Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory arthritic and potentially disabling condition, mainly affecting women of middle age and having characteristic clinical features. Various microbial agents were implicated in the causation of RA. Extensive literature based on the results of various genetic, microbiological, molecular, and immunological studies carried out by independent research groups supports the role of Proteus mirabilis bacteria in the etiopathogenesis of RA. New diagnostic markers and criteria and the use of a novel therapeutic protocol in the form of antibiotic and dietary measures are suggested to be used together with current treatments in the management of RA. Prospective longitudinal studies with the use of antimicrobial measures in patients with RA are required to establish the therapeutic benefit of this microbe–disease association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory polyarthritic disease associated with remissions and exacerbations and characteristic genetic, clinical, pathological, and immunological features. The typical clinical presentation of RA is a symmetrical peripheral joint arthritis of insidious onset with positive rheumatoid factors (RF) and progressive erosions of the affected joints occurring predominantly in women of middle age groups. It is a relatively common rheumatic disease with a worldwide distribution. RA causes significant clinical problems and is also responsible for substantial economic and social costs, particularly from work-related disability [1]. In the years between 1979 and 1998, it was observed that more than 20% of deaths from various rheumatic conditions occurred in patients with RA [2].
There are as yet no biochemical or immunological tests with combined high sensitivity and specificity to diagnose and/or monitor the disease activity in RA. This would appear to limit the management of RA patients during the early stages of the disease, especially before the occurrence of irreversible arthritic sequelae. In this review paper we have examined the evidence linking Proteus microbes to RA and tried to exploit this association to develop novel diagnostic criteria, which could be helpful in the therapeutic management of patients, especially during the early stages of this disease.
Etiopathogenesis
There is a general consensus that RA results from the interplay of genetic and environmental factors.
Genetic factors
During the last three decades many studies were carried out on the role of genetic factors in the pathogenesis of RA [3].
RA-associated HLA markers
For decades, a significant genetic contribution for the development of RA was widely accepted and is estimated to account for nearly 60% of the disease risk [4]. Despite extensive studies, no genes other than HLA-DRB1 have yet been more clearly demonstrated to be involved in RA. The association of RA with HLA-Dw4 was first reported in 1976 [5]. With the use of more sensitive techniques, it was later discovered that various HLA-DR4 and non-DR4 alleles are associated with RA among different populations [3]. Of these alleles, HLA-DR4 subtypes (DRB1*0404 and *0405), HLA-DR1 (DRB1*0101), and HLA-DR6 (DRB1*1402) are associated, while other HLA-DR4 subtypes (DRB1*0402 and 0403) would appear not to be linked to RA [6]. This discrepancy in the association of different HLA-DRB1 genes with RA was found to be due to the presence of a conserved hexameric amino acid sequence in the third hypervariable regions of all RA-associated HLA-DRB1 alleles involving positions 69–74 and consisting of glutamic acid, glutamine, lysine or arginine, arginine, alanine, and alanine “EQK/RRAA.” This common sequence in RA was given the name of the “shared epitope” (SE) [7]. More than 90% of RA patients were reported to possess one or more of these alleles, which carry the “SE” moiety [8]. Any theory about the origin of RA must explain this highly significant HLA–disease association. The SE contribution to the development of RA is proportional to the degree of heterogeneity in RA-associated HLA-DR alleles, where it was shown that the relative risk for the development of RA is considerably increased in those with higher numbers of SE-positive sequences [9] present within different RA-associated HLA-DRB1 molecules. In a meta-analytic study of 3,240 patients with RA, an increased association of the SE with the development of erosive disease was observed among many ethnic groups [10].
However, the concordance rate among monozygotic twins with RA is quite low (<15%) [11, 12], suggesting the role of other nongenetic, environmental factors in this condition.
Environmental factors
Among various environmental factors, which were implicated in the causation of RA, infectious agents are the most likely culprits. A variety of viral and bacterial candidates were proposed, but most of these were later discarded because of insufficient evidence.
-
1.
Viruses: Viral infection due to Epstein–Barr virus (EBV), human parvovirus B19, or rubella viruses, each of which was suggested to have a role in RA etiopathogenesis, usually results in acute polyarthritis of sporadic and self-limiting nature. Furthermore, these viruses are ubiquitous and affect most, if not all, people at some stages in their life.
