Abstract
The Sejnane reservoir in northeast Tunisia provides drinking and irrigation water. Long-term water quality monitoring data including precipitation, evaporation, temperature, pH, conductivity, dissolved oxygen, turbidity, total suspended solids, major anions and cations, fluoride, BOD5, NO3 −, NO2 −, NH4 +, P tot, fecal coliform bacteria, boron and heavy metals (Fe, Zn, Cu, Ni, Pb, Cr and Cd) are reported. The appropriateness for irrigation was estimated by the SAR and Na percentage and the water quality assessed using the Canadian Water Quality Index as good to excellent, which confirmed its suitability for drinking, aquatic life and irrigation purposes.
Résumé
Le barrage Sejnane, situé au nord est de la Tunisie, a pour objectif de contribuer à la satisfaction des besoins en eau potable et l’irrigation des terres agricoles. L’article présente un suivi à long terme des paramètres de la qualité des eaux: précipitation, évaporation, température, pH, conductivité, oxygène dissous, turbidité, matières en suspension, Na+, K+, Ca2 +, Mg2+, Cl−, F−, SO =4 , HCO3 −, BOD5, NO3 −, NO2 −, NH4 +, P tot, coliformes fécaux, bore et métaux lourds (Fe, Zn, Cu, Ni, Pb, Cr et Cd). La compilation de ces résultats a été réalisée avec une technique d’analyse multivariable pour la réduction des données. La convenance pour l’irrigation a été évaluée par les indices SAR et le pourcentage de sodium. Cette étude démontre la conformité des eaux aux règles de potabilité, de vie aquatique et d’irrigation. La qualité de l’eau a été évaluée via l’Indice canadien de qualité d’eau (WQI) et qualifiée de bonne à excellente.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface water reservoirs are increasingly being developed in Tunisia to regulate the seasonal stream flows and to provide water to meet expanding demands for agriculture, energy development and domestic needs. For this reason, many reservoirs in central and north Tunisia have been extensively studied, e.g. Sidi Salem (Sternick 1990; Ben Othmen 2003), Sidi Saad (Bouden 1995), Bir M’cherga (Gaabab 1997). Most of their characteristics are relatively well known, except the Sejnane, Joumine and Sidi El Barrak reservoirs which are poorly understood.
This paper reports a water quality monitoring program for the Sejnane reservoir and discusses factors which may have caused some of the observed variations in water quality.
Study area and geological setting
The Sejnane reservoir is located in the northeast of Tunisia, 40 km west of Bizerte town (longitudes between 9°29′ and 9°22′30″ and latitudes between 37°8′ and 37°11′30″, Fig. 1). The earth dam of the reservoir was constructed in the middle of the Sejnane River in 1994 to provide water for drinking and irrigation. The reservoir has a total surface area of about 7.35 km2 and a storage capacity of 122 million m3 (Zouabi 2003).
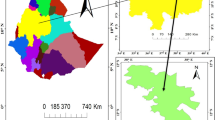
The geology of the catchment includes Triassic to Quaternary deposits (Fig. 2). The Sejnane area is dominantly a succession of reddish sandstone and silty clays (Crampon 1971). Each sandstone horizon is approximately 100 m thick and consists of fine to medium grained sand with some lenses of coarse conglomerate (250–300 mm in diameter). The interbedded argillaceous layers are generally greenish to greyish in colour and about 300 mm thick (Castany 1953; Rouvier 1977).
Geological settings of the Sejnane dam watershed area (Zouabi 2003)
The watershed receives an average annual rainfall exceeding 400 mm. From late autumn to early spring, the precipitation is equal to or exceeds the evaporation. During the rest of the year, the evaporation is significantly higher than the rainfall. Meteorological data of precipitation and atmospheric temperature were recorded by a weather station at the dam (see Fig. 3).
Materials and methods
Monthly monitoring of water quality parameters at the Sejnane reservoir was undertaken from 1995 to 2006 inclusive. Of the nine sites routinely sampled, only site B1 is discussed here.
