Abstract
Due to the special soil-forming environment and process, grain-size distribution, and mineral composition, loess has some poor engineering properties, such as high compressibility, strong water sensitivity, and severe collapsibility. These poor properties lead to frequent occurrence of geological disasters and engineering problems in loess area, so loess is often strengthened in engineering projects. Compared with the traditional methods, the use of stabilizing agents has the advantages of saving time, reducing cost, and increasing efficiency. Therefore, more and more attention has been paid to the research on soil stabilizing agents. In this review, the non-traditional stabilizing agents which can effectively improve the physical and mechanical properties of loess are summarized, including nanomaterials, industrial waste residues, water-hardening inorganic materials, polymer, ion stabilizing agents, and so on. The composition, mechanism, effect, and application of each stabilizing agent are introduced. The mechanisms of strengthening mainly include filling, cementation, ion exchange, and wrapping. One or several of these mechanisms work together when loess is strengthened with any stabilizing agent. Based on this review, it is recognized that it is still meaningful to find eco-friendly and cost-effective loess stabilizing agents, and the focuses are now still on interpretation of the mechanism of each stabilizing agent, as well as determination of the physical and mechanical properties of strengthened loess. Attention should be paid to the degradation in the mechanical properties of strengthened loess and the technical innovation in application, as well as to solving the problems related to practical application of these stabilizing agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Loess soils are widely distributed all over the world, accounting for about 10% of the land area of the world. China, Russia, the USA, New Zealand, and some other mid-latitude countries have large areas of loess distribution (Ryashchenko et al. 2008; Nouaouria et al. 2008; Li et al. 2016, 2019a; Costantini et al. 2018; Yates et al. 2018). Among these countries, China has the largest area of loess distribution; about 6% of the land area of the country is covered by loess. The Loess Plateau in northwest China has the thickest loess strata in the world (Sun et al. 2006). Loess is a typical aeolian soil, which is formed by deposition of sand and dust blown by the wind from the Gobi Desert (Dijkstra et al. 1994). Therefore, from the northwest to the southeast of the Loess Plateau, the average size of loess particles decreases gradually, forming sandy loess zone, silty loess zone, and clayey loess zone (as shown in Fig. 1). Loess usually has some poor engineering properties such as strong water sensitivity and severe collapsibility because of the inherent metastable structure (Houston et al. 1988; Liu et al. 2015). These properties lead to frequent occurrence of geological disasters and engineering problems, such as ground subsidence, ground fissure, landslide, and mudflow, which not only bring great harm to people’s lives and properties, but also affect the function and safety of infrastructures built on/in loess (Wang et al. 2018, 2019). Therefore, loess soils are often strengthened to eliminate the collapsibility or reduce the compressibility using various methods in engineering practice.
Physical methods (such as dynamic compaction method and stone column method) and chemical methods (i.e., adding stabilizing agents into soil) have been extensively used to strengthen the subsoils in loess area (Behnood 2018). As the preferred methods in engineering practice, physical methods have some shortcomings. For example, dynamic compaction method produces unbearable noises and affects residents nearby. It also causes great vibration which may lead to failure of slopes and disruption of buildings nearby (Feng et al. 2015). In addition, physical methods have some limitations because large mechanical equipment used has high requirements for size and flatness of the site. The essence of chemical methods is adding stabilizing agents which may react with the minerals or water in soil or filling soil pores directly and then cement soil particles and make the soil structure denser (Semkin et al. 1986). Owning to rapid speed, easy construction, and stable performance after treatment, chemical methods have gained attentions of a growing number of researchers and engineers. Lime, cement, and some other traditional stabilizing agents are usually used in engineering practice to improve the mechanical properties of loess (Mariri et al. 2019; Garakani et al. 2019), while these stabilizing agents may not provide a satisfactory result or have negative effects. For example, lime-treated loess has relatively low strength and poor water resistance (Jha and Sivapullaiah 2015; Gao et al. 2018). Cement-treated loess is susceptible to shrinking and cracking although it has high strength and excellent water resistance (Ghadakpour et al. 2020). Besides, production of cement costs a lot, and it will pollute the environment (Zhang et al. 2017). Given that traditional stabilizing agents have defects, it is meaningful to find eco-friendly and cost-effective stabilizing agents for strengthening loess and to study the mechanism and effect of strengthening systematically.
Soil stabilizing agents appeared in the 1940s and have been widely accepted in Western countries since the 1970s (Ikeagwuani and Nwonu 2019). In the 1990s, soil stabilizing agents began to be introduced into China. After 30 years of research and application, abundant theoretical achievements and practical experience have been accumulated. However, there is still a lack of systematic and in-depth researches on the stabilizing agents which are dedicated for loess. It might be because loess is mainly distributed in Northwest China where the economy and society were relatively backward, and there were relatively few engineering construction activities in the early years. This review summarizes the non-traditional stabilizing agents that have been studied or applied to strengthen loess soils. The mechanism of strengthening is analyzed, and the physical and mechanical properties of strengthened loess are described. In addition, the problems and shortcomings in research and application are put forward and analyzed. Such a review is expected to provide helpful guidance for research and application of loess stabilizing agents.
Loess stabilizing agents
This part introduces seven non-traditional stabilizing agents which are capable of improving the mechanical and hydraulic properties of loess, including nanomaterials, red mud, HEC (High Strength and Water Stability Earth Consolidator), hydrophobic stabilizing agent, EN-1 (a highly concentrated acidic solution composed of a variety of inorganic and organic chemicals), SH (a polymer material made up mainly of chemical wastes and taking polyacrylic acid as the base), and lignin. Most of them are still studied in the laboratory. The effect of each stabilizing agent on the mechanical and hydraulic properties of loess is illustrated by the collection and analysis of the published data. The mechanism of each non-traditional stabilizing agent for strengthening loess is put forward. In addition, the problems and shortcomings in research and application of each stabilizing agent are carefully discussed.