-
1.1
EBV: EBV is one of the most likely candidates because of the existing evidence for molecular mimicry and positive immune responses associated with these viral agents in patients with RA [13]. However, there are certain problems with this hypothesis. First, it is known that EBV establishes persistent infection in more than 90% of the human adult population worldwide early in childhood [14]. Therefore, it is not unexpected to find enhanced humoral and cellular immune responses in the majority of patients with RA. Second, EBV is the cause of various nonrheumatic diseases, such as infectious mononucleosis and malignancies like Burkitt’s lymphoma and nasopharyngeal carcinoma, and no clear-cut and constant relationship has yet been shown between these conditions and RA [15]. Third, in a prospective study no significant association could be found between positive polymerase chain reaction (PCR) for EBV or CD8 T cell response to EBV and RA disease activity [16]. Fourth, the onset of RA starts predominantly in middle-aged females, while EBV exposure usually occurs in young children before the age of 5 years. Finally, others could not find increased EBV particles in the joint tissues of RA patient [17, 18]. Therefore, the presence of EBV-positive lymphocytes in the synovial membrane is likely to be an epiphenomenon unrelated to the pathogenesis of RA.
-
1.2
Parvovirus B19: Clinical and immunological human [19] and experimental animal [20] studies have supported the role of parvovirus B19 in the pathogenesis of RA, but this could not be confirmed by others [21]. Apart from certain clinical disorders like erythema infectiosum and hydrops fetalis, parvovirus B19 was also implicated in other rheumatic diseases such as systemic lupus erythematosus and being the triggering agent in the production of various nonorgan specific autoantibodies, such as antinuclear, anti-DNA, anti-SSA/SSB, and antiphospholipid antibodies [22].
-
1.3
Rubella and other viruses: A similar lack of evidence occurs with rubella [23], mumps, and measles viruses [24] in RA. In a recent study, patients with RA and those with psoriatic arthritis and reactive arthritis were found to possess signs of replicative viral infections involving EBV and cytomegaloviruses [25].
-
1.1
-
2.
Bacteria
Amid bacterial candidates, apart from Proteus microbes, three other bacterial agents have mainly attracted attention in recent years as possible etiological triggers in RA. These include, mycobacteria [26], mycoplasma [27], and Escherichia coli [28], but there are some limitations in the proposed role for these bacteria.
-
2.1
Mycobacteria: One of the mycobacterial species is the cause of tuberculosis (TB), a chronic infectious disease affecting the lungs that usually responds to treatment with antimycobacterial therapy. Although no strong epidemiological link has yet been discovered between RA and TB, a mild increase in the risk of encountering TB in patients with RA was reported more recently, but this increase was found to be even higher among patients receiving biological therapies [29]. The suggested triggering mycobacterial antigens in RA, heat shock proteins 65 kD (hsp65), which are also found in other bacterial species [30], showed significant differences in sequences and epitopes with human hsp [31]. Similar immune responses to hsp65 were also found at other inflammatory sites, such as in pleural effusions, which indicates that enhanced reactivity against mycobacterial antigens seems to be a common feature of chronic inflammation [32]. Furthermore, using a PCR gene amplification method, no significant DNA extracts from five mycobacterial species could be detected in the synovial fluid and tissue samples from RA patients [33].
-
2.2
Mycoplasma: Some groups have reported an increased frequency of systemic mycoplasma infection in RA compared to controls [34]. However, a similar prevalence for infection by mycoplasma was also reported in patients with other chronic inflammatory rheumatic diseases, such as spondyloarthropathy [35]. Apart from serological evidence of previous mycoplasma exposure in RA patients and in disease controls, other groups were not able to detect mycoplasma DNA in the synovial samples of these patients [36].
-
2.3
E. coli and other microbes: E. coli shows molecular similarity with RA-associated HLA antigens [37] and patients with RA were shown to have elevated immune responses to the hsp60 antigens of this microbe [38]. However, no significant associations between humoral immune responses to E. coli and RA could be shown in more than six other investigations (Table 1).
In a previous study, no clear or specific relationship was found between DNA extracts from single or multiple bacterial species in the synovium from 237 patients with various arthritides, including RA. Ten percent of patient samples were PCR-positive in panbacterial screening assays. Bacterial species identified belonged to the genera Neisseria, Acinetobacter, Moraxella, Salmonella and Pseudomonas. Thirty-five percent of PCR-positive patients showed the presence of DNA from more than a single bacterial species in synovium [39]. Hence, it seems that RA is not due to the direct invasion of the joints by microbial agents or particles, but could result from the effects of immune responses to triggering microbes residing at remote sites.