All samples were collected from the surface next to the riser on the principal axis of the reservoir (Fig. 1). Temperature, pH, conductivity and turbidity (expressed in nephelometric turbidity units (NTU)) were measured in the field using portable devices. In order to assess the water quality, separate samples for dissolved oxygen (DO) determination were taken in plain glass bottles; the acid modification of the Winkler method was used to analyze the sample (APHA 1975). The samples were retained at a temperature of 4°C (Rodier 1978; Hounslow 1995) and analysis for the major elements (Na+, K+, Ca2+, Mg2+, Cl−, HCO3 − and SO4 2−) was undertaken. All nutrients were analyzed using colorimetric methods. Sodium and potassium concentrations were measured using the emission flame spectrophotometer. Calcium, magnesium and chloride were determined by titrimetry, while SO4 2− was obtained by the gravimetric method. The fecal coliform (FC) bacterial densities were determined by a membrane filter technique. Potentiometry with ion-selective electrodes and emission flame photometry methods were used to determine whether specific toxic ions (such as fluoride and boron) were present while atomic absorption spectrophotometry (AAS) was used to analyze concentrations of the heavy metals iron, zinc, copper, nickel, lead, chromium and cadmium (APHA 1995).
The water sampling protocol was conducted according to the APHA standards (1995). Sample containers were carefully packed with an appropriate packing material and transported in a cooler. To measure the precision of the analytical process in the laboratory, intra-lab (blind-coded) duplicate samples were used.
Principal component analysis (PCA)
The PCA was used to analyze the water quality data (Davis 1986) using the XLStat-pro program (Addinsoft 1995–2002). The objective of PCA is both to achieve parsimony and to reduce dimensionality by extracting the smallest number of components p′ (p′ ≤ p) that account for most of the variation in the original multivariate data and to summarize them by two or three transformed principal components (PCs) that can be displayed graphically with minimal loss of information (Davis 1986).
The 19 variables selected included chemical parameters (Na+, K+, Ca2+, Mg2+, Cl−, SO4 2−, HCO3 −, BOD5, P tot, NH4 +, NO2 − and NO3 −), physical parameters (T, pH, conductivity, DO, turbidity and total suspended solids) and precipitation. The years in which the observations of each variable were carried out constitute the 12 individuals. The standard variations and correlation matrix used were those proposed by Chatfield and Collins (1980); the Pearson correlation matrix was then produced, as shown in Table 1.
Results of statistical analyses
Seven uncorrelated PCs are extracted in decreasing order of importance, so that the first PC accounts for as much of the variation as possible and each successive component accounts for a little less (Chatfield and Collin 1980). An eigenvalue greater than 1 indicates that PCs account for more variance than one of the original variables in the standardized data. This is used as a cutoff point for which PCs are retained. Using this procedure, two components which account for a large proportion of the total data were identified (Table 2).
The first eigenvalue (E 1) equals 12.39 and represents an acceptable amount of the data variability of the first PC (65.24%). The percentage of variability represented by the first two PCs (F1 and F2) is very high (80.08%), indicating that the maps based on the first two PCs are a good quality projection of the initial multidimensional table. As a consequence, it was not necessary to complement the results with a third PC, as shown graphically in the scree test of Raymond B. Cattel (Fig. 4). The rate of decline tends to be fast first then levels off. The point corresponding to the curve-bend is considered to indicate the maximum number of PCs from which to extract PC1 and PC2.
The original data demonstrates relatively great redundancy particularly in precipitation and conductivity (r = −0.946), sodium and potassium (r = 0.892) and calcium and potassium (r = 0.945), hence some of those variables can be removed without a significant effect on the quality of interpretation. In this way the original data is reduced from 19 to only 7 factors associated with non-trivial eigenvalues.
The position of the variables relative to the correlation circle is shown in Fig. 5. This is consistent with the correlation matrix in Table 1 and indicates they are equally effective in modeling the variability of the dataset. The figures also indicate a high correlation between conductivity and most of the major ions: Mg2+ (r = 0.952), Ca2+ (r = 0.947), SO4 2− (r = 0.923), temperature (r = 0.911), HCO3 − (r = 0.907) and Na+ (r = 0.852).