Nanomaterials
Nanomaterials refer to materials with at least one dimension not exceeding 100 nm in three-dimensional space or materials composed of nanomaterials. Nanomaterials are used in various industries because of their special structure, large specific surface area, and high surface activity (Krishnan and Shukla 2019). Nanomaterials have been used in the field of civil engineering to improve the strength of concrete and reduce energy consumption and environmental pollution (Amin and Abu El-Hassan 2015). Nanomaterials also have been used in the fields of geotechnical engineering and geo-disaster prevention to improve the soil strength (Hosseini et al. 2019). Pham and Nguyen (2014) found that nanosilica can reduce the expansibility of montmorillonite effectively. Ng and Coo (2015) added nanoparticles (gamma-aluminum oxide and copper oxide) into clay and found that the permeability of clay decreased. Iranpour and Haddad (2016) confirmed the effectiveness of nanomaterials (nanoclay, nanocopper, nanoalumina and nanosilica) in treating collapsible soils. Taha et al. (2018) reduced the swelling-shrinkage property of clays by adding nanocarbon to them. However, at present, researches on loess stabilization with nanomaterials are very limited. Only about a dozen researchers around the world are studying the use of nanosilica and nanoclay (K10 montmorillonite) to strengthen loess.
Nanosilica has characteristics of small particle size and high surface energy which make nanosilica powders function as fillings and cementations when they are mixed with loess. Owing to the strong surface activity and high adsorption capacity, nanosilica particles associate themselves into aggregates that can fill macropores and cement coarse particles. Therefore, the accumulation of aggregations of nanoparticles will change the distribution of soil pores, transforming large pores into small pores and endowing the soil a denser structure (Huang and Wang 2016). In addition, nanosilica has the pozzolanic activity due to the large specific surface area and high surface energy; it can react with the calcium ions in soil to generate hydrated calcium silicates (C-S–H) gel that can cement or wrap soil particles (Ghasabkolaei et al. 2017). The physical and mechanical properties of treated loess are thus different from that of untreated loess. Kong et al. (2018) measured the mechanical properties of loess treated with nanosilica with an average particle size of 30 nm. The results show that when the dosage of nanosilica (ratio of the weight of stabilizing agent to the dry weight of soil, s denotes the dosage thereafter) ranges between 0 and 2%, the unconfined compressive strength (UCS) of treated loess increases monotonously with the dosage or curing time. They considered that the increase in the soil strength after treatment with nanosilica is due to the changes in the soil structure rather than chemical reactions. Sarli et al. (2019) carried out a study on loess strengthened with nanosilica and recycled polyester. The results indicate that the addition of nanosilica with an average particle size of 30 nm can increase the shear strength of loess. The maximum shear strength corresponds to the dosage of 4%, above which the shear strength no longer increases much with the dosage. This could be related to the high adsorption capacity of nanoparticles. As the dosage increases, nanoparticles are more likely to aggregate themselves; then, the effect of strengthening could be affected. To reduce this impact, some researchers proposed to add nanomaterial into soil in the form of sol. Lv et al. (2018) used sols made from nanosilica particles with different average sizes (i.e., 10 nm, 29 nm, 100 nm) to strengthen loess. The treated loess was cured for 28 days before being compressed to determine its UCS. Figure 2 compares the UCSs of loess treated with sols made from nanosilica particles with different average sizes. It is observed that the curves showing the relationship between the UCS and dosage of nanosilica sol can be fitted by exponential function, i.e., UCS = a∙exp(b∙s), where a and b are fitting parameters, and s is the dosage. It can be seen from Fig. 2 that parameter a varies a little with the average size of nanosilica particles. While parameter b decreases with the average size of nanosilica particles, there is a good linear relationship between them. In that case, the UCS of loess treated with nanosilica sols made from nanosilica particles of any average size (for example, between 10 and 100 nm) can be predicted based on the above test results. This linear relationship needs to be further validated, which is however beyond the scope of this study. In addition, for large dosages, accurate predictions could not be provided since the UCS does not always increase exponentially with the dosage. The variation of the UCS of loess treated with nanosilica particles is also shown in the figure. Two sets of data show very different rates at which the UCS increases with the dosage. That is because the definition of the dosage varies in two studies; one is related to the weight of nanosilica particles, and the other is related to the weight of nanosilica sol. Moreover, loess soils used in two studies are different. Nevertheless, two sets of data show that the UCS of treated loess increases monotonously with the dosage. The UCSs of loess treated with nanosilica sols do not decrease even in the cases of high dosage (> 4%).
It can be seen that whether loess is treated with nanosilica particles or nanosilica sol, the UCS of treated loess increases with the dosage. The smaller the average particle size of nanosilica, the higher the UCS of loess treated with nanosilica sol. Figure 3 depicts the relationship between the UCS, dosage, and curing time of loess treated with nanosilica particles. It can be seen that the UCS of treated loess increases with the increase of dosage or extension of curing time. The UCS of treated loess can be expressed as Eq. (1), where s refers to the dosage and the value should be multiplied by 100 before being put into the equation; in addition, the UCS of untreated loess does not change with the time, so let us define s ≠ 0; t refers to the curing time, and the unit is day; when t < 7, the predicted UCS is lower than that of untreated loess, which does not match with the fact; in addition, when t exceeds 60 days, the UCS increases slowly with the curing time and tends to be constant, which cannot be indicated by the equation. For this reason, 7 ≤ t ≤ 60, t0 = 1 day; pa refers to the atmospheric pressure, pa = 101.33 kPa.
With respect to nanoclay, Tabarsa et al. (2018) studied the effect of nanoclay on strengthening loess through a comprehensive experimental study, including measurements of Atterberg limits, standard Proctor compaction tests, unconfined compressive strength tests, unconsolidated undrained triaxial tests, collapse tests, pinhole dispersion tests, and an in situ test at the Gonbad dam in Iran. The results suggest that the addition of nanoclay can increase the plasticity index, shear strength and UCS of loess, and decrease the collapsibility and dispersibility. The in situ test shows that the addition of 2% nanoclay can endow the irrigation canal excellent resistance to erosion and corrosion. Figure 4 compares the Atterberg limits of loess soils treated with nanoclay and nanosilica sol. Both liquid limit and plastic limit of loess treated with either nanoclay or nanosilica increase with the dosage. This indicates that the resistance to liquefaction of loess has been improved.
Since silica and clay are two major minerals in natural loess, the use of nanosilica and nanoclay to strengthen loess has little impact on the soil environment and ecological environment. They are eco-friendly and have promising application prospect. However, according to the current research status, research on the mechanism of strengthening loess with nanomaterials is not thorough, and the relevant experimental studies are quite lacking. Therefore, there is a strong need to study the physical and chemical reactions between nanoparticles and soil particles or water molecules at atomic or molecular level. In addition, the physical and mechanical properties of loess treated with nanomaterials should be thoroughly studied. The mechanical properties of treated loess under complex stress paths and under the influence of various environmental factors considering the time effect should be studied systematically.
