Abstract
We report on the unique effects and benefits of autologous stem cell transplantation in childhood systemic lupus erythematosus (SLE) and describe this procedure in two young girls with severe and refractory disease. The patients’ stem cells were mobilized with granulocyte colony-stimulating factor (G-CSF) and collected by CS-3000 Blood Cell Separator (Baxter Healthcare, Round Lake, Ill., USA), and the CliniMACS CD34+ cell selection device (Miltenyi Biotech, Bergisch Gladbach, Germany) was used to obtain CD34+ cells. A total of 1.7×106 and 1.0×106/kg CD34+ cells were obtained, with 2.0×105 and 1.0×104/kg of CD3+ cells remaining, respectively. The conditioning regimen consisted of cyclophosphamide (50 mg/kg per day for 4 days) plus antithymocyte globulin (ATG-Fresenius, 5 mg/kg per day for 3 days). Neutrophil counts recovered within 9 days in both cases. Within 15 days, the platelet counts recovered and were sustained over 100×109/l. Cushingoid features disappeared completely 3 months after transplantation because of the removal of corticosteroid medication. One 13-year-old child increased her height by 5 cm in 6 months after stopping steroids. She had not increased her height in her previous 7 years of disease. As of the time of this report, the first patient remains in clinical and laboratory remission for nearly 4 years, while the second suffered a relapse of thrombocytopenia 9 months post-transplantation. One residual effect of their treatment is that their CD4+ cell counts remained in the lower range after one year of transplant. The effect of this conditioning regimen plus CD34+ autologous stem cell transplantation on these two children with refractory SLE was beneficial, but long-term follow-up data and additional experience with this procedure are required. Autologous stem cell transplantation may limit the long-term toxicity of therapy in childhood SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current therapies for autoimmune diseases are limited by toxicity and inefficacy. By the end of the 1990s, investigators from Europe and the United State began to treat autoimmune diseases in adults with autologous hematopoietic stem cell transplantation and achieved some success [1, 2]. However, there has to date been little published in regard to this kind of treatment in younger patients or children with autoimmune diseases [3–8]. Systemic lupus erythematosus (SLE) is an autoimmune disease which can manifest severe multiorgan involvement beginning in childhood. To further describe stem cell transplantation in younger patients with refractory SLE, we report herein on the CD34+ autologous stem cell transplantation of two young patients.
Cases
Case 1. An 18-year-old girl had suffered from recurrent menorrhagia and petechiae since age 13 (April 1996). She was initially diagnosed with idiopathic thrombocytopenic purpura (ITP), but 2 years later developed lower extremity edema and was found to have nephrotic range proteinuria (9.8 g of protein/24 h urine). Her renal biopsy revealed WHO class IV lupus nephritis, and she had a positive test for antinuclear antibodies (ANA) as well as anti-Ro antibodies. Her treatments included corticosteroids, 6-mercaptopurine, and cyclophosphamide, but she had no lasting improvement in her symptoms or proteinuria. In addition, she developed hypovolemic shock twice from menorrhagia prior to being referred for autologous stem cell transplantation in April 2001.
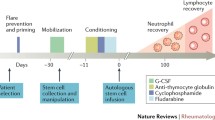
After admission, informed consent of the patient and family as well as Institutional Review Board approval were obtained. Then, a subcutaneous injection of 5–10 μg/kg filgrastim (granulocyte colony-stimulating factor, G-CSF) was given for 5 consecutive days for stem cell mobilization. A CS-3000 Blood Cell Separator (Baxter Healthcare, Round Lake, Ill., USA) was used for two separate peripheral blood stem cell collections; 1.7×108/kg mononuclear cells were collected. Platelets were removed by centrifugation, and CD34+ stem cells were obtained with the CliniMACS system (Miltenyi Biotech, Bergisch Gladbach, Germany) as has been described in previous reports [9]. An amount of 1.7×106/kg CD34+ cells was collected and then cryopreserved at −80°C with 2.0×105/kg CD3+ cells remaining in the graft.