The F1 axis seems to be a factor of mineralization. It is defined by the conductivity and the concentrations of major anions and cations (Ca2+, Mg2+, Na+, K+ and SO4 2−). Precipitation, temperature and evaporation play a key role in the temporal evolution of this phenomenon. Based on the squared cosines of the variables (Table 3), the F2 axis is defined by optic characteristics of the water body [turbidity (0.495), TSS (0.424), P tot (0.48) and NH4 + (0.406)]. The amount of variability explained by the F1 and F2 axes is 80.08%, which is considered acceptable to describe the initial data without significant information loss.
Calcium and magnesium result from passage through or over limestones, dolomites of Middle and Upper Continental Pleistocene (alluvial and calcareous crusts) and Oligocene-Aquitanian (clayey-sandy flyschs) age.
Magnesium and sulphates are positively correlated (r = 0.83) which suggests that the magnesium originated not only from the Triassic dolomites but also from dissolution of the dolomitic limestones which frequently occurs. This would also explain the high correlation recorded between Mg2+ and Ca2+ (r = 0.939). The high degree of correlation between HCO3 − and Ca2+ (r = 0.921) confirms that the fluctuation of bicarbonate content is controlled by the level of saturation of the water with calcite and the variation in CO2 pressure.
Chloride, sodium and potassium result from the dissolution of Triassic evaporates, dominantly at the periphery of the watershed, and from the decomposition of aluminum silicates, potassium feldspar and similar minerals in the clay which are common in all the geological formations in the study area (Zouabi 2003). Not surprisingly, therefore, there was a high degree of correlation between sodium and potassium (r = 0.98), which suggests that a common mechanism is involved in the regulation of the concentrations of these two elements.
The hydrochemical facies were determined using the Piper diagram (Piper 1944; Back 1961; Back and Hanshaw 1965). As seen in Fig. 6, based on the water classification scheme of Back and Hanshaw (1965) the water is characterized by the predominance of chlorinated–sulphated sodium–calcium facies.
The watershed is lithologically heterogeneous and composed typically of sedimentary rocks, with a relatively low salts content, always less than 0.5 g/l. This may to some extent be related to the climate and relief.
Three groups of climate scenarios occur in the Sejnane region: wet and cold; dry and hot; and moderate. As can be seen in Fig. 7, plotting the characteristics of the individual years against the F1 and F2 axes indicated they are strongly linked to precipitation and annual mean of temperature.
Water quality assessment
Calcium and magnesium
The average concentrations of calcium and magnesium are 52.18 mg/l and 9.77 mg/l, respectively, in the reservoir (Appendix 1). These values are well within the permissible limits for drinking-water prescribed by World Health Organization (WHO 1996, 2004) and do not damage boilers, pipes and cooking utensils (Brooks et al. 1997).
Sodium and chloride
Sodium concentrations of around 67.64 mg/l are acceptable, especially for the segment of the population that may be on severely restricted diets requiring limitation of their sodium intake. However, the low chloride concentration levels (75.65 mg/l) may affect domestic plumbing, water heaters and municipal waterworks equipment.
Nitrogen compounds and total phosphorus
Nitrate is the most abundant form of nitrogen compounds in the reservoir (0.489 mg/l) but still complies with WHO, CEC and FAO recommendations (Appendix 1). Ammonium was found in small amounts (0.025 mg/l) and also nitrite (0.007 mg/l). The sources of nitrate in the reservoir are mainly atmospheric and the geological formations containing soluble nitrogen compounds.
No agricultural fertilizers or domestic sewage were identified during the sampling period and there is no elevated health risk such as methemoglobinemia or blue baby syndrome (Antweiler et al. 1995).
Finally, a concentration of P tot around 0.034 mg/l is consistent with healthy aquatic life in the water body (Brooks et al. 1997).
Dissolved oxygen
The average DO concentration level of about 7.78 mg/l complies with WHO and CEC standards and is considered good to sufficient for human consumption and most aquatic biota (Wilcock et al. 1995).
Specific conductivity
When acidic water flows over rocks containing calcite (CaCO3), such as the calcareous shales of Maastrichtian age (Fig. 2), calcium (Ca2+) and carbonate (CO3 2−) ions will easily dissolve into the water and its specific conductivity will increase. However, as the water passes mainly over quartzose and other resistant rocks such as the Pliocene sandstone, this is unlikely to be important in the climatic conditions of the study area (Zouabi 2003).