Red mud
Red mud is a kind of reddish-brown alkaline waste residue produced during the extraction of alumina from bauxite. The physical, chemical, and mineralogical properties of red mud are varied because the technical level and extraction process of alumina and the storage time of red mud are varied. According to the extraction process of alumina, red mud can be divided into Bayer red mud, sintered red mud, and combined red mud (Wang and Liu 2012). Bayer red mud has the largest output and is the most used for soil stabilization among three types of red mud (Liu and Wu 2012). The average particle size of red mud is less than 10 μm, and the specific surface area is approximately 64–187 m2/g (Xue et al. 2016). When red mud and cementitious material (for example, cement) are used to strengthen loess together, the addition of red mud can increase not only the alkalinity of the mixture but also the number of active ions, promoting the hydration and pozzolanic reaction of cement to generate more hydrated calcium silicates (C-S–H), hydrated calcium aluminosilicates (C-A-S–H), and ettringite (i.e., product in the form of crystal which is helpful to increase the early strength of cement and can also be fillings) (Yu et al. 2019). Red mud may also be hydrated to generate hydrated dicalcium silicates (C2-S–H). These cementitious hydration products (i.e., C-S–H, C-A-S–H, C2-S–H), ettringite, and red mud particles can fill large pores and cement soil particles, resulting in a lower porosity and a denser soil structure (Chen et al. 2019). For this reason, the strength of loess stabilized with red mud is higher and grows faster than that of loess stabilized with cement only; accordingly, the permeability of stabilized loess is about an order of magnitude lower than that of loess without any stabilizing agent (Wan et al. 2009). In summary, the cementation effect of cementitious hydration products of cement and red mud and the filling effect of cementitious hydration products and red mud can make the loess structure more compact and stable. Mi et al. (2016) carried out a series of unconfined compressive strength tests on a cement-loess (the weight of cement is 5% of the weight of loess particles) strengthened with red mud. They found that the UCS of strengthened cement-loess reaches the peak when the dosage of red mud is 5%. As the dosage of red mud exceeds 5%, the UCS decreases, even lower than that of cement-loess without red mud, see Fig. 5a. Chen et al. (2018) found that the maximum dynamic elastic modulus, shear strength, and UCS of cement-loess strengthened with red mud are greatly higher than that of cement-loess without red mud. However, their experimental results revealed that the optimum dosage of red mud is about 15%. Excessive red mud can lead to significant degradation of the mechanical properties of cement-loess, see Fig. 5a. It might be due to that redundant red mud particles adhere to the surfaces of soil particles, which prevents the hydration products from cementing soil particles, leading to a reduction of the integrity and stability of the soil structure.
Figure 5b shows the relationship between the UCS of cement-loess (5% cement) strengthened with red mud, dosage of red mud, and curing time. It shows that the UCS of red mud-strengthened cement-loess increases at first and then decreases with the dosage of red mud. In addition, the longer the curing time, the greater the UCS of strengthened cement-loess. The UCS of strengthened cement-loess can be expressed as Eq. (2), where s refers to the dosage of red mud and it is dimensionless; the value should also be multiplied by 100 before being put into the equation, and s ≠ 0; t refers to the curing time, and 7 ≤ t ≤ 28 days, t0 = 1 day; pa refers to the atmospheric pressure, pa = 101.33 kPa. The reasons for limiting the values of s and t (i.e., s ≠ 0, 7 ≤ t ≤ 28) are the same as mentioned earlier. The optimum dosages of red mud obtained in two studies are quite different, which might be because both loess soil and red mud used in one study are different from that in the other. It suggests that the optimum dosage of red mud is influenced by the properties of loess and the type of red mud.
It is certain that red mud can improve the physical and mechanical properties of loess, while there are very few researches on the deterioration of red mud-strengthened loess due to freezing, thawing, drying, and wetting (Xu et al. 2020). More importantly, red mud needs to be dried and grinded before mixed with loess, which will undoubtedly increase the input of mechanical devices and human resources, and increase the project budget. Even worse, the harmful substances in red mud, for example, heavy metals, may cause pollution to the soil environment due to stockpiling of red mud or use of red mud in loess stabilization. These harmful substances may migrate to groundwater under conditions of rainfall infiltration and groundwater table rising, bringing harm to the ecological environment. Therefore, whether red mud can be used in loess engineering should be seriously considered to comprehensively evaluate the feasibility of using red mud to stabilize loess subgrade, loess slope, and loess subsoil.
HEC
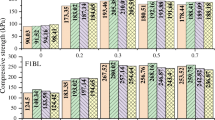
HEC is a powdered water-hardening inorganic cementitious material taking industrial waste residues (such as fly ash, slag, gypsum) as raw materials. HEC and its cementitious hydration products (i.e., hydrated calcium aluminates, C-A-H) can cement loess particles at room temperature, improving the mechanical properties of loess. Zhang et al. (2009a) stated that the addition of HEC can effectively increase the strength and resistance to disintegration, and reduce the permeability (by about an order of magnitude) and collapsibility of loess. Ma et al. (2018) confirmed that HEC can increase the compressive strength and reduce the permeability of loess significantly. They also suggested that the effect of strengthening loess with HEC and cement together is better than using either of them alone. This may be because both HEC and cement can be hydrated, producing various cementitious hydration products which work together and show a better effect. Figure 6 compares the UCSs of loess soils strengthened with HEC, cement, and both. It can be seen that the UCS of loess strengthened with HEC and cement together is the highest, and the UCS of loess strengthened with HEC is higher than that of loess strengthened with cement.
The HEC-treated loess has the characteristics of high early strength, stable later strength, excellent water stability, and high durability. However, like cement, HEC is a water-hardening inorganic cementitious material; the side effects of them for soil stabilization are also similar, such as high cost and environmental pollution. For these reasons, there are few researches on HEC and soils strengthened with it up to now. Nevertheless, taking account of that the strengthening effect of HEC is better than that of cement (see Fig. 6), HEC may be a good alternative for strengthening the subsoils in important projects and treating urgent engineering disasters caused by water seepage in loess area.