-
2.1
“RA” and Proteus
Many studies support the role of Proteus mirabilis in the etiopathogenesis of RA, and these involve investigations on the microbiological, molecular, and immunological characteristics of these bacteria in patients with RA carried out by independent groups:
-
(1)
Sera from rabbits immunized with HLA-DR4 positive lymphocytes were found to bind only to Proteus species in comparison to 18 other microbes [40].
-
(2)
HLA-DR4 allogeneic tissue typing sera were binding more significantly to P. mirabilis than to E. coli microbes [41].
-
(3)
Molecular similarity was observed between the “ESRRAL” amino acid sequences present in hemolysins of P. mirabilis and the “EQ/KRRAA” SE motif present in the RA-associated HLA-DR molecules [42].
-
(4)
Molecular similarity was also found between the “LRREI” amino acid sequences present in Proteus urease enzymes and the “IRRET” amino acid motif found in type XI collagen [43].
-
(5)
Antiserum against a 15-mer synthetic peptide containing the RA susceptibility sequence EQ/KRRAA was shown to bind more significantly to a 16-mer peptide comprising the ESRRAL amino acid sequence from P. mirabilis hemolysin molecules, and anti-ESRRAL serum reacted similarly to the EQ/KRRAA peptides [44].
-
(6)
Anti-ESRRAL peptide antibodies were found to bind to mouse fibroblast transfectant cell lines expressing HLA-DRB1*0401, but not to HLA-DRB*0402, which is not expressing the SE sequence [44].
-
(7)
Using ELISA and immunoblotting methods, analysis of urine from 76 patients with RA and 48 healthy controls showed that only 4% of the control urines but 33% of those from the RA patients were infected. The commonest infecting organism in the RA patients’ urine was P. mirabilis, which occurred twice as frequently as E. coli microbes. P. mirabilis was found in 52% of the infected urines of the RA patients. Comparison of antibody levels to P. mirabilis showed that RA patients had significantly increased levels of IgA, IgG, and IgM antibodies in their sera and urine compared with the control group [45].
-
(8)
IgG antibodies from RA patients were found to have cytotoxic activities against HLA-DR4-peptide-bearing cells as shown by increased hemolysis for sheep red blood cells coated with HLA-DRB1*0404 peptides comprising the “EQRRAA” amino acid sequences when compared to patients with ankylosing spondylitis and healthy control subjects [46].
-
(9)
During the last three decades many serological studies were carried out by different independent groups on microbial antibodies against various microbial antigens in patients with RA and other rheumatic diseases. Antibodies against P. mirabilis were found to be significantly elevated among a total of 1,375 RA patients, but not in other diseases or healthy subject controls from more than 15 different countries [47]. The high titers of anti-Proteus antibodies in RA patients would appear to be specific because there was no such elevation in antibodies against 27 other microbial agents (Table 1).
Upper urinary tract as the main source of Proteus infection in RA
A number of studies support a link between upper urinary tract infection (UTI) due to Proteus microbes and RA.
-
(1)
Microbiological studies carried out on 89 RA patients showed that P. mirabilis microbes were isolated from the urine of 63% female and 50% male patients, compared to a frequency of isolation in 32% and 11% of healthy women and men, respectively [48]. Furthermore, anti-Proteus antibody levels were found to be increased in the urine of RA patients [45].
-
(2)
Serum anti-Proteus antibody levels were found to correlate significantly with the isolation rate of P. mirabilis from the midstream urine specimens of RA patients [49].
-
(3)
P. mirabilis microbes especially those of proticine type 3 strains have a higher affinity to affect the upper urinary tract or kidneys [50].
-
(4)
P. mirabilis is considered to be the second most common causative microbe of UTIs after E. coli [51].
-
(5)
There are reports of increased incidence of UTIs in patients with RA [52, 53]. However, these observations were not confirmed by other groups [54]. This discrepancy in the epidemiological link between urinary infections and RA could be due to the occurrence of subclinical or occult infections, which are merely characterized by bacteriuria. This was recognized in the results of two independent studies in which RA patients were found to have asymptomatic Proteus bacteriuria more frequently than healthy controls [45, 48].
It is relevant to note that RA occurs three to four times more often in women than men and women are ten times more likely to have UTIs than men, hence, these observations could explain why RA is more prevalent in women than men.