During the present monitoring, the conductivity seems to vary related to the watershed lithology, precipitation, temperature and evaporation (Zouabi 2003). However, the conductivity is relatively low (621 μS/cm) indicating a high water quality.
Fecal coliform
Fecal coliform content is exemplified by the concentration of Escherichia coli (E. coli). Although FC were detected, their concentration is generally low (<25 E. coli counts per 100 ml) and presents little health risk (WHO 1996). However, as it does not meet the bacteriological standard purity for drinking-water (Appendix 1) the reservoir water was treated with chlorine disinfection (chlorination), after which no FC was detected in distribution system.
Heavy metals and specific toxic elements
During the monitoring period, the measured concentrations of boron, iron, zinc, copper, nickel, lead, chromium and cadmium contents were considerably lower than the permitted international standards (Appendix 1) and hence, in this respect, the water is safe for drinking, irrigation and aquatic life.
Use for irrigation purposes
Excess amounts of sodium in irrigation waters may change the soil properties and reduce its fertility due to salinization and alkalization processes (Richards 1954; Cheverry 1972; Dehayer et al. 1997). The United States Salinity Laboratory Staff (1954) has determined that calcium and magnesium in water are important in modifying the effect of sodium on the soil and has introduced the sodium adsorption ratio (SAR) which expresses the relative activity of sodium ions in the exchange reaction with soil by
with [Na], [Ca] and [Mg] ion concentrations of sodium, calcium and magnesium. All concentration values are expressed in equivalents per million.
Data for water samples (Table 4) have been plotted in a Wilcox diagram for classification of irrigation waters (Wilcox 1948), based on specific conductance (at 25°C). As can be seen in Fig. 8, the water plots in the C2S1 class, i.e. with a medium salinity hazard (C2) and low sodium hazard (S1). This indicates they are suitable for irrigation and can be used on almost all soil types with little danger of harmful levels of exchangeable sodium developing or a change in the soil structure (Fig. 9).
The quality of water in relation to salinity and sodium hazard (after US Salinity Laboratory 1954)
Relationship between SAR and ECi of irrigation water for prediction of soil structure stability (after US Salinity Laboratory 1954)
Percent sodium ratio (Na%)
The sodium percentage (Table 4) is plotted against conductivity in the Wilcox diagram in Fig. 10. This indicates an excellent to good water quality (Wilcox 1948).
The quality of water in relation to electrical conductivity and Na% (Wilcox 1948)
Water quality trend
The Canadian Water Quality Index (WQI) is a 100 point scale which summarizes the variables of water quality (CCME 1999, 2001a, b). The WQI is used here to evaluate the overall quality of water in the Sejnane reservoir with respect to the guidelines for drinking-water (given in WHO 1996), irrigation (FAO 1994) and aquatic life (CEC 1978). As seen in Fig. 11, during the 10 year test period all the measured indices varied but showed good to excellent quality water.
-
(a)
Fecal coliform and turbidity levels are slightly in excess of the good condition such that the natural raw water did not fully comply with the WHO guideline of excellent (Appendix 1).
-
(b)
Turbidity, iron and to a lesser degree copper and chromium levels occasionally failed to comply with the CEC standard of excellent for aquatic life.
-
(c)
The salinity status, expressed mainly by the relatively high sodium concentrations, seems to play a key role in the index fluctuation.
The water quality showed a marked stabilization for aquatic and irrigation indices although the drinking index trend is not as clear.
Although some heavy metals (boron, iron, zinc, copper, nickel, lead, chromium and cadmium) and specific toxic elements (fluoride and chloride) were identified in small amounts, they are to believed to be mostly of natural origins and do not pose a real threat to human consumption, aquatic life and irrigation.
Comparison with other reservoirs in Tunisia
Drinking-water supply
The untreated waters from the Sejnane, Joumine and Sidi Salem reservoirs have TSS and turbidity values of 7–12.43 mg/l) and 3.56–5.09 NTU, respectively. However, those from the Sidi Saad reservoir have high values of both TSS (23 mg/l) and turbidity (6.4 NTU). The mean concentrations of nitrate of all reservoirs are generally consistent with the WHO standard for drinking-water (Appendix 1); the lowest values being recorded at Sejnane (0.49 mg/l) and the highest at Joumine reservoir (3.93 mg/l).
