Abstract
Oxidative stress is suggested to be involved in the pathogenesis of systemic sclerosis (SSc). The aim of the present study was to clarify such a hypothesis by determination of four different plasmatic parameters of oxidative stress, and to define its role in the microvascular damage, assessed by nailfold capillaroscopy (NC). Plasma samples of 18 patients with SSc were analyzed. The biomarkers measured were: total antioxidant capacity, hydroperoxides (ROOHs), and sulfhydryl (SH) and carbonyl (CO) groups. Each patient had a detailed clinical assessment and underwent an NC. The results showed significantly increased ROOHs in SSc patients compared to control group (5.02 ± 0.24 vs 3.28 ± 0.19 μmol/l; p < 0.05). Plasmatic levels of SH groups were significantly lower in SSc (0.466 ± 0.08 mmol/l) compared to control group (0.542 ± 0.04 mmol/l; p < 0.002). Plasma levels of ROOHs correlated with the capillaroscopy semiquantitative rating scale score (p < 0.05) and with the rating system for avascular areas (p < 0.03). The levels of CO groups inversely correlated with modified Rodnan’s skin score (p < 0.039) and were lower in patients with pulmonary fibrosis (p < 0.045), while the levels of SH groups were lower in those presenting gastrointestinal involvement (p < 0.029). The obtained data indicate augmented free radical-mediated injury in SSc and also show correlations among oxidative abnormalities, some clinical findings, and signs of a more severe microvascular involvement. These results give more evidence to the connection between oxidative impairment and SSc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a generalized multisystem disorder characterized by microvascular damage leading to tissue fibrosis accompanied by severe complications. Its etiology and pathogenesis have not been fully understood, and it is believed that the causes are rather complex. The vascular disease is one of the main aspects under investigation, and it is well known that endothelial abnormalities occur early and may drive the fibrotic disease process; what initiates these abnormalities is not known [1]. Changes of the vascular system lead to a dysfunction of the control of vascular tone, i.e., Raynaud’s phenomenon, triggering a cascade of events in which there is increasing evidence to suggest that oxidative stress (OS), mediated by free radicals, is one of the major players [2, 3]. The way reactive oxygen and nitrogen species (RONS) can contribute to the pathogenesis of vascular disease is very extensive. They may damage endothelial cells’ function directly by chemical modification of macromolecules via peroxidation of lipids and oxidation of proteins or by activating various proinflammatory cytokines which further initiate cascades of processes leading to activation of fibroblasts and immune cells.
In most SSc patients, Raynaud’s phenomenon is the first symptom to occur, usually preceding the development of the disease by months or even years. Provoking frequent episodes of hypoxia reperfusion, it is able to induce the production of RONS responsible for endothelial injury [4]. However, Raynaud’s phenomenon and related events are not the only possible source of free radicals in the microvasculature. Other factors include activated polymorphonuclears, which can produce high amounts of RONS along with proteases and also inducible NO synthase, which can generate pathological excess of NO [5, 6]. These early events are often followed by small vessel structural changes and ischemia. The birth of a vicious circle of RONS generation along with related inflammatory processes leads to further endothelial damage, obliteration of microvasculature, and fibrosis [2].
Among other possible concurrent factors, an abnormal susceptibility to oxidative damage, mostly due to deteriorated antioxidant defense system, has been proposed in the determinism of SSc vascular damage; thus, generated RONS cannot be effectively scavenged due to alterations in physiological antioxidant defenses. Moreover, the evidence that RONS may directly alter DNA and proteins from apoptotic cells and thus contribute to the development of autoimmune responses gives further effort to the hypothesis that free radicals might provide a link between the vascular and immune abnormalities in SSc [4, 7].
The extent of free radical-mediated injury is reflected in increased levels of different products of oxidative reactions. Because RONS can attack all biological macromolecules, there has been a wide range of assays determining various OS parameters in vivo. The first sensitive targets for attacks of RONS are unsaturated fatty acids of cells’ lipid membranes, yielding many products including hydroperoxides (ROOHs); free radical-mediated oxidation of proteins may similarly lead to many end-products, such as carbonyl groups (CO) [8, 9].
To clinically evaluate the severity of microvascular involvement in SSc, nailfold capillaroscopy (NC) proved to be a noninvasive technique able to detect microcirculatory abnormalities that may have diagnostic and prognostic value [10]. Specific NC features have been described in SSc patients, and different patterns have been proposed to better assess the microvascular damage [11].