Molecular mimicry as a possible pathogenetic mechanism in RA
Rheumatic fever is the prototypic disease for molecular mimicry, which is suggested to be one of the main mechanisms involved in the pathogenesis of other immune-mediated rheumatic diseases.
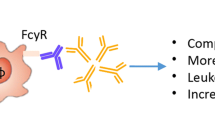
Molecular similarities or cross reactivities exist between P. mirabilis and various self-antigens (Fig. 1). Antibodies against these bacterial antigens bind to the tissues carrying these self-antigens in vitro [46].
It should be noted that only a small percentage of HLA-DR4/1 positive individuals are prone to develop RA. It is only individuals with high titers of antibodies against Proteus microbes are liable to undergo joint inflammation with consequent pathological damages and development of RA. Patients with inactive (having normal ESR and/or CRP levels) RA, however, do not have elevated anti-Proteus antibodies [55].
A positive correlation exists between the levels of antibodies against P. mirabilis and the concentration of acute phase proteins in patients with RA [56]. Activation of complements and other inflammatory cascades can only be accomplished in those patients with high anti-bacterial antibody titers because two Fc molecules of IgG are required to activate the C1q component of the complement system.
Failure to detect microbial DNA products at the main pathological sites, articular tissues, when analyzed by gas chromatography–mass spectrometry and panbacterial PCR [57] supports the suggestion that the immune system in patients with RA could be triggered by microbes residing at remote sites.
Repeated infections with Proteus microbes, which are most likely to be subclinical will result in the production of high titers of bacterial antibodies. The binding of these antibodies to the cross-reactive self-antigens will then trigger a cascade of inflammatory responses sequentially evoking production of various cytokines, chemokines, complement, and proteinases leading to a local inflammation with or without systemic effects. In addition, such activation may have a “bystander” effect on other immune cells, including T lymphocytes, which might result in further enhancement of the inflammatory immune responses, with the development of arthritis and extraarticular and systemic features of this disease.
Proteus infection can explain some prominent features in RA
The involvement of Proteus, but not other microbes in RA, could explain some of the characteristic features of this disease.
RA-specific features
-
(1)
Women in their 30s to 50s are mainly affected by RA and UTIs are more common in females in these age groups.
-
(2)
RA is most commonly associated with HLA-DR alleles, which carry the SE moieties, and these antigens are cross-reactive with Proteus hemolysin.
-
(3)
RF forms part of the American Council of Rheumatology (ACR) diagnostic criteria for RA. Extensive evidence supports the notion that RFs are produced as the result of triggering microbial infections [58, 59] and Proteus microbe could be one of them.
-
(4)
Characteristic small joints involvement occurs in RA. Elevated levels of antibodies to LRREI peptides present in the Proteus urease enzymes, which are cross reactive with IRRET amino acids present in collagen type XI, a component of hyaline cartilage, could explain the small joints involvement.
New diagnostic criteria and therapeutic protocol
Diagnosis
Currently, the diagnosis of either RA is based on the presence of certain clinical and radiological features, where patients can be categorized into different groups according to the ACR criteria [60]. The requirement for using sensitive and specific laboratory diagnostic markers are of crucial importance to identify patients and/or monitor disease activity status during treatment. Various laboratory markers, including RFs, were proposed to fulfill these requirements in RA [61]. These proposed diagnostic markers should be able to identify patients during the early stages of the disease, before the appearances of irreversible pathological sequelae.
We propose that individuals presenting with inflammatory mono/polyarthritis of more than 3 months could be identified as patients with “early RA” if they possess positive SE genetic markers and high anti-Proteus hemolysin and urease peptide antibodies [62] (Table 2). These diagnostic criteria could be supported further with an increased isolation of Proteus from urinary tract in RA patients. The appearance of other clinical and radiological features of these diseases would be of further help in reaching a final diagnosis.
Treatment
Drugs used in RA are of various types from nonsteroidal anti-inflammatory drugs and disease modifying antirheumatic drugs [63] to biological agents such as antitumor necrosis factor antagonists [64]. However, most, if not all, of these drugs are considered as nonspecific in preventing or stopping the pathological processes in RA. Some of these, especially the biological agents, are expensive [65] and might possess deleterious or even lethal side effects [66]. Management of RA could be improved through the use of more specific methods aimed at the eradication of Proteus microbes, and this could be achieved through the use of antimicrobial therapies involving antibiotics and/or dietary manipulation.