Cyclophosphamide (Cytoxan, CTX) 50 mg/kg per day for 4 days and antithymocyte globulin (ATG, manufactured by Fresenius S) 5 mg/kg per day for 3 days were used for bone marrow conditioning as described by Traynor et al. [5]. The only difference between our conditioning regimen and the Traynor et al. regimen was that the high-dose methylprednisolone was omitted as our patient had already received high-dose methylprednisolone pulse therapy prior to admission. Lower dose methylprednisolone (1 mg/kg per day) was used for prevention of side effects associated with ATG infusion. Forty-eight hours after infusion of the last dosage of CTX, the autologous CD34+ stem cells as well G-CSF 5 μg/kg per day were given to promote the rapid recovery of neutrophil counts. Trimethoprim-cotrimoxazole (50 mg/kg per day, two times per day, 3 days/week) was used for Pneumocystis carinii prophylaxis and intravenous immune globulin (IVIG, 500 mg/kg) were supplemented on the day of stem cell infusion.
The conditioning regimen was complicated by diabetes mellitus and ketoacidosis as well as Klebsiella pneumonia septicemia. Of note the patient had manifested some glycosuria with previous high-dose methylprednisolone therapy. Hematopoietic recovery occurred 9 days after transplantation, and the lowest neutrophil count appeared at 4 days and was 0.1 k/μl. Six units of packed red blood cells and three single-donor units of platelets were required. As expected, the conditioning regimen had a significant effect on measured immune cell populations. The effect was seen particularly on CD4+ and CD19+ cells and was least noticeable on CD56+ cells (Tables 1, 2; Fig. 1). The percentage of CD19+ cells (B lymphocytes) returned to the normal range 3 months after transplantation, while the number of CD4+ cells still remained in the lower range 1 year after transplantation.
Post-transplantation SLE-related symptoms resolved and the patient has remained in clinical remission, with no detectable autoantibodies (anti-double-stranded DNA antibodies remained negative throughout her pre- and post-transplantation course). Her Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score prior to transplantation was 6 and was reduced to 0 post-transplantation. There has been no recurrence of any degree of proteinuria or thrombocytopenia with the exception of a transient decrease in platelet count in association with a rubella viral infection. All corticosteroids were stopped immediately post-transplantation, and cushingoid features disappeared within 3 months. Her follicle-stimulating hormone (FSH) level remained in the normal range 6 months after transplantation at 6 IU/l. She has continued to do well and remains in clinical and laboratory remission now more than 44 months post-transplantation.
Case 2. A 13-year-old girl had been diagnosed with SLE at age 6 when she presented with eyelid edema and proteinuria and was found to have WHO class III lupus nephritis (6.5 g of protein/24 h urine). Laboratory work-up also revealed positive ANA, antineutrophil cytoplasmic autoantibodies (ANCA), and LE cells in her peripheral blood. Corticosteroids were ineffective in suppressing her proteinuria. In August 2001, she presented with fevers, pleurisy, and thrombocytopenia (platelet 20×109/l). When cyclophosphamide and pulse methylprednisolone were ineffective in controlling her symptoms, she was referred for consideration of autologous stem cell transplantation.
The patient and family provided informed consent for transplantation, and Institutional Review Board approval was obtained. The mobilization and conditioning regimens in this case were identical to those used in case 1. In this case 4.3×108/kg peripheral mononuclear cells were obtained on initial collection and 1.0×106/kg of CD34+ stem cells were obtained after CliniMACS (Miltenyi Biotech, Bergisch Gladbach, Germany) separation, with 1.0×104/kg of CD3+ cells remaining in the graft.
As this patient displayed evidence of occult diabetes on oral glucose tolerance testing, insulin was supplemented during the conditioning regimen, and there were no apparent complications of her transplantation. Hematopoietic recovery occurred 7 days after transplantation, with a neutrophil nadir count of 0.1 k/μl on day 4 (identical to case 1). Four units of packed red blood cells were required for support in the immediate post-transplant period. During the conditioning her platelets increased gradually so she did not need any platelets at all. Her lymphocyte populations were affected in the same way as in patient 1 (Tables 1, 2; Fig. 1).