The role of free radicals in the pathogenesis of SSc, and their correlation with other features of the disease, is not completely clarified. The objective of the present study was to assess four different parameters reflecting OS status changes in plasma samples of 18 SSc patients, to compare them with healthy controls, evaluating the main clinical and laboratory parameters involved in the disease expression, as well as the NC abnormalities, and to look for the first time to possible connections among these features, thereby help better clarifying the OS involvement in SSc pathogenesis.
Materials and methods
Eighteen consecutive outpatients fulfilling the SSc criteria proposed by the American College of Rheumatology [12] were recruited from the Division of Rheumatology of Rome University, giving their informed consent.
Patients had a detailed clinical assessment, and their organ involvement was investigated. The severity of skin thickening was determined using modified Rodnan’s skin score [13]. Organ system involvement was defined as previously described [14]. Antinuclear antibodies (ANA) including anticentromere antibodies were detected by indirect immunofluorescence (Bio-Rad, Redmond, WA, USA). Antibodies against topoisomerase I (anti-Scl70) were measured using an enzyme-linked immunosorbent assay (Diamedix, Miami, FL, USA).
Nailfold capillaroscopy
Each patient underwent NC, performed according to the standard method [10] by the same investigator (VR). Only capillaries in the distal row were analyzed and scored, and fingers affected by recent local trauma were not analyzed. The following morphological parameters were considered, according to previous classifications: presence of enlarged and giant capillaries, hemorrhages, loss of capillaries (avascularity), disorganization of the vascular array, ramified/bushy capillaries, and sludge of blood [10, 15]. A semiquantitative rating scale was adopted to score these changes, according to previous studies [11]: score 0 = no changes; 1 = few = <4 alterations; 2 = some = between 4 and 6 alterations; 3 = frequent = >6 alterations per linear millimeter. The mean score for each subject was obtained from analysis of all the fingers.
The rating system for avascular areas (avascularity of the capillary bed) was classified as grade 0=normal= no obvious avascular areas; grade 1 = mild = one or two discrete areas of vascular deletion; grade 2 = moderate = more than two discrete areas of vascular deletion; grade 3 = severe = the presence of large, confluent avascular areas [16].
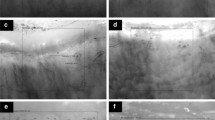
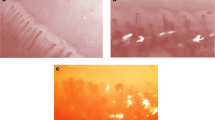
Patients were distributed into a proper NC pattern, as already reported [11]. The patterns were (a) “early” (few giant capillaries, few hemorrhages, relatively preserved capillary distribution, no evident loss of capillaries); (b) “active” (frequent giant capillaries, frequent hemorrhages, moderate loss of capillaries with some avascular areas, mild disorganization of the capillary architecture, absent or some ramified capillaries); (c) “late” (irregular enlargement of the capillaries, few or absent giant capillaries, absence of hemorrhages, severe loss of capillaries with large avascular areas, severe disorganization of the normal capillary array, frequent ramified/bushy capillaries).
Samples
The assays we employed to measure four different oxidative stress parameters were a ferric reducing antioxidant power assay for total antioxidant capacity (TAC); a ferrous ion oxidation assay measuring ROOHs, a marker of lipid peroxidation; assays for determination of sulfhydryl (SH) groups/thiols and CO groups, both reflecting oxidation of proteins, performed by spectrophotometric methods, as previously described [17, 18].
Venous blood was collected into sodium citrate-coated tubes for ROOHs and TAC measurements or EDTA tubes for the measurement of CO and SH groups. Plasma was separated within 30 min, and samples were analyzed within 3 h of collection. The rest of plasma samples were divided into aliquots and stored at −70°C within 1 h after collection for later measurements. TAC, ROOHs, and SH groups were measured in fresh samples, while CO groups were assessed on the next day, according to previous report [18].
Controls consisted of 16 healthy volunteers with no acute or chronic inflammatory disease and under no medication.
Statistical analysis
Statistical analysis was performed using the program SigmaStat version 3.0 for Windows (SPSS, Chicago, IL, USA). Categorical variables were analyzed by χ 2 test or Fisher’s exact test. All the differences were expressed as mean ± standard deviation. The significance of the differences was determined using independent samples t test. The significance of any correlation was determined by Pearson’s correlation test. Values of p less than 0.05 were considered significant.
Results
The clinical, capillaroscopy, and laboratory data reported in this study were obtained at the time the blood samples were drawn. Summary of patients’ characteristics is shown in Table 1.
A capillaroscopic score = 3 was found in eight patients (44%), and nine (50%) had an avascular areas score >grade 1. The “early” NC pattern was observed in six patients (33%), the “active” pattern was found in seven patients (39%), and the “late” pattern in five cases (28%; Figs. 1, 2, and 3).
