Abstract
The aim of this study was to compare the socioeconomic consequences of early and late rheumatoid arthritis in Belgium and to assess the patient out-of-pocket contributions. This multicentre longitudinal study in Belgium evaluated patients with rheumatoid arthritis. Early disease was defined as diagnosis since less than 1 year. At baseline sociodemographic and disease characteristics were assessed and during the following year patients recorded all healthcare- and non-healthcare-related direct costs and out-of-pocket contributions. The study included 48 patients with early and 85 patients with late rheumatoid arthritis. Mean disease duration was 0.5 vs 12.5 years in patients with early and late rheumatoid arthritis, respectively. The disease activity score (DAS28) was comparable between both groups (4.1 vs 4.5, p=0.14), but physical function (Health Assessment Questionnaire, HAQ) was more impaired in patients with long-standing disease (1.0 vs 1.7, p<0.001). Work disability had increased from 2% in patients with early to 18% in patients with late disease. The annual societal direct costs per patient were € 3055 (median: € 1518) opposed to € 9946 (median: € 4017) for early and late rheumatoid arthritis, respectively. The higher direct cost for patients with long-standing disease was seen for all categories, but especially for physiotherapy and need for devices and adaptations. Patients with early as well as late disease contribute out of pocket about one-third to the direct healthcare costs. Within each group, HAQ was a strong determinant of costs. In Belgium, patients with long-standing rheumatoid arthritis are nine times more likely to be work disabled than patients with less than 1 year disease duration and have a threefold increase in costs. Differences in healthcare consumption between patients could be mainly explained by differences in physical function (HAQ).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last 10 years limited financial resources resulted in increasing national regulations aimed at cost containment in all Western societies. To assure high-quality care for the patients, physicians realize their pivotal role in negotiations with the decision makers to safeguard sufficient financial resources. Insight into data on costs of the disease and its treatment on the one hand and outcome of disease on the other hand are essential tools for negotiations on budgets and reimbursement decisions [1, 2]. Cost-of-illness (COI) studies are a first step to be informed on the financial implications of disease [3]. In chronic diseases such as rheumatoid arthritis (RA), they can help to identify the cost categories with high expenditures and clarify demographic or disease-related variables associated with high costs. Since no data were available on socioeconomic consequences for RA in Belgium, this study aimed to examine the economic consequences of the disease for the society and for the patients. Since it was hypothesized that such effects would be different for patients with early opposed to late disease, the differences in costs between these groups of patients were compared.
Methods
Data were collected alongside a prospective multicentre study in Belgium.
Patients
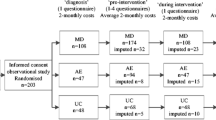
A random sample of consecutive outpatients was recruited in 2000 from six centres in Belgium. There was a mix of patients coming from private rheumatology practices and university hospitals. Patients were eligible if they were older than 16 years and fulfilled American College of Rheumatology (ACR) criteria for RA [4]. Patients with a known history of poor compliance, severe comorbidity (other than diabetes, osteoporosis, hypercholesterolaemia, hypertension or depression) and patients participating in a drug intervention study were excluded. Before the start of the study it was decided to enrol one-third of the patients with early disease, defined as diagnosed according to the ACR criteria within 1 year, and two-thirds of the patients with late RA. Since more late patients had been included towards the end of the study, centres were contacted to only proceed including patients with early RA. The study was approved by the Ethics Committees of all participating centres and all patients had to give informed consent.
Assessments
At baseline, an interview by a study nurse was performed to record the sociodemographic characteristics and drug history while patients completed a Health Assessment Questionnaire (HAQ) and a visual analogue scale on disease activity. The treating rheumatologist performed a clinical examination including painful and swollen joint counts. Finally, a laboratory assessment was performed to determine erythrocyte sedimentation rate (ESR). From the joint counts, the ESR and patient disease activity score, the DAS28, was calculated. Patients were instructed to collect accounts or record expenditures (whatever was applicable), for all health-related resources consumed without making a distinction whether this was RA related or not. Patients received a costing map divided into the three sections of healthcare consumption with clear instructions on how to complete each section of the map. Each section contained questions on different categories of costs. Patients had to distinguish the total costs and the personal contribution. In the first section, costs for physician visits, physiotherapist visits, medication, alternative medicine and technical procedures, aids and appliances and community home help had to be recorded. The second section had to include the accounts for adaptations to home and car and accounts of all types of hospitalizations (acute care hospital, rehabilitation hospital and nursing home). In the third section, transportation costs and hours of help received from family or friends (informal care) were recorded. Sections one and three of the costing map had to be completed by patients with early RA for three periods of 3 months during the 1-year follow-up period and by patients with late RA for two periods of 3 months. Section three of the costing map had to be sent in after the full year of follow-up and patients were instructed to send in late coming accounts to the study nurse. To calculate the costs of informal help from family or friends for which no direct payments were performed the unit price of € 8.70/h, which is the opportunity cost for this category of worker in 2000, was applied. To calculate the total direct costs, the summed costs of the observation periods were annualized.