Similarly to patient 1, all SLE-related antibodies and symptoms resolved (anti-double-stranded DNA antibodies remained negative throughout her course), and for 9 months there was no recurrence of any significant thrombocytopenia or proteinuria, with the exception of a brief episode of 1+ dipstick proteinuria that occurred in association with a urinary tract infection (UTI) 3 months after transplantation. Her SLEDAI score decreased to 0 from 12 pre-transplantation. Also similarly to patient 1, corticosteroids were discontinued immediately after transplantation, and there was a rapid resolution of cushingoid features. The abdominal circumference was reduced from 77 cm before transplantation to 64 cm post-transplantation. In addition, this patient, who had had significant growth retardation during her 7 years of SLE therapy, gained 5 cm in height in the first 6 months post-transplant (Fig. 2). Sex hormone levels also remained within normal limits 6 months post-transplantation (FSH 1.2 IU/l). Unfortunately, 9 months post-transplantation, the patient relapsed with thrombocytopenia and fever and presented for immunosuppressive treatment to another hospital, where she was subsequently lost to follow-up.
Stature-for-age compared to southern Chinese children—patient 2. Adapted from Chang et al. [10]
Discussion
Our treatment regimen differed from previously reported cases in the use of lower doses of methylprednisolone, as mentioned above, and in the avoidance of cyclophosphamide for peripheral blood progenitor cell mobilization. In most cases of autologous stem cell transplantation for autoimmune diseases, both cyclophosphamide and G-CSF are used in the mobilization regimen out of concern for the possibility of G-CSF-induced disease flares. As these two girls had recently received CTX and high-dose pulse methylprednisolone treatment and obtained a degree of improvement in clinical condition before transplantation, G-CSF alone was used for stem cell mobilization. Perhaps for this reason, we were only able to collect and infuse a lower number of CD34+ cells than has been typically infused—fortunately the time to recovery of neutrophil counts in both patients was similar to what has been previously reported [3, 6].
With regard to treatment-related complications, our cases may serve as a reminder of the possibility of treatment-associated diabetic ketoacidosis. In addition, in both cases steroid and other immunosuppressive medications were stopped immediately after transplantation without appreciable clinical deterioration, suggesting that a prolonged steroid taper is not required for disease quiescence. However, delayed recurrence of SLE post-transplantation, as occurred in case 2, is a well-recognized phenomenon. In their recent review of 53 patients undergoing autologous stem cell transplantation for SLE, Jayne et al. [11] reported delayed recurrence of disease in 32% of patients who had achieved initial disease remission. Whether routine post-transplantation maintenance therapy with less toxic immunosuppressants such as azathioprine or mycophenolate mofetil (recently studied with regard to lupus nephritis remission maintenance) can reduce relapse rates remains to be seen [12].
The purpose of CD34+ autologous stem cell transplantation is to reduce populations of autoreactive lymphocytes, while reconstituting lymphocyte populations from precursor cells that are not autoreactive. The number of memory T cells (CD3+) in the transplant could directly influence the response to transplantation. In the cases reported, 3–4 logs of CD3+ cells were removed, and it is possible that the durability of response may be modifiable by the extent to which these cells are reduced. That lesser degrees of immunodepletion may also allow repopulation of the immune system with non-autoreactive lymphocytes is suggested by recent trials of high-dose CTX alone [13, 14]. Ideally repopulation with such “healthy” lymphocytes by any method might lead to long-term remissions of SLE or even clinical cures.
The possible benefits of prolonged SLE remission are self-evident with regard to disease symptoms, but are also particularly important with regard to the minimization of treatment-related toxicity. As demonstrated by our two cases [17], this is of particular relevance in SLE of young womanhood and childhood SLE. Growth retardation secondary to prolonged high-dose corticosteroid use can be avoided with successful remission induction, as demonstrated in case 2. In addition, ovarian toxicity induced by CTX is felt to be at least in part targeted at developing follicles, and therefore the preservation of gonadal function in CTX use may be more related to duration of therapy than to dose [15, 16]. Young women spared repeated or prolonged courses of CTX by transplant-induced SLE remission might therefore be more likely to maintain fertility.
SLE can be a profoundly life-limiting and life-threatening disease, and even in “controlled” SLE, long-term medication side effects can contribute significantly to morbidity and mortality. Autologous stem cell transplantation, both to control refractory disease and to reduce long-term morbidity from chronic high-dose immunosuppression, is an option that can be successful for an as yet undefined period of time, and cessation of significant immunosuppression in children or adolescent patients carries unique potential benefits. Prospective trials comparing standard pulse CTX regimens and autologous stem cell transplant regimens as primary therapy in severe SLE are warranted and will hopefully better characterize the possible short- and long-term risks and benefits of these two types of therapy [3].