In SSc patients, TAC, although higher, was not significantly different from the control group (0.380 ± 0.08 mmol/l vs 0.326 ± 0.07 mmol/l), while ROOH levels, measured in 15 patients only, were significantly increased (5.02 ± 0.24 μmol/l vs 3.28 ± 0.19 μmol/l; p < 0.05). Besides, plasma SH levels were decreased as compared with healthy controls (0.466 ± 0.08 mmol/l vs 0.542 ± 0.04 mmol/l; p < 0.002), while the levels of CO groups in our patients did not differ from the controls (2.27 ± 0.83 nmol/mg of proteins vs 2.11 ± 0.83 nmol/mg of proteins) (Table 2). These data have been partly described in our recent report concerning different systemic rheumatic diseases, although no clinical or microvascular aspect was investigated [19].
In our patients, plasma ROOH levels correlated with the capillaroscopy semiquantitative rating scale score [11] (p < 0.05) and with the rating system for avascular areas [19] (p < 0.03).
The levels of CO groups inversely correlated with modified Rodnan’s skin score (p < 0.039; Fig. 4) and were significantly lower in patients with pulmonary fibrosis (p < 0.045), while the levels of SH groups were significantly lower in those presenting gastrointestinal involvement (p < 0.029).
Discussion
The present study investigated and confirmed that increased OS occurs in SSc. In our 18 SSc patients, two of four investigated parameters were found significantly different from the control group. Namely, we found higher ROOHs and lower SH groups suggesting that free radical-mediated injury occurs in SSc. Elevated ROOHs also confirm previously reported increase in lipid peroxidation, although other studies evaluated different markers [3]. The decrease in plasmatic thiols indicates oxidation of proteins, and a similar reduction in SH groups has been also previously reported [20]. However, this fact is not supported by our CO group-evaluating assay where no significant difference was found. Up to our knowledge, there is only one previous study regarding CO values in SSc, which reports higher values in these patients compared to controls [21].
Inconsistently with above mentioned findings implicating increased OS in SSc, TAC was not decreased, but even higher compared to healthy controls. It goes also against the assumption based on other authors’ studies describing impairment in antioxidant defenses in SSc [3, 22]. A hypothetical explanation of the resulted TAC may lie in some unknown feedback mechanism being a response to increased oxidative stress [23]. Likewise adaptation to increased OS may induce an overproduction of antioxidant enzymes and other endogenous molecules such as ceruloplasmin or glutathione to manage the rise of RONS [24]. The antioxidant/chelating properties of these substances may partly be able to keep the TAC higher.
More interestingly, the increase in ROOH levels in those patients showing more severe NC changes, such as higher capillaroscopic score and avascular areas score, suggests that this OS parameter modifies along with the worsening of the state of microvasculature, as assessed by NC.
No connection with plasma levels of TAC, SH, and CO groups was found in this respect, though. Defective oxidative function, in our patients, includes deterioration of antioxidant defenses as defined by decreased TAC, oxidation of proteins as assessed by reduced SH groups, and also lipid peroxidation with elevated ROOHs. We previously reported the same oxidative changes to be present in other chronic diseases, such as rheumatoid arthritis and psoriatic arthritis [19]; thus, disease specificity is not clear, but these findings together support the hypothesis that alterations in physiological antioxidant defenses can generate reactive oxygen products within differently inflamed tissues, provoking similar impairment in different conditions [25].
Besides, we showed for the first time that some of the evaluated OS parameters had interesting associations with differently relevant clinical aspects of SSc, indicator of disease severity, such as higher skin score and presence of pulmonary fibrosis and gastrointestinal involvement. The significant association of OS changes with a more severe cutaneous and visceral involvement clearly supports the role for oxidative damage in the determinism of some of the disease features, leading to the hypothesis of a possible role for free radical-mediated injury in the assessment of the disease.
In conclusion, our data support previous findings on the occurrence of OS in SSc, showing some differences regarding the oxidative biomarkers hereby examined. The significant correlation among the plasmatic levels of these substances, some clinical findings, and the severity and extent of the microvascular involvement in our patients, shown by NC findings, further emphasizes the relationship between oxidative impairment and SSc.
Finally, our findings also imply a possible benefit for antioxidant treatment on the vascular architecture in SSc. Future trials focused on patients in earlier stages, along with improved study design, can clarify the real clinical effect of antioxidant therapy.