Hydrophobic stabilizing agent
Hydrophobic stabilizing agent includes liquid agent C444 and solid agent SD. C444 is a yellow colloidal organic chemical. It can destroy the bound water film adsorbed on the surfaces of clays, reduce the distance between clay particles, and increase the attraction between clay particles (Eren and Filiz 2009). In addition, the hydrophobic molecules in C444 can enhance the water repellency of soil, which helps to maintain the internal structure of soil dry. SD is a grayish-white powdered polymer. When SD is added to loess, a polymer network will be formed on the surfaces of soil particles, which can wrap soil particles and weaken the softening effect of water on particle connections, thus increasing the cohesion of soil. The addition of liquid agent C444 and solid agent SD in different proportions can achieve the goals of strengthening different types of soils. In short, hydrophobic stabilizing agent can improve the shear strength and water repellency of soil, and accelerate the petrification of soil. Zhang et al. (2016a) observed that the liquid limit, plastic limit, UCS, and water repellency of loess strengthened with hydrophobic stabilizing agent are greatly higher than that of intact loess. Peng et al. (2017) carried out an infiltration test and observed the microstructure of strengthened loess using X-Ray diffraction, scanning electron microscopy (SEM), and mercury intrusion porosimetry (MIP) techniques, to investigate the mechanism of hydrophobic stabilizing agent for strengthening loess. In the infiltration test, 0.05 ml distilled water was dropped on the surface of soil sample using a standard buret and the time required for complete infiltration (i.e., time from the beginning to the disappearance of droplet) was recorded. The result shows that the time required for complete infiltration increases with the dosage of hydrophobic stabilizing agent, which indicates that the surface free energy of soil particles is reduced and the water repellency of soil is improved. The microscopic observation shows that fine particles (clays) are aggregated after adding hydrophobic agent, while the arrangement of coarse particles (including silts and aggregates) is almost unchanged (see Fig. 7); thus, the macro-level structure (i.e., the arrangement of silts and aggregates) of loess is little influenced. When dividing soil pores in loess into four groups according to the pore diameter, namely, micropores (< 1 μm), small pores (1–4 μm), mesopores (4–16 μm), and macropores (> 16 μm), it can be seen from the results of mercury intrusion tests, as shown in Fig. 8, that micro- and small pores in strengthened loess are increased greatly, while meso- and macropores do not change much. Figure 9 shows the relationship between the permeability coefficient of strengthened loess and dosage of hydrophobic agent. It is shown that with the increasing dosage of hydrophobic agent, the permeability coefficient of strengthened loess decreases slightly rather than increases. That is probably due to the increase in the complexity of the pore structure (a large increase in micro- and small pores is not accompanied by an apparent decrease in meso- and macropores, which indicates that the complexity of the pore structure is increased). For these reasons, hydrophobic stabilizing agent is suggested to be capable of improving the water repellency (or the water stability) of loess while maintaining the soil macro-level structure unchanged, which makes it a good choice to strengthen loess or reduce the collapsibility while maintaining the permeability of loess.
EN-1
EN-1 is non-volatile, but has corrosivity and an irritating sour smell. Diluted EN-1 is a polymer composite which has little impact on the environment. The strong oxidants, solvents, and dispersants in EN-1 can promote the decomposition and recrystallization of the minerals in soil to form insoluble metal salts (Shan et al. 2010). Those metal salts can fill soil pores and enhance the bonding force between soil particles. In addition, the cations with higher exchange capacity in EN-1 can exchange with the cations adsorbed on the surfaces of clays intensely, thin the bound water film, decrease the surface potential of particles, and strengthen the connection between particles, thus improving the shear strength and reducing the permeability of loess (Zhang and Jing 2011). Shan et al. (2010) carried out a model test of loess subgrade strengthened with EN-1. They reported that EN-1 can be used to strengthen loess subgrade, but it weakens the water retention capacity of loess. In addition, it is often to tamp the mixture when mixing EN-1 with loess so that the additive can fully contact and react with loess particles. Therefore, this stabilizing agent may not improve the resistance to erosion of loess and have negative effect on the growth of vegetation on loess slope.
SH
SH has high hydrophilia and can dissolve in water infinitely. The main chains in SH are macromolecular chains connected by hydrophobic C–C bonds, and there are two hydrophilic groups on the macromolecular chains, hydroxyl (–OH) and carboxyl (–COOH) groups. Researchers speculated that a series of physical interactions (such as adsorption and flocculation) and chemical interactions (such as ion exchange and bonding) may take place between this polymer material and loess (Li et al. 2018). In terms of physical interactions, SH can not only fill soil pores, but also form a network to wrap soil particles, preventing water from infiltrating into soil and increasing the water stability of loess. With respect to chemical interactions, the hydrogen ions in hydroxyl or carboxyl groups in SH can replace the alkaline-earth metal ions (such as Mg2+ and Ca2+) on the surfaces of clay particles, the thickness of the double-layer and the distance between particles are then reduced (i.e., the exchange capacity of H+ is higher than Ca2+ and Mg2+; the higher the exchange capacity of the cation, the thinner the thickness of the double-layer). As a result, the attraction between soil particles is increased (Wang et al. 2011). The carboxyl (–COOH) on the macromolecular chains can also react with the hydroxyl (–OH) on the surfaces of silicates to form a hydrogen bond. The test results of Wang (2016) show that the addition of SH can improve the shear strength, resistance to deformation related with freezing and thawing, and water stability and reduce the compressibility and eliminate the collapsibility of loess. It is worth mentioning that the extension of curing time also helps to improve the mechanical and hydraulic properties of loess. For example, the permeability coefficient of SH-strengthened loess after 28 days curing is about an order of magnitude lower than that of SH-strengthened loess without curing.
SH has the advantages of low cost, good effect, and little environmental pollution. However, there is still a lack of experimental research on it at present. More attention should be paid to the development and variation of the mechanical properties of SH-strengthened loess under the influence of environmental factors (meteorological, biological, and so on), to provide a theoretical basis for the application and popularization of SH in practical engineering.
Lignin
Lignin is a three-dimensional polymer compound composed of phenylpropanoid structural units connected by C–C bonds and ether bonds (Calvo-Flores and Dobado 2010). Lignin is widely found in the xylem of terrestrial plants, while natural lignin is not available (Vinod et al. 2010). The lignin used to strengthen loess is a derivative of lignin in fact, i.e., lignosulfonate (Kim et al. 2012). Lignosulfonate includes calcium lignosulfonate and sodium lignosulfonate. Compared with other stabilizing agents, both traditional and non-traditional, lignosulfonate has the advantages of non-toxicity, rich reserve, low cost, strong reproducibility, and stable chemical properties (Zhang et al. 2016b).