In conclusion, extensive scientific evidence suggests an important role of Proteus in RA. The presence of HLA antigens and elevated Proteus antibodies in RA could be helpful in the identification of patients with these conditions during early stages of disease. The use of new therapeutic modalities in the form of antimicrobial therapies in conjunction with the currently available treatments should be evaluated in longitudinal studies.
References
Cooper NJ (2000) Economic burden of rheumatoid arthritis a systematic review. Rheumatology 39:28–33
Sacks JJ, Helmick CG, Langmaid G (2004) Deaths from arthritis and other rheumatic conditions, United States, 1979–1998. J Rheumatol 31:1823–1828
Turesson C, Mattesson EL (2006) Genetics of rheumatoid arthritis. Mayo Clin Proc 81:94–101
MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K et al (2000) Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 43:30–37
Stastny P (1976) Mixed lymphocyte cultures in rheumatoid arthritis. J Clin Invest 57:1148–1157
Watanabe Y, Tokunaga K, Matsuki K, Takeuchi F, Matsuta K, Maeda H et al (1989) Putative amino acid sequence of HLA-DRβ chain contributing to rheumatoid arthritis susceptibility. J Exp Med 169:2263–2268
Gregersen PK, Silver J, Winchester RJ (1987) The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 30:1205–1213
Wallin J, Hillert J, Olerup O, Carlsson B, Strom H (1991) Association of rheumatoid arthritis with a dominant DR1/Dw4/Dw14 sequence motif, but not with T cell receptor beta chain gene alleles or haplotypes. Arthritis Rheum 34:1416–1424
Gonzalez-Gay MA, Garcia-Porrua C, Hajeer AH (2002) Influence of human leukocyte antigen-DRB1 on the susceptibility and severity of rheumatoid arthritis. Semin Arthritis Rheum 31:355–360
Gorman JD, Lum RF, Chen JJ, Suarez-Almazor ME, Thomson G, Criswell LA (2004) Impact of shared epitope genotype and ethnicity on erosive disease: a meta-analysis of 3,240 rheumatoid arthritis patients. Arthritis Rheum 50:400–412
Silman AJ, MacGregor AJ, Thomson W, Holligan S, Carthy D, Farhan A et al (1993) Twin concordance rates for rheumatoid arthritis: results from a nation-wide study. Br J Rheumatol 32:903–907
Svendsen AJ, Holm NV, Kyvik K, Petersen PH, Junker P (2002) Relative importance of genetic effects in rheumatoid arthritis: historical cohort study of Danish nationwide twin population. Br Med J 324:264–266
Sawada S, Takei M (2005) Epstein–Barr virus etiology in rheumatoid synovitis. Autoimmun Rev 4:106–110
Rickinson AB, Kieff E (1996) Epstein–Barr virus. In: Fields BN, Knipe DM, Howley PM et al (eds) Fields virology. Lippincott-Raven, Philadelphia, pp 2397–2446
Callan MF (2004) Epstein-Barr virus, arthritis, and the development of lymphoma in arthritis patients. Curr Opin Rheumatol 16:399–405
Berthelot JM, Saulquin X, Coste-Burel M, Peyrat MA, Echasserieau K, Bonneville M et al (2003) Search for correlation of CD8 T cell response to Epstein–Barr virus with clinical status in rheumatoid arthritis: a 15 month follow-up pilot study. J Rheumatol 30:1673–1679
Fox RI, Chilton T, Rhodes G, Vaughan JH (1986) Lack of reactivity of rheumatoid arthritis synovial membrane DNA with cloned Epstein–Barr virus DNA probes. J Immunol 137:498–501
Niedobitek G, Lisner R, Swoboda B, Rooney N, Fassbender HG, Kirchner T et al (2000) Lack of evidence for an involvement of Epstein–Barr virus infection of synovial membranes in the pathogenesis of rheumatoid arthritis. Arthritis Rheum 43:151–154
Murai C, Munakata Y, Takahashi Y, Ishii T, Shibata S, Muryoi T et al (1999) Rheumatoid arthritis after human parvovirus B19 infection. Ann Rheum Dis 58:130–132
Takasawa N, Munakata Y, Ishii KK, Takahashi Y, Takahashi M, Fu Y et al (2004) Human parvovirus B19 transgenic mice become susceptible to polyarthritis. J Immunol 173:4675–4683