References
Tyndall A, Koike T (2002) High-dose immunoablative therapy with hematopoietic stem cell support in the treatment of severe autoimmune disease: current status and future direction. Intern Med 41:608–612
Marmont A, Gratwohl A, Vischer T et al (1995) Haemopoietic precursor cell transplants for autoimmune disease. Lancet 345:978
Traynor AE, Barr WG, Rosa RM, Rodriguez J, Oyama Y, Baker S et al (2002) Hematopoietic stem cell transplantation for severe and refractory lupus. Analysis after five years and fifteen patients. Arthritis Rheum 46:2917–2923
Dong L, Chen H, Jiang M (2000) Selected CD34+ cells transplantation: a primary clinical report. Zhonghua Yi Xue Za Zhi 80:841–844
Traynor AE, Schroeder J, Rosa RM, Cheng D, Stefka J, Mujais S et al (2000) Treatment of severe systemic lupus erythematosus with high-dose chemotherapy and haemopoietic stem-cell transplantation: a phase I study. Lancet 356:701–707
Burt RK, Traynor AE, Pope R, Schroeder J, Cohen B, Karlin KH et al (1998) Treatment of autoimmune disease by intense immunosuppressive conditioning and autologous hematopoietic stem cell transplantation. Blood 92:3505–3514
Wulffraat NM, Sanders EAM, Kamphuis SS, Rijkers GT, Kuis W, Lilien M et al (2001) Prolonged remission without treatment after autologous stem cell transplantation for refractory childhood systemic lupus erythematosus. Arthritis Rheum 44:728–731
Trysberg E, Lindgren I, Tarkowski A (2000) Autologous stem cell transplantation in a case of treatment resistant central nervous system lupus. Ann Rheum Dis 59:236–238
McNiece I, Briddel R, Stoney G, Kern B, Zilm K, Recktenwald D et al (1997) Large-scale isolation of CD34+ cell using the Amgen cell selection device results in high levels of purity and recovery. J Hematother 6:5–11
Chang KSF, Lee MMC, Low WD, Chui S, Chow M (1965) Standards of height and weight of southern Chinese children. Far East Med J 1:101–109
Jayne D, Passweg J, Marmont A, Farge D, Zhao X, Arnold R et al (2004) Autologous stem cell transplantation for systemic lupus erythematosus. Lupus 13:168–176
Contreras G, Pardo V, Leclerq B, Lenz O, Tozman E, O’Nan P et al (2004) Sequential therapies for proliferative lupus nephritis. N Engl J Med 350:971–980
Petri M, Jones RJ, Brodsky RA (2003) High-dose cyclophosphamide without stem cell transplantation in systemic lupus erythematosus. Arthritis Rheum 48:166–173
Gladstone DE, Prestrud AA, Pradhan A, Styler MJ, Topolsky DL, Crilley PA et al (2002) High-dose cyclophosphamide for severe systemic lupus erythematosus. Lupus 11:405–410
Slater CA, Liang MH, McCune JW, Christman GM, Laufer MR (1999) Preserving ovarian function in patients receiving cyclophosphamide. Lupus 8:3–10
McDermott EM, Powell RJ (1996) Incidence of ovarian failure in systemic lupus erythematosus after treatment with pulse cyclophosphamide. Ann Rheum Dis 55:224–229
Chen J, Gu LJ, Zhao HJ, Xue HL, Zheng Y, Xie XJ et al (2003) Application of CD34+ autologous peripheral progenitor cell transplant in the treatment of children with refractory SLE (in Chinese). Zhonghua Er Ke Za Zhi 41:426
Acknowledgments
The authors would like to thank Drs. H. James Williams and Hong-Hua Mu for their critical review and assistance with the preparation of this report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Wang, Y., Kunkel, G. et al. Use of CD34+ autologous stem cell transplantation in the treatment of children with refractory systemic lupus erythematosus. Clin Rheumatol 24, 464–468 (2005). https://doi.org/10.1007/s10067-004-1065-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-004-1065-6