He et al. (2017) studied the mechanical properties of loess soils treated with calcium lignosulfonate and sodium lignosulfonate, respectively. The results show that the compressive strength, tensile strength, shear strength, and resistance to disintegration of calcium lignosulfonate-strengthened loess are all increased when the dosage of calcium lignosulfonate is increased up to 1%. In contrast, sodium lignosulfonate cannot improve the mechanical properties of loess; even worse, it will aggravate the salinization of soil. As for the mechanism, mixing calcium lignosulfonate with loess will generate filamentous materials, which can not only fill pores, but also connect soil particles, thus improving the strength and stability of loess. Meanwhile, the hydrophilic group on the molecular chain of calcium lignosulfonate can exchange with the metal ions on the surfaces of loess particles, the thickness of the bound water film is then reduced, and the attraction between particles is increased. The molecular chain of calcium lignosulfonate can wrap soil particles and form a net structure, reducing the damage brought by water to the soil structure and improving the water stability of loess. Figure 10 shows the relationship between the UCS of strengthened loess and dosage of calcium lignosulfonate or sodium lignosulfonate. It is shown that the UCS of loess treated with sodium lignosulfonate decreases linearly with the dosage, while the UCS of loess treated with calcium lignosulfonate increases at first and then decreases with the growing dosage. Increasing the curing time is also beneficial to improve the strength of strengthened loess, while when the dosage of calcium lignosulfonate is greater than 1%, the extension of curing time will have a negative effect on the UCS of strengthened loess. Therefore, for different loess soils, determination of the optimum dosage is the premise for application of lignin in loess stabilization. The permeability coefficient of loess is greatly reduced after being treated with calcium lignosulfonate (it can be reduced by an order of magnitude when the dosage is 3%); with the extension of curing time, the coefficient of permeability decreases further.
Other loess stabilizing agents
In addition to the above-mentioned stabilizing agents, Table 1 summarizes some other loess stabilizing agents with less research and application, including LD, BCS, BTS, CONAID and LUKANG, ISS, Coal gangue, and SSA. Their composition, mechanism, and effect of strengthening, advantages, or disadvantages are described briefly.
Discussion
Discussion on the mechanism of strengthening
Based on the introduction and analysis of various loess stabilizing agents in previous sections, it could be concluded that there are several mechanisms of strengthening loess with these stabilizing agents, including filling, cementation, cementation of hydration products, ion exchange, and wrapping of polymer. Table 2 provides an in-depth interpretation of these mechanisms by using schematic diagrams.
Discussion on the effect of strengthening
Figure 11 shows the permeability coefficients of loess soils strengthened with different stabilizing agents. It can be seen that calcium lignosulfonate and red mud are the most effective in improving the impermeability of loess, i.e., the permeability coefficient of loess can be reduced by an order of magnitude at most. SH and HEC also perform well in reducing the permeability of loess, while when the dosage reaches a certain value, the permeability coefficient of SH-treated loess no longer decreases obviously with the increase of dosage. The permeability coefficient of loess treated with HEC decreases monotonously with the growing dosage. The effect of hydrophobic stabilizing agent is similar to that of SH on reducing the permeability of loess at low dosages, while as the dosage increases further, the permeability coefficient of loess strengthened with hydrophobic agent increases, i.e., the difference in the permeability coefficient between treated and untreated loess decreases (see Fig. 11).
Different stabilizing agents have different effects on reducing the permeability of loess, which can be attributed to differences in the component, state, and strengthening mechanism of various stabilizing agents. Ion exchange occurs when mixing calcium lignosulfonate with loess particles, which makes the double-layer on the particle surfaces thinner, increasing the attraction between particles and making the soil structure more compact (Li et al. 2019b). Calcium lignosulfonate can also react with clay minerals in loess to generate siliceous cementations and carbonate cementations (He et al. 2017). In addition, calcium lignosulfonate molecules can fill the voids between particles. These mechanisms act together, making the porosity of calcium lignosulfonate-strengthened loess greatly lower than that of intact loess, and the permeability coefficient of loess decreases significantly as well. Red mud itself may be hydrated and generate hydrated dicalcium silicates C2-S–H; the strong alkaline environment and the active ions provided by red mud can promote the hydration and pozzolanic reaction of cement to generate cementitious hydration products, such as C-S–H and C-A-S–H gel. These cementitious gels fill soil pores and cement soil particles, leading to the flow channels in soil plugged; thus, the permeability is greatly decreased (Chen et al. 2018). Ion exchange occurs when loess is strengthened with SH, which reduces the thickness of the double-layer and the distance between soil particles. As a result, the porosity and permeability of loess are decreased (Wang et al. 2011). In comparison, the wrapping effect of the network formed by the macromolecular chains is not as effective as the filling effect on reducing the permeability. Therefore, the permeability of SH-strengthened loess is not decreasing with the dosage as significantly as that of calcium lignosulfonate- and red mud-strengthened loess. HEC can also be hydrated, producing cementitious substances that can fill soil pores, but the hydration rate of HEC is slow and the hydration products are limited (Zhang et al. 2009a). Besides, there is neither ion exchange like that in calcium lignosulfonate-strengthened loess to make the soil structure denser nor alkaline environment like that in red mud-strengthened loess to promote the hydration of cement. So, the permeability reduction of HEC-strengthened loess is less than that of calcium lignosulfonate- or red mud-strengthened loess. With regard to hydrophobic stabilizing agent, it has little impact on macro- and mesopores, but can increase micro- and small pores greatly, so the complexity of the pore structure increases and the permeability of soil decreases slightly. This stabilizing agent is suggested to be able to improve the water stability of soil while maintaining the soil macro-level structure unchanged (Peng et al. 2017).
Among the various mechanisms, the filling effect is the most effective to improve the impermeability of loess, i.e., to reduce the permeability coefficient. The filling effect could be played by individual particles or aggregates of granular stabilizing agents (such as nanoparticles, red mud, HEC, calcium lignosulfonate, coal gangue), liquid polymer materials, hydration products of stabilizing agents (such as red mud and HEC) or other additives (typically cement), and products of the reaction between stabilizing agent and minerals in soil (for example, EN-1).