Nikkari S, Luukkainen R, Mottonen T, Meurman O, Hannonen P, Skurnik M et al (1994) Does parvovirus B19 have a role in rheumatoid arthritis? Ann Rheum Dis 53:106–111
Meyer O (2003) Parvovirus B19 and autoimmune diseases. Joint Bone Spine 70:6–11
Bosma TJ, Etherington J, O’Shea S, Corbett K, Cottam F, Holt L et al (1998) Rubella virus and chronic joint disease: is there an association? J Clin Microbiol 36:3524–3526
Zhang D, Nikkari S, Vainionpaa R, Luukkainen R, Yli-Kerttula U, Toivanen P (1997) Detection of rubella, mumps, and measles virus genomic RNA in cells from synovial fluid and peripheral blood in early rheumatoid arthritis. J Rheumatol 24:1260–1265
Mehraein Y, Lennerz C, Ehlhardt S, Remberger K, Ojak A, Zang KD (2004) Latent Epstein–Barr virus (EBV) infection and cytomegalovirus (CMV) infection in synovial tissue of autoimmune chronic arthritis determined by RNA- and DNA-in situ hybridization. Mod Pathol 17:781–789
Holoshitz J, Klajman A, Drucker I, Lapidot Z, Yaretzky A, Frenkel A et al (1986) T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet 2:305–309
Horowitz S, Evinson B, Borer A, Horowitz J (2000) Mycoplasma fermentans in rheumatoid arthritis and other inflammatory arthritides. J Rheumatol 27:2747–2753
Auger I, Escola JM, Gorvel JP, Roudier J (1996) HLA-DR4 and HLA-DR10 motifs that carry susceptibility to rheumatoid arthritis bind 70-kD heat shock proteins. Nat Med 2:306–310
Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Coster L et al (2005) Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum 52:1986–1992
Van Eden W, Thole JE, van der Zee R, Noordzij A, van Embden JD, Hensen EJ et al (1988) Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 331:171–173
Gaston JS, Life PF, Bailey LC, Bacon PA (1989) In vitro responses to a 65-kilodalton mycobacterial protein by synovial T cells from inflammatory arthritis patients. J Immunol 143:2494–2500
Res PC, Telgt D, van Laar JM, Pool MO, Breedveld FC, de Vries RR (1990) High antigen reactivity in mononuclear cells from sites of chronic inflammation. Lancet 336:1406–1408
Van der Heijden IM, Wilbrink B, Schouls LM, van Embden JD, Breedveld FC, Tak PP (1999) Detection of mycobacteria in joint samples from patients with arthritis using a genus-specific polymerase chain reaction and sequence analysis. Rheumatology 38:547–553
Haier J, Nasralla M, Franco AR, Nicolson GL (1999) Detection of mycoplasma infections in blood of patients with rheumatoid arthritis. Rheumatology 38:504–509
Schaeverbeke T, Gilroy CB, Bebear C, Dehais J, Taylor-Robinson D (1996) Mycoplasma fermentans, but not M. penetrans, detected by PCR assays in synovium from patients with rheumatoid arthritis and other rheumatic disorders. J Clin Pathol 49:824–828
Hoffman RW, O’Sullivan FX, Schafermeyer KR, Moore TL, Roussell D, Watson-McKown R et al (1997) Mycoplasma infection and rheumatoid arthritis: analysis of their relationship using immunoblotting and an ultrasensitive polymerase chain reaction detection method. Arthritis Rheum 40:1219–1228
Albani S, Tuckwell JE, Esparza L, Carson DA, Roudier J (1992) The susceptibility sequence to rheumatoid arthritis is a cross-reactive B cell epitope shared by the Escherichia coli heat shock protein dnaJ and the histocompatibility leukocyte antigen DRB10401 molecule. J Clin Invest 89:327–331
Handley HH, Yu J, Yu DT, Singh B, Gupta RS, Vaughan JH (1996) Autoantibodies to human heat shock protein (hsp) 60 may be induced by Escherichia coli groEL. Clin Exp Immunol 103:429–435
Gerard HC, Wang Z, Wang GF, El-Gabalawy H, Goldbach-Mansky R, Li Y et al (2001) Chromosomal DNA from a variety of bacterial species is present in synovial tissue from patients with various forms of arthritis. Arthritis Rheum 44:1689–1697
Ebringer A, Ptaszynska T, Corbett M, Wilson C, Macafee Y, Avakian H et al (1985) Antibodies to Proteus in rheumatoid arthritis. Lancet ii:305–307