Belgian social security system
In Belgium an obligatory social security system has existed for all inhabitants since the second part of the twentieth century. On the macroeconomic level, 35% of healthcare is financed through social contributions included in the taxes, 41% through public health insurance premiums and 18% through direct patient contributions. For the elderly, severely handicapped, widows without income and orphans the level of the patient’s contribution is lower. Possibilities for reinsurance for patients’ contributions are limited to the costs of hospitalization. Physicians and other healthcare providers working in academic hospitals are salaried. General practitioners, nonacademic physicians and physiotherapists are paid on a fee for service basis. Depending on the service consumed, patients pay only the personal contribution (technical procedures and drugs) or pay the whole amount and are reimbursed afterwards (physician visits). Access to specialist care is not limited by the gatekeeper role of the general practitioner.
Statistical analyses
In case there were missing data for one of the questions on resource utilization, the value reported for the available time point (or the mean value of the two other time points in early disease) in the same patient was imputed. The total costs per patient were then annualized (by multiplying the costs per patient with early disease by factor 1.33 and with late disease by factor 2.0). Demographic and disease characteristics between the group with early and late RA were compared by general linear model (GLM) for continuous and Fisher’s exact test for categorical variables. Determinants of costs were performed by linear regression analyses on log transformed costs. Data were entered in a TriaXS system and statistical analyses were performed in SAS.
Results
Disease and sociodemographic characteristics of patients
The study included 48 patients with early and 85 patients with late RA who had returned at least one (late) or two (early) cost questionnaires. For the first cost questionnaire 73% of the patients had complete data and for the second and third questionnaires 76 and 68%, respectively. Overall, 70% of the cost data were complete. Table 1 presents the disease characteristics of patients at baseline. Mean disease duration was 0.5 and 12.5 years for patients with early and late disease, respectively. Remarkably, patients with early disease were significantly older. While disease activity was comparable, physical function measured by HAQ was significantly worse in patients with late disease. In the early as well as in the late RA group, 93% of patients were on disease-modifying antirheumatic drugs (DMARDs) while 43 and 56%, respectively, were on low-dose steroids. Tumor necrosis factor (TNF) blocking drugs were not yet reimbursed at the time the study was conducted. In the early RA group 82% of patients were taking nonsteroidal anti-inflammatory drugs (NSAIDs) compared to 73% in the late RA group. Previous surgery for RA was noted in 0 and 18% of the patients, respectively, in the early and the late RA patients. Work disability increased from 2 to 18% in patients with early opposed to long-standing disease, respectively. In those with paid work, 13% were on sick leave at the baseline evaluation in the early and 26% in the late group.
Costs
Table 2 presents the annual costs per patient for the total direct costs and for each resource category separately in patients in the early opposed to late disease. The total direct costs of patients in the group with long-standing RA were 3.2-fold higher than in patients with less than 1 year disease duration. The increase in costs was noticed for all categories of costs, but especially relevant for physiotherapy visits, community home care and devices and adaptations to the house. For visits to physiotherapists, need for aids and appliances and adaptations at the house there was a striking increase of the proportion of patients using these. The need for community home help already early in the disease was remarkable (20%) and likely reflects the impact of the disease on women to perform the heavy household tasks. The increase in costs of community home help likely has to be attributed to an increase in the number of hours of help required per patient requiring help. A similar trend could be seen for the need for informal care (unpaid help from family or friends). The costs of informal care were the cost drivers in early as well as in late disease. In patients with early RA, medication costs were the next most important contributor to costs while in patients with long-standing disease the costs for adaptations and medications were equally important additional contributors.
Table 3 presents the patients’ contributions for the direct healthcare resources. Total out-of-pocket expenditures were € 465/year for patients with less than 1 year disease duration and € 1098 for patients with long-standing disease. The costs of medications accounted for 51 and 41% of the total personal contributions in patients in the early and late groups, respectively. Further, contributions to physician visits were the next most important cost (24% of total out-of-pocket costs) in patients with early RA while costs for physician visits and admissions to hospitals and rehabilitation facilities were equally contributing as the second most important cost in patients with late RA. Patients with early as well as long-standing disease pay 30% of the total societal healthcare costs.
Multivariate analyses showed that the differences in costs between patients with early and late disease but also within the patients with either early or long-standing RA were mainly explained by the physical function assessed by HAQ.