Irrigation
The Sejnane and Joumine reservoirs have similar values of SAR (3 and 2.3) and conductivity (621 and 650 μS/cm), but for the Sidi Salem reservoir they were considerably higher (11.45 and 1,700 μS/cm) while the Sidi Saad reservoir gave values of 41.15 and 37,345 μS/cm.
Protection of aquatic life
Temperature, TSS, DO, pH, turbidity and to a lesser degree ammonium and nitrate levels recorded in all reservoirs are in the order of those required by the standards of the Commission of European Communities for aquatic life protection (Appendix 1). From the available data, the natural waters from the Sejnane and Joumine reservoirs are often of good quality and meet the standards established for drinking, irrigation and aquatic life protection. However, those from Sidi Salem and Sidi Saad do not always meet the irrigation standards especially for salts concentration. As a consequence, their use should be limited to only some soil types to avoid modification of the sediment structure.
Conclusions
The following conclusions can be drawn, based on the analysis and tests carried out during the study period:
-
1.
The climatic conditions and the topographic and lithological characteristics of the watershed are the main controls on the water quality status in the reservoir. This explains why the waters have a unique chemical hydro facies type Na−(Ca)−Cl−(SO4).
-
2.
The measured physicochemical parameters (temperature, pH, dissolved O2, NO3 −, NO2 −, NH4 +, P tot, Na+, K+, Ca2+, Mg2+, Cl−, F−, SO4 2−, HCO3 −, etc), boron, heavy metals (Fe, Zn, Cu, Ni, Pb, Cr and Cd) and tests (FC, alkalinity, hardness and conductivity) confirm that the water of the Sejnane reservoir generally conforms to international standards for drinkability, aquatic life and irrigation.
-
3.
Based on Na%, SAR and ECi, the water has an excellent to good quality with no effect on the structure and stability of the soil when used for irrigation of arable lands.
-
4.
According to the Canadian WQI, the Sejnane reservoir water is clean and rated as “good” to “excellent” throughout the 10 year study period. The water is able to support a high diversity of aquatic life without excessive algae growth. In addition, it is suitable for all forms of recreation, including those involving direct contact with the water (e.g. swimming).
-
5.
To ensure this situation is maintained, a long-term program for preserving the water quality should be considered.
References
Addinsoft (1995–2002) XLStat-pro. 5.1 Version 4, Paris, France
Antweiler RC, Goolsby DA, Taylor HE (1995) Nutrients in the Mississippi River. US Geological Survey Circular 1133. USGS, Denver
APHA (1975) Standard methods for the examination of water and waste water, 14th edn. American Public Health Association, Washington DC
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Back W (1961) Techniques for mapping of hydrochemical facies. USGS Prof. Pap., vol 424D, pp 380–382
Back W, Hanshaw BB (1965) Chemical geohydrology. Adv Hydrosci 2:49–109
Ben Othmen D (2003) Etude de la qualité des eaux du barrage Sidi Salem. Thesis, Fac. Sci., Tunis, p 150
Bouden S (1995) Etude de la qualité des eaux du barrage Sidi Saad. Thesis, Fac. Sci., Tunis, p 92
Brooks KN, Ffolliott PF, Gregersen HM, DeBano LF (1997) Hydrology and the management of watersheds, 2nd edn. Iowa State University Press, Ames, p 502
Canadian Council of Ministers of the Environment (CCME) (1999) Water Quality Index 1.0, Technical report, p 5
Canadian Council of Ministers of the Environment (CCME) (2001a) Canadian water quality index 1.0—technical report. Canadian water quality guidelines for the protection of aquatic life. Canadian environmental quality guidelines, p 13
Canadian Council of Ministers of the Environment (CCME) (2001b) Canadian Water Quality Index 1.0—user’s manual. Canadian water quality guidelines for the protection of aquatic life. Canadian environmental quality guidelines, p 5