Figure 12 compares the cohesion and internal friction angles of loess soils strengthened with different stabilizing agents. In comparison, the cohesion and internal friction angle of HEC-strengthened loess are increased most significantly with the dosage, followed by SH-strengthened loess. The cohesion of loess strengthened with nanoclay increases with the dosage, while the internal friction angle decreases. In general, the shear strength of loess strengthened with nanoclay is increased (Tabarsa et al. 2018), while not as much as that of the former two, i.e., HEC- and SH-strengthened loess. With the increase of dosage, the cohesion and internal friction angle of calcium lignosulfonate-strengthened loess increase at first and then decrease. The two shear strength parameters (i.e., cohesion and internal friction angle) reach the highest when the dosage of calcium lignosulfonate is 1%.
The cohesion of soil depends mainly on the cementation between soil particles, electrostatic attraction, and capillary pressure. The internal friction angle is related to the relative density, mineral composition, particle shape, and bound water film adsorbed on the surfaces of soil particles (Liu et al. 2020). HEC is the most effective to improve the shear strength of loess among these stabilizing agents (see Fig. 12). HEC is a water-hardening inorganic cementitious material; HEC and its hydration products can cement soil particles, which have a considerable promotion on the cohesion and internal friction angle of soil (Ma et al. 2018). As for SH, it strengthens loess through the ion exchange effect and the wrapping effect of polymer, and the shear strength of SH-strengthened loess increases a little (Wang 2016). Addition of nanoclay is equivalent to increase the content of fine particles. Nanoparticles accumulate at the particle contacts and cement soil particles, thus increasing the cohesion of soil. However, the effect of nanoclay on improving the shear strength of loess is not as good as that of SH and HEC. This is because the coating effect of nanoparticles weakens the intergranular interlocking effect when the dosage of nanoparticles is relatively high, and the internal friction angle of soil is then decreased (Tabarsa et al. 2018). Overall, the shear strength of loess treated with nanoclay is greater than that of untreated loess. Like nanoclay, the effect of calcium lignosulfonate on improving the shear strength of loess is also affected by the dosage. When the dosage of calcium lignosulfonate is small (less than 1%), the ion exchange effect makes the soil structure denser, the cohesion and internal friction angle of soil are increased. However, when too much calcium lignosulfonate is added (more than 1%), the distance between soil particles increases and the interparticle attraction decreases due to the increase of large-sized calcium lignosulfonate molecules. It is getting easier for soil particles to slide over each other; the shear strength of soil is thus degraded (He et al. 2017).
Among the stabilizing agents mentioned above, the stabilizing agents that can produce cementitious substances by hydration are the most effective to improve the shear strength of loess. That is because cementitious hydration products can not only cement soil particles to improve the cohesion of soil, but also fill the voids between particles to increase the internal friction angle of soil. Some stabilizing agents can make the double layers of clays thinner and the soil structure denser by ion exchange, such as hydrophobic stabilizing agent, calcium lignosulfonate, and BTS, resulting in a little increase in the cohesion and internal friction angle of soil. Some other stabilizing agents may have negative influence on the shear strength of loess when the dosage is very high.
Conclusions
This review summarizes the non-traditional stabilizing agents that can effectively improve the physical and mechanical properties of loess, including nanomaterials, red mud, HEC, hydrophobic stabilizing agent, EN-1, SH, calcium lignosulfonate, and some other loess stabilizing agents with less research and application. The composition, mechanism, effect, and application of each stabilizing agent are introduced. These stabilizing agents are found to strengthen loess through the following mechanisms, namely, filling, cementation, cementation of hydration products, ion exchange, and wrapping of polymer. Filling is to increase the density and uniformity of soil by filling soil pores with stabilizing agents or hydration products of them or other additives. Cementations can be produced in two cases: one is that stabilizing agents absorb the water in soil pores to generate viscous gel, and the other is that the hydration of stabilizing agents or other additives produces cementitious substances. Both can cement soil particles and enhance the connections between soil particles. Ion exchange is that the cations with higher exchange capacity in stabilizing agent can replace the cations on the surfaces of clay particles, reducing the thickness of the double-layer and increasing the attraction between soil particles. Wrapping of polymer is that the macromolecular chains in polymer that are crossed and connected can form a network to wrap soil particles, thereby improving the stability and strength of soil. The effects of these stabilizing agents on loess include increasing the soil density, reducing the soil permeability, increasing the soil strength, and improving the resistance to deformation. One or several of them work together when loess is treated with any stabilizing agent. Among the mechanisms, the filling effect is the most effective to reduce the permeability, and the effect of cementation is the most effective to improve the shear strength of loess. When strengthening loess with a stabilizing agent, the dosage is a very important variable since some stabilizing agents may have negative effect when the dosage is very high. For this reason, determination of the optimum dosage is the premise for application of any stabilizing agent.
Although there are already dozens of stabilizing agents that can improve the physical and mechanical properties of loess, it is still meaningful to find eco-friendly and cost-effective stabilizing agents for strengthening loess. In addition, at present, researches on most stabilizing agents are not thorough, the related experimental studies are quite lacking, and the results for the same stabilizing agent are not consistent. Even worse, understanding of the mechanisms of most stabilizing agents is not in-depth and specific enough. So, research focuses are now still on interpretation of the mechanism of each stabilizing agent, in terms of physical, chemical, and biological process, respectively, as well as determination of the physical and mechanical properties of strengthened loess. Strengthened loess could be regarded as a new soil; its mechanical properties under complex stress paths and under the influence of various environmental factors should be studied systematically. In addition, attention should be paid to the degradation in the mechanical properties of strengthened loess. Furthermore, the effect of strengthening loess with two or more stabilizing agents together might be better than that of any one alone since they may stimulate the activity of each other. As long as the stabilizing agents do not react with and restrain each other, the use of two or more stabilizing agents at the same time or in stages can make full use of the advantage of each stabilizing agent. Last but not the least, special attention should be given to model tests and field tests carried out in large-scale sites which can reveal the effect of strengthening more accurately and find the problems related to practical application of these stabilizing agents.