References
Kahaleh MB, LeRoy EC (1999) Autoimmunity and vascular involvement in systemic sclerosis. Autoimmunity 31:195–214
Murrell DF (1993) A radical proposal for the pathogenesis of scleroderma. J Am Acad Dermatol 28:78–85
Simonini G, Matucci Cerinic M, Generini S, Zoppi M, Anichini M, Cesaretti C, Pignone A, Falcini F, Lotti T, Cagnoni M (1999) Oxidative stress in systemic sclerosis. Mol Cell Biochem 196:85–91
Herrick AL, Matucci Cerinic M (2001) The emergin problem of oxidative stress and the role of antioxidants in systemic sclerosis. Clin Exp Rheumatol 19:4–8
Yamamoto T, Katayama I, Nishioka K (1998) Nitric oxid production and inducible nitric oxide synthase expression in systemic sclerosis. J Rheumatol 25:314–317
Matucci Cerinic M, Kahaleh MB (2002) Beauty and the beast. The nitric oxide paradox in systemic sclerosis. Rheumatology (Oxford) 41:843–847
Ahsan H, Ali A, Ali R (2003) Oxigen free radicals and systemic autoimmunity. Clin Exp Immunol 131:398–404
Pratico D (2001) In vivo measurement of the redox state. Lipids 36(suppl):45–47
Deaney CL, Feyi K, Forrest CM, Freeman A, Harman G, McDonald MS, Petrie A, Shaw SJ, Stone TW, Stoy N, Darlington LG (2001) Levels of lipid peroxidation products in a chronic inflammatory disorder. Res Commun Mol Pathol Pharmacol 110(1–2):87–95
Maricq HR (1981) Widefield capillary microscopy: technique and rating scale for abnormalities seen in scleroderma and related disorders. Arthrits Rheum 24:1159–1165
Cutolo M, Sulli A, Pizzorni C, Accardo S (2000) Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 27(1):155–160
Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee (1980) Preliminary Criteria for the Classification of Systemic Sclerosis (Scleroderma). Arthritis Rheum 23(5):581–590
Kahaleh MB, Sultany GL, Smith EA, Huffstutter JE, Loadholt CB, Le Roy EC (1986) A modified scleroderma skin scoring method. Clin Exp Rheumatol 4(4):367–369
Steen VD, Powell DL, Medsger TA (1988) Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum 31:196–203
Kabasakal Y, Elvins DM, Ring EFJ, McHugh NJ (1996) Quantitative nailfold capillaroscopy findings in a population with connective tissue disease and in normal healthy controls. Ann Rheum Dis 55:507–512
Lee P, Leung FYK, Alderice C, Armstrong SK (1983) Nailfold capillary microscopy in the connective tissue diseases: a semiquantitative assessment. J Rheumatol 10:930–938
Firuzi O, Giansanti L, Vento R, Seidert C, Petrucci R, Marrosu G, Agostino R, Saso L (2003) Hypochlorite scavenging activity of hydroxycinnamic acids evaluated by a rapid microplate method based on the measurement of chloramines. J Pharm Pharmacol 55:1021–1027
Firuzi O, Mladenka P, Riccieri V, Spadaro A, Petrucci R, Marrosu G, Saso L (2006) Parameters of oxidative stress status in healthy subjects; their correlations and their stability after sample collection. J Clin Lab Anal 20:139–148
Firuzi O, Fuksa L, Spadaro C, Bousova I, Riccieri V, Spadaro A, Petrucci R, Marrosu G, Saso L (2006) Oxidative stress parameters in different systemic rheumatic diseases. J Pharm Pharmacol 58:951–957
Lau CS, O’Dowd A, Belch JJ (1992) White blood cell activation in Raynaud’s phenomenon of systemic sclerosis and vibration induced white finger syndrome. Ann Rheum Dis 51:249–252
Borderie D, Allanore Y, Meune C, Devaux JY, Ekindjian OG, Kahan A (2004) High ischemia-modified albumin concentration reflects oxidative stress but not myocardial involvement in systemic sclerosis. Clin Chem 50:2190–2193
Herrick AL, Rieley F, Schofield D, Hollis S, Braganza JM, Jayson MIV (1994) Micronutrient antioxidant status in patients with primary Raynaud’s phenomenon and systemic sclerosis. J Rheumatol 21:1477–1483
Prior RL, Cao G (1999) In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med 27:1173–1181
Morita A, Minami H, Sakakibara N, Sato K, Tsuji T (1996) Elevated plasma superoxide dismutase activity in patients with systemic sclerosis. J Dermatol Sci 11:196–201
Halliwell B (1995) Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann Rheum Dis 54:505–510
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riccieri, V., Spadaro, A., Fuksa, L. et al. Specific oxidative stress parameters differently correlate with nailfold capillaroscopy changes and organ involvement in systemic sclerosis. Clin Rheumatol 27, 225–230 (2008). https://doi.org/10.1007/s10067-007-0769-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-007-0769-9