Khalafpour S, Ebringer A (1987) Cross-reactivity between HLA-DR4 and Proteus mirabilis. Period Biol (Zagreb) 89(Suppl 1):203
Ebringer A, Cunningham P, Ahmadi K, Wrigglesworth J, Hosseini R, Wilson C (1992) Sequence similarity between HLA-DR1 and DR4 subtypes associated with rheumatoid arthritis and Proteus/Serratia membrane haemolysins. Ann Rheum Dis 51:1245–1246
Wilson C, Ebringer A, Ahmadi K, Wrigglesworth J, Tiwana H, Fielder M et al (1995) Shared amino acid sequences between major histocompatibility complex class II glycoproteins, type XI collagen and Proteus mirabilis in rheumatoid arthritis. Ann Rheum Dis 54:216–220
Tiwana H, Wilson C, Alvarez A, Abuknesha R, Bansal S, Ebringer A (1999) Cross-reactivity between the rheumatoid arthritis-associated motif EQKRAA and structurally related sequences found in Proteus mirabilis. Infect Immun 67:2769–2775
Senior BW, Anderson GA, Morley KD, Kerr MA (1999) Evidence that patients with rheumatoid arthritis have asymptomatic ‘non-significant’ Proteus mirabilis bacteriuria more frequently than healthy controls. J Infect 38:99–106
Wilson C, Rashid T, Tiwana H, Beyan H, Hughes L, Bansal S et al (2003) Cytotoxicity responses to peptide antigens in rheumatoid arthritis and ankylosing spondylitis. J Rheumatol 30:972–978
Ebringer A, Rashid T, Wilson C (2003) Rheumatoid arthritis: proposal for the use of anti-microbial therapy in early cases. Scand J Rheumatol 32:2–11
Ebringer A, Wilson C, Ahmadi K, Corbett M, Rashid T, Shipley M (1993) Rheumatoid arthritis as a reactive arthritis to Proteus infection: Prospects for therapy. In: Machtey I (ed) Progress in rheumatology, sixth international seminar on the treatment of rheumatic diseases, vol. 5, pp 77–83
Wilson C, Thakore D, Isenberg D, Ebringer A (1997) Correlation between anti-Proteus antibodies and isolation rates of P. mirabilis in rheumatoid arthritis. Rheumatol Int 16:187–189
Senior BW (1979) The special affinity of particular types of Proteus mirabilis for the urinary tract. J Med Microbiol 12:1–8
Ronald AR, Nicolle LE (2001) Infections of the upper urinary tract. In: Schlier RW (ed) Diseases of the kidney and urinary tract. Williams & Wilkins, Philadelphia, pp 941–969
Lawson AA, Maclean N (1966) Renal disease and drug therapy in rheumatoid arthritis. Ann Rheum Dis 25:441–449
Tishler M, Caspi D, Aimog Y, Segal R, Yaron M (1992) Increased incidence of urinary tract infection in patients with rheumatoid arthritis and secondary Sjogren’s syndrome. Ann Rheum Dis 51:601–606
Vandenbroucke JP, Kaaks R, Valkenburg HA, Boersma JW, Cats A, Festen JJ et al (1987) Frequency of infections among rheumatoid arthritis patients, before and after disease onset. Arthritis Rheum 30:810–813
Wanchu A, Deodhar SD, Sharma M, Gupta V, Bambery P, Sud A (1997) Elevated levels of anti-Proteus antibodies in patients with active rheumatoid arthritis. Indian J Med Res 105:39–42
Rashid T, Darlington G, Kjeldsen-Kragh J, Forre O, Collado A, Ebringer A (1999) Proteus IgG antibodies and C-reactive protein in English, Norwegian and Spanish patients with rheumatoid arthritis. Clin Rheumatol 18:190–195
Chen T, Rimpilainen M, Luukkainen R, Mottonen T, Yli-Jama T, Jalava J et al (2003) Bacterial components in the synovial tissue of patients with advanced rheumatoid arthritis or osteoarthritis: analysis with gas chromatography–mass spectrometry and pan-bacterial polymerase chain reaction. Arthritis Rheum 49:328–334
Hara Y, Kaneko T, Yoshimura A, Kato I (1996) Serum rheumatoid factor induced by intraperitoneal administration of periodontopathic bacterial lipopolysaccharide in mice. J Periodontal Res 31:502–507
Posnett DN, Edinger J (1997) When do microbes stimulate rheumatoid factor? J Exp Med 185:1721–1723