References
Amin M, Abu El-Hassan K (2015) Effect of using different types of nano materials on mechanical properties of high strength concrete. Constr Build Mater 80:116–124
Behnood A (2018) Soil and clay stabilization with calcium- and non-calcium-based additives: a state-of-the-art review of challenges, approaches and techniques. Transp Geotech 17:14–32
Calvo-Flores FG, Dobado JA (2010) Lignin as renewable raw material. Chem Sus Chem 3(11):1227–1235
Chen RF, Tian GY, Mi DY, Dong XQ (2018) Study on basic engineering properties of red mud stabilized loess. Rock Soil Mech 39(S1):89–97 (in Chinese)
Chen RF, Dong XQ, Mi DY, Puppala AJ, Duan W (2019) Mechanical properties and micro-mechanism of loess roadbed filling using by-product red mud as a partial alternative. Constr Build Mate 216:188–201
Costantini EA, Carnicelli S, Sauer D, Priori S, Andreetta A, Kadereit A, Lorenzetti R (2018) Loess in Italy: genesis characteristics and occurrence. CATENA 168:14–33
Dijkstra TA, Rogers CDF, Smalley IJ, Derbyshire E, Li YJ, Meng XM (1994) The loess of north-central China: geotechnical properties and their relation to slope stability. Eng Geol 36(3–4):153–171
Eren S, Filiz M (2009) Comparing the conventional soil stabilization methods to the consolid system used as alternative admixture matter in Isoarta Daridere material. Constr Build Mater 23(7):2473–2680
Feng SJ, Du FL, Shi ZM, Shui WH, Tan K (2015) Field study on the reinforcement of collapsible loess using dynamic compaction. Eng Geol 185:105–115
Gao YY, Qian H, Li XY, Chen J, Jia H (2018) Effects of lime treatment on the hydraulic conductivity and microstructure of loess. Environ Earth Sci 77:529. https://doi.org/10.1007/s12665-018-7715-9
Garakani AA, Mohsen HS, Desai CS, Mohammad HSG, Sadollahzadeh B, Senejani HH (2019) Testing and constitutive modeling of lime-stabilized collapsible loess. I: experimental investigations. Int J Geomech 19(4):1–10
Ghadakpour M, Choobbasti AJ, Kutanaei SS (2020) Experimental study of impact of cement treatment on the shear behavior of loess and clay. Arab J Geosci 13(4):184. https://doi.org/10.1007/s12517-020-5181-7
Ghasabkolaei N, Choobbasti AJ, Roshan N, Ghasemi SE (2017) Geotechnical properties of the soils modified with nanomaterials: a comprehensive review. Arch Civ Mech Eng 17(3):639–650
He ZQ, Fan HH, Wang JQ, Liu G, Wang ZN, Yu JH (2017) Experimental study of engineering properties of loess reinforced by lignosulfonate. Rock Soil Mech 38(03):731–739 (in Chinese)
Hosseini A, Haeri SM, Mahvelat S, Fathi A (2019) Feasibility of using electrokinetics and nanomaterials to stabilize and improve collapsible soils. J Rock Mech Geotech Eng 11(5):1055–1065
Houston SL, Houston WN, Spadola DJ (1988) Prediction of field collapse of soils due to wetting. J Geotech Eng 114(1):40–58
Huang Y, Wang L (2016) Experimental studies on nanomaterials for soil improvement: a review. Environ Earth Sci 75(6):497. https://doi.org/10.1007/s12665-015-5118-8
Ikeagwuani CC, Nwonu DC (2019) Emerging trends in expansive soil stabilisation: a review. J Rock Mech Geotech Eng 11(2):423–440
Iranpour B, Haddad A (2016) The influence of nanomaterials on collapsible soil treatment. Eng Geol 205:40–53
Jha AK, Sivapullaiah PV (2015) Mechanism of improvement in the strength and volume change behavior of lime stabilized soil. Eng Geol 198:53–64
Kim S, Gopalakrishnan K, Ceylan H (2012) Moisture susceptibility of subgrade soils stabilized by lignin-based renewable energy coproduct. J Transp Eng 138(11):1283–1290
Kong R, Zhang FY, Wang GH, Peng JB (2018) Stabilization of loess using nano-SiO2. Materials 11(6):1014
Krishnan J, Shukla S (2019) The behaviour of soil stabilised with nanoparticles: an extensive review of the present status and its applications. Arab J Geosci 12(14):436
Li CQ, Liu QB, Xiang W, Wang QE, Qiao Y (2018) Anti-erodibility of loess subgrade slope reinforced with SH-polymer and inorganic material. J Yangtze River Sci Res Inst 35(8):90–94 (in Chinese)
Li JY, Wang JM (2019) Comprehensive utilization and environmental risks of coal gangue: a review. J Clean Prod 239:117946. https://doi.org/10.1016/j.jclepro.2019.117946
Li GY, Hou X, Mu, YH, Ma W, Wang F, Zhou Y, Mao YC (2019b) Engineering properties of loess stabilized by a type of eco-material calcium lignosulfonate. Arab J Geosci 12(22). https://doi.org/10.1007/s12517-019-4876-0
Li P, Xie WL, Pak RYS (2019a) Vanapalli SK. Microstructural evolution of loess soils from the loess plateau of China. CATENA 173:276–288
Li P, Vanapalli SK, Li TL (2016) Review of collapse triggering mechanism of collapsible soils due to wetting. J Rock Mech Geotech Eng 8(2):256–274
Liu DY, Wu CS (2012) Stockpiling and comprehensive utilization of red mud research progress. Materials 5(7):1232–1246
Liu JW, Fan HH, Song XY, Yang XJ (2020) Characteristics of shear strength and deformation of compacted Q3 loess. Soil Mech Found Eng 57(1):65–72
Liu SJ, Fan HH, Shi X, He ZQ (2014) Durability of stabilized soil by BCS. Journal of Northwest A & F University (Natural Science Edition) 42(12):214–220 (in Chinese)
Liu Z, Liu FY, Ma FL, Wang M, Bai XH, Zheng YL, Yin H, Zhang GP (2015) Collapsibility, composition, and microstructure of a loess in China. Can Geotech J 53(4):676–686
Lv QF, Chang CR, Zhao BH, Ma B (2018) Loess soil stabilization by means of SiO2 nanoparticles. Soil Mech Found Eng 4(6):409–413
Ma WJ, Wang BL, Wang X, Jiang DJ, Li ZY (2018) Experimental study on mechanical properties of modified loess. Water Resour Hydropower Eng 49(10):150–156 (in Chinese)
Mariri M, Ziaie MR, Kordnaeij A (2019) Stress–strain behavior of loess soil stabilized with cement, zeolite, and recycled polyester fiber. J Mater Civ Eng 31(12). https://doi.org/10.1061/(ASCE)MT.1943-5533.0002952