Castany G (1953) Le Tyrrhénien de la région de Bizerte. Bull Soc Sci Nat t VI:1952–1953
Chatfield C, Collin AJ (1980) Introduction to multivariate analysis. Chapman & Hall/Methuen, New York. ISBN 0-412-16030-7
Cheverry C (1972) Exemple d’application des travaux de l’US Salinity Laboratory (1963–1968) sur l’alcalinisation des sols soumis à l’action d’eaux carbonatées, Cah., ORSTOM., Serv., Pédol., 10, no. 2, pp 193–203
Commission of European Communities (CEC) (1978) Council Directive of 18 July 1978 on the quality of fresh waters needing protection or improvement in order to support fish life, (78/659/EEC). Official J L/222:1–10
Crampon N (1971) Etude géologique de la bordure des Mogods, du pays de Bizerte et du Nords des Hedil (Tunisie septentrionale). Thesis, Sci. Natur., Nancy I, tome 1, Stratigraphie, pp 1–228
Davis JC (1986) Statistics and data analysis in Geology, 3rd edn, p 638
Dehayer R, Diatloff N, Gordon I (1997) Irrigation water quality salinity and soil structure stability, Water facts, ISSN 1327–5364
Food and Agriculture Organization (1994) Water quality for agriculture, 29 Rev. 1, p 174. ISBN 92-5-102263-1
Gaabab F (1997) Etude géochimique des eaux du basin versant du barrage Bir M’cherga. Thesis, Fac. Sci., Tunis, p 135
Hadj Zekri S (2003) Evaluation de la qualité des eaux de deux hydrosystèmes du nord de la Tunisie: les retenues des barrages Joumine et Sejnène. 3rd cycle thesis, National Institute of Agronomy of Tunis (INAT), p 161
Hounslow AW (1995) Water quality data analysis and interpretation. Lewis Publishers, New York, p 397
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–932
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Agriculture handbook, vol 60. US Department of Agriculture, Washington DC
Rodier J (1978) L’analyse de l’eau, Eaux naturelles, eaux résiduaires et eau de mer, DUNOD, Ed, Paris
Rouvier H (1977) Géologie de l’extrême Nord tunisien: Tectoniques et paléogéographies superposées à l’extrémité orientale de la Chaîne nord maghrébine. Thesis, Univ. Pierre et Marie Curie, p 427
United States Salinity Laboratory Staff (1954) Diagnosis and improvement of saline and alkali soils, USDA agriculture handbook no. 60, US Gov
Sternick K (1990) La trophie et l’eutrophisation du barrage Sidi Salem. Ann. Lim., Tunis, p 40
Wilcock RJ, McBride GB, Nagels JW, Northcott GL (1995) Water quality in a polluted lowland stream with chronically depressed dissolved oxygen: causes and effects. New Zealand J Mar Freshw Res 29:277–288
Wilcox LV (1948) The quality of water irrigation. US Dept Agric Tech Bull 962:1–40
World Health Organization (WHO) (1996) Chapman D (ed) Water quality assessments—a guide to use of biota, sediments and water in environmental monitoring, 2nd edn. UNESCO/WHO/UNEP. ISBN 0 419 21590 5
World Health Organization (WHO) (2004) Guidelines for drinking-water quality, vol 1, 3rd edn, 540 p. ISBN 92 4 154638 7
Zouabi B (2003) Etude de la qualité des eaux du Barrage Sejnane. Thesis, Fac. Sci., Tunis, p 109
Acknowledgments
The authors would like to thank the staff of technicians (Laboratory of geochemistry—Ben Arous) for their assistance in the acquisition of samples and chemical analysis, Samia ZOUABI and specially Tahar ALOUI for their assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
See Table 5.
Rights and permissions
About this article
Cite this article
Zouabi Aloui, B., Gueddari, M. Long-term water quality monitoring of the Sejnane reservoir in northeast Tunisia. Bull Eng Geol Environ 68, 307–316 (2009). https://doi.org/10.1007/s10064-009-0186-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10064-009-0186-1
Keywords
- Water quality
- Water Quality Index
- Sodium adsorption ratio
- Sodium percent
- Irrigation
- Principal component analysis