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Maddison PJ, Huey P (2004) Serological profile. In: Isenberg DA, Maddison PJ, Woo P, Glass DN, Breedveld FC (eds) Oxford textbook of rheumatology. Oxford University Press, Oxford, pp 491–499
Rashid T, Ebringer A, Wilson C, Bansal S, Paimela L, Binder A (2006) The potential use of antibacterial peptide antibody indices in the diagnosis of rheumatoid arthritis and ankylosing spondylitis. J Clin Rheumatol 12:11–16
Roberts LJ, Cleland LG, Thomas R, Proudman SM (2006) Early combination disease modifying antirheumatic drug treatment for rheumatoid arthritis. Med J Aust 184:122–125
Van der Heijde D, Klaresdog L, Rodriguez-Valverde V, Codreanu C, Bolosiu H, Melo-Gomes J et al (2006) Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis. Two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum 54:1063–1074
Merkesdal S, Ruof J, Mittendorf T, Zeidler H (2004) Cost-effectiveness of TNF-alpha-blocking agents in the treatment of rheumatoid arthritis. Expert Opin Pharmacother 5:1881–1886
Fleischmann RM, Iqbal I, Stern RL (2004) Considerations with the use of biological therapy in the treatment of rheumatoid arthritis. Expert Opin Drug Saf 3:391–403
Khalafpour S, Ebringer A, Abuljadayel I, Corbett M (1988) Antibodies to Klebsiella and Proteus microorganisms in ankylosing spondylitis and rheumatoid arthritis patients measured by ELISA. Br J Rheumatol 27(Suppl II):86–89
Deighton CM, Gray SW, Biant AJ, Walker DJ (1992) Specificity of the Proteus antibody response in rheumatoid arthritis. Ann Rheum Dis 51:1206–1207
Subair H, Tiwana H, Fielder M, Binder A, Cunningham K, Ebringer A et al (1995) Elevation in anti-Proteus antibodies in patients with rheumatoid arthritis from Bermuda and England. J Rheumatol 22:1825–1828
Fielder M, Tiwana H, Youinou P, Le Goff P, Deonarian R, Wilson C et al (1995) The specificity of the anti-Proteus antibody response in tissue-typed rheumatoid arthritis (RA) patients from Brest. Rheumatol Int 15:79–82
Tiwana H, Wilson C, Cunningham P, Binder A, Ebringer A (1996) Antibodies to four gram-negative bacteria in rheumatoid arthritis which share sequences with the rheumatoid arthritis susceptibility motif. Br J Rheumatol 35:592–594
Tiwana H, Wilson C, Walmsley RS, Wakefield AJ, Smith MS, Cox NL et al (1997) Antibody response to gut bacteria in ankylosing spondylitis, rheumatoid arthritis, Crohn’s disease and ulcerative colitis. Rheumatol Int 17:11–16
Tani Y, Tiwana H, Hukuda S, Nishioka J, Fielder M, Wilson C et al (1997) Antibodies to Klebsiella, Proteus and HLA-B27 peptides in Japanese patients with ankylosing spondylitis and rheumatoid arthritis. J Rheumatol 24:109–114
Blankenberg-Sprenkels SHD, Fielder M, Feldkamp TEW, Tiwana H, Wilson C, Ebringer A (1998) Antibodies to Klebsiella pneumoniae in Dutch patients with ankylosing spondylitis and acute anterior uveitis and to Proteus mirabilis in rheumatoid arthritis. J Rheumatol 25:743–747
Newkirk MM, Goldbach-Mansky R, Senior BW, Klippel J, Schumacher HR Jr, El-Gabalawy HS (2005) Elevated levels of IgM and IgA antibodies to Proteus mirabilis and IgM antibodies to Escherichia coli are associated with early rheumatoid factor (RF)-positive rheumatoid arthritis. Rheumatology 44:1433–1441
Rashid T, Leirisalo-Repo M, Tani Y, Hukuda S, Kobayashi S, Wilson C et al (2004) Antibacterial and antipeptide antibodies in Japanese and Finnish patients with rheumatoid arthritis. Clin Rheumatol 23:134–141
Acknowledgement
We thank the Trustees of the Middlesex Hospital, the Arthritis Research Campaign (grant no. EO514) and “American Friends of King’s College London” for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rashid, T., Ebringer, A. Rheumatoid arthritis is linked to Proteus—the evidence. Clin Rheumatol 26, 1036–1043 (2007). https://doi.org/10.1007/s10067-006-0491-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-006-0491-z