivudine prophylaxis, so we are withholding rebiopsy at this point.
Although information is accumulating regarding TNF-alpha blockade in patients coaffected with rheumatologic diseases and chronic viral hepatitis, a lot of unanswered issues remain including the effect of concurrent treatment with antiviral and anti-TNF alpha therapy. Serial histological evaluation along with serological markers will provide valuable information regarding the treatment effect of TNF-alpha antagonism on chronic hepatitis B and C, but we favor further evaluation.
References
Fang JW, Shen WW, Meager A, Lau JY (1996) Activation of the tumor necrosis factor-alpha system in the liver in chronic hepatitis B virus infection. Am J Gastroenrol 91(4):748–753
Kallinowski B, Haseroth K, Marinos G, Hanck C et al (1998) Induction of tumour necrosis factor (TNF) receptor type p55 and p75 in patients with chronic hepatitis C virus (HCV) infection. Clin Exp Immunol 111:269–277
Nelson DR, Lim HL, Marousis CG, Fang JW et al (1997) Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig Dis Sci 42:2487–2494
Magrin S, Craxi A, Fabiano C et al (1994) Hepatitis C viremia in chronic liver disease: relationship to interferon-alpha or corticosteroid treatment. Hepatology 19(2):273–279 (Feb)
Ostuni P, Botsios C, Punzi L, Sfriso P et al (2003) Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis 62:686–687
Michel M, Duvoux C, Hezode C, Cherqui D (2003) Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset still’s disease. J Rheumatol 30(7):1624–1625 (Jul)
Oniankitan O, Duvoux C, Challine D, Mallat A et al (2004) Infliximab therapy for rheumatic diseases in patients with chronic hepatitis B or C. J Rheumatol 31:107–109
Calabrese LH, Zein N, Vassilopoulos D (2004) Safety of antitumour necrosis factor (anti-TNF) therapy in patients with chronic viral infections: hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis 63(Suppl 2):ii18–ii24
Sakellariou GT, Chatzigiannis I (2007) Long term anti-TNF alpha therapy for ankylosing spondylitis in two patients with chronic HBV infection. Clin Rheumatol 26:950–952
Roux C, Brocq O, Breuil V et al (2006) Safety of anti-TNF-alpha therapy in rheumatoid arthritis and spondylarthropathies with concurrent B or C chronic hepatitis. Rheumatology 45:1294–1297
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, P.P., Chan, V.C. & Berney, S.N. Histological evaluation of liver in two rheumatoid arthritis patients with chronic hepatitis B and C treated with TNF-alpha blockade: case reports. Clin Rheumatol 27, 1069–1071 (2008). https://doi.org/10.1007/s10067-008-0896-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-008-0896-y