Discussion
The direct costs of patients with RA in Belgium are important and remarkably higher in patients with long-standing disease compared with patients with early disease. Differences in costs could be mainly explained by differences in physical functioning (HAQ) as seen in other studies [3, 5]. The important effect of progression of the disease on socioeconomic consequences is also reflected in the increasing work disability and the magnitude of the need for help from family and friends. Not only the direct societal costs are striking, also the patient’s out-of-pocket costs for healthcare are high. Patients with early as well as late disease contribute about 30% of the healthcare costs.
Comparison with the literature is difficult since no stratification for disease duration was performed in most publications and can explain that the costs in this study for patients with long-standing disease were higher than reported in other prevalent studies. In line with data from the literature, the costs of hospitalization are no longer the cost driver as was the case in the earlier studies [6]. One recent publication from the Netherlands [7] presented direct COI stratified for patients with increasing disease duration. In patients with early RA (0–2 years), the average annual costs were € 5235 (median: € 2923) and in patients with more than 10 years of disease € 8243 (median: € 3778) and this is quite comparable with our data. The major differences from this study, however, were that in our study also the unpaid help from informal caregivers was valued, as recommended in the societal perspective, and that in our study all causes and not only the RA-related costs were included.
Indirect costs were not included in the COI calculation since numbers of days on sick leave were not collected prospectively. However, substantial work disability and sick leave at baseline suggest an important impact of increasing disease duration on the ability to perform paid work which would add substantially to the total costs of illness.
Our cost-of-illness study has certainly limitations.
The patients studied are only a small sample of the Belgian RA patients but were coming from all over the country as well from private practice and university centres. The relatively high age at disease onset in the early compared to the late RA group, however, is in line with data from the literature suggesting a higher age at disease onset in more recent patient cohorts [8, 9].
When collecting data, only costs were recorded by the patients and not resource use, hampering comparisons with other studies. Moreover, the recorded healthcare costs were tariffs and not true opportunity costs as recommended in guidelines on costing studies. However, in Belgium, as in most other countries, no true costing data exist and tariffs are used as a surrogate for the true societal costs. Striking were the high out-of-pocket costs for the patients: amounting to € 1098/year in patients with late RA, independent of the lost wages! However, one has to be very careful when interpreting these patient contributions out of pocket since the premiums for social security in Belgium to be paid by the patients are low. Nevertheless, this reflects the low solidarity principle in the Belgian social security system of the healthy with the chronically ill. At the present time, policy makers are trying to decrease the financial burden of illness for patients with chronic diseases.
It will be of interest to see how early intensive treatment and the use of biologics will influence the cost of illness in RA in the future for patients as well as society.
References
Cooper NJ (2000) Economic burden of rheumatoid arthritis: a systematic review. Rheumatology 39:28–33
Yelin E, Wanke LA (1999) An assessment of the annual and long term direct costs of rheumatoid arthritis: the impact of poor functioning and functional decline. Arthritis Rheum 42:1209–1218
Van Jaarsveld CH, Jacobs JW, Schrijvers AJ et al (1998) Direct cost of rheumatoid arthritis during the first six years: a cost of illness study. Br J Rheumatol 37:837–847
Arnett FC, Edworthy SM, Bloch DA, Mc Shane DJ, Fries JF, Cooper NS et al (1988)The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Lajas C, Abasolo L, Bellajdel B et al (2003) Costs and predictors of costs in rheumatoid arthritis: a prevalence based study. Arthritis Rheum 49:64–70
Pugner KM, Scott DI, Holmes JW, Hieke K (2000) The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum 29:305–320
Verstappen SMM, Verkleij H, Bijlsma JWJ et al (2004) Determinants of direct costs in Dutch rheumatoid arthritis patients. Ann Rheum Dis 63:817–824
Kaipiainen-Sepanen O, Aho K, Isomaki H, Laakso M (1996) Incidence of rheumatoid arthritis in Finland during 1980–1990. Ann Rheum Dis 55:608–611
Imanaka T, Shichikawa K, Inoue K, Shimaoka Y, Takenada Y, Wakitani S (1997)Increase in age at onset of rheumatoid arthritis in Japan over a 30 year period. Ann Rheum Dis 56:313–316
Acknowledgements
The authors wish to thank Dr. Veerle Taelman, Dr. Kristien Maenaut, Dr. Kathleen Declerck, Dr. Ellie Kruithof and all the participating patients for their tremendous effort to collect all the data. This study was financially supported by an Educational Grant from Aventis Belgium.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Westhovens, R., Boonen, A., Verbruggen, L. et al. Healthcare consumption and direct costs of rheumatoid arthritis in Belgium. Clin Rheumatol 24, 615–619 (2005). https://doi.org/10.1007/s10067-005-1119-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-005-1119-4