Mi DY, Song ZW, Huang FF, Dong XQ (2016) Influence of red mud on electrical resistivity and strength characteristics of loess solidified by cement. Sci Technol Eng 16(15):268–272 (in Chinese)
Ng CWW, Coo JL (2015) Hydraulic conductivity of clay mixed with nanomaterials. Can Geotech J 52(6):808–811
Nouaouria MS, Guenfoud M, Lafifi B (2008) Engineering properties of loess in Algeria. Eng Geol 99(1–2):85–90
Peng Y, Zhang HY, Lin CB, Wang XW, Yang L (2017) Engineering properties and improvement mechanism of loess soil modified by consolid system. J Rock Mech Eng 36(03):762–772 (in Chinese)
Pham H, Nguyen QP (2014) Effect of silica nanoparticles on clay swelling and aqueous stability of nanoparticle dispersions. J Nanopart Res 16(1):2137. https://doi.org/10.1007/s11051-013-2137-9
Ryashchenko TG, Akulova VV, Erbaeva MA (2008) Loessial soils of Priangaria Transbaikalia Mongolia and Northwestern China. Quat Int 179(1):90–95
Sarli JM, Hadadi F, Bagheri RA (2019) Stabilizing geotechnical properties of loess soil by mixing recycled polyester fiber and nano-SiO2. Geotech Geol Eng 38(2):1151–1163. https://doi.org/10.1007/s10706-019-01078-7
Semkin VV, Ermoshin VM, Okishev ND (1986) Chemical stabilization of loess soils in Uzbekistan to prevent building deformations. Soil Mech Found Eng 23(5):196–199
Shan ZJ, Zhang XC, Zhao WX, Chen ZG (2010) Effect of EN-1 stabilizing agent on soil anti-erodibility. Sci Soil Water Conserv 24(05):6–9 (in Chinese)
Sun Y, Lu HY, An ZS (2006) Grain size of loess palaeosol and Red Clay deposits on the Chinese Loess Plateau: Significance for understanding pedogenic alteration and palaeomonsoon evolution. Palaeogeogr Palaeoclimatol Palaeoecol 241(1):129–138
Tabarsa A, Latifi N, Meehan CL, Manahiloh KN (2018) Laboratory investigation and field evaluation of loess improvement using nano-clay - a sustainable material for construction. Constr Build Mater 158:454–463
Taha MR, Alsharef JMA, Al-Mansob RA, Khan TA (2018) Effects of nano-carbon reinforcement on the swelling and shrinkage behaviour of Soil. Sains Malays 47(1):195–205
Vinod JS, Indraratna B, Mahamud MAA (2010) Stabilization of an erodible soil using a chemical admixture. Water Energy Int 163:43–51
Wan JH, Sun HH, Wang YY (2009) Effect of red mud on mechanical properties of loess-containing aluminosilicate based cementitious materials. Mater Res 610:155–160
Wang JD, Xu YJ, Ma Y, Qiao SN, Feng KQ (2018) Study on the deformation and failure modes of filling slope in loess filling engineering: a case study at a loess mountain airport. Landslides 15(12):2423–2435
Wang JD, Zhang DF, Wang NQ, Gu TF (2019) Mechanisms of wetting-induced loess slope failures. Landslides 16(5):937–953
Wang P, Liu DY (2012) Physical and chemical properties of sintering red mud and Bayer red mud and the implications for beneficial utilization. Materials 5(10):1800–1810
Wang SJ, Han WF, Wang YM (2003) Testing study on loess consolidated by LD serial rock-soil cemedin. Chin J Rock Mech Eng C2:2888–2893 (in Chinese)
Wang YM, Li C, Teng F, Gao LC (2011) New research on chemical solidification of collapsible loess foundation. Appl Mech Mater 90:2466–2471
Wang YM (2016) Shear strength characteristics of loess stabilized by new polymer materials. Low Temp Archit Technol 212(02):122–124 (in Chinese)
Xue SG, Zhu F, Kong XF, Wu C, Huang L, Huang N, Hartley W (2016) A review of the characterization and revegetation of bauxite residues (red mud). Environ Sci Pollut Res Int 23(2):1120–1132
Xu J, Li YF, Wang SH, Wang QZ, Ding JL (2020) Shear strength and mesoscopic character of undisturbed loess with sodium sulfate after dry-wet cycling. Bull Eng Geol Environ 79(3):1523–1541
Yates K, Fenton CH, Bell DH (2018) A review of the geotechnical characteristics of loess and loess-derived soils from Canterbury South Island New Zealand. Eng Geol 236:11–21
Yu JC, Qian JS, Tang JY, Ji ZW, Fan YR (2019) Effect of ettringite seed crystals on the properties of calcium sulphoaluminate cement. Constr Build Mater 207:249–257
Zhang CL, Jiang GL, Su LJ, Zhou GD (2017) Effect of cement on the stabilization of loess. J Mt Sci 11(14):2325–2336
Zhang HY, Peng Y, Wang XW, Lin CB (2016a) Study on water entry and loss capacity of loess stabilized by hydrophobic agent. Rock Soil Mech 37(S1):19–26 (in Chinese)
Zhang T, Cai G, Liu S, Puppala AJ (2016b) Engineering properties and microstructural characteristics of foundation silt stabilized by lignin-based industrial by-product. KSCE J Civ Eng 20(7):2725–2736
Zhang LJ, Li Y, Liu HH (2016c) Experimental study on loess stabilization by composite BTS agent. Subgrade Eng 6:125–128 (in Chinese)
Zhang WF, Liu QB, CAI ST, (2009a) Testing study on loess consolidated by HEC soil stabilizer. Yangtze River 40(03):56–59 (in Chinese)
Zhang LP, Zhang XC, Sun Q (2009b) Study on capacity of improving loess anti-shear strength and anti-permeability of two kinds of ionic soil solidified agent. Water Sav Irrig 5:35–38 (in Chinese)
Zhang ZQ, Jing YL (2011) Mechanical properties of road base with roadbood EN-1. Adv Mater Res 255:3366–3370
Funding
The funding was received from the National Natural Science Foundation of China (42007251), the National Key Research and Development Plan (2018YFC1504703), the Shaanxi Key Laboratory of Loess Mechanics and Engineering (LME201803), the China Postdoctoral Science Foundation (2019M653883XB), and the Education Department of Shaanxi Provincial Government (18JK0785).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, Y., Li, P. & Wang, J. Review of chemical stabilizing agents for improving the physical and mechanical properties of loess. Bull Eng Geol Environ 80, 9201–9215 (2021). https://doi.org/10.1007/s10064-021-02486-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10064-021-02486-x