Abstract
Loss of function mutations in CCM1/KRIT1, CCM2/MGC4607, and CCM3/PDCD10 gene are identified in about 95 % of familial cases of cerebral cavernous malformations and 2/3 of sporadic cases with multiple lesions. In this study, 279 consecutive index patients referred for either genetic counseling or for diagnosis of cerebral hemorrhage of unknown etiology were analyzed for the three cerebral cavernous malformations (CCM) genes by direct sequencing and quantitative studies, to characterize in more detail the mutation spectrum associated with cerebral cavernous malformations and to optimize CCM gene screening. Analysis of the cDNA was performed when possible to detect the consequences of the genomic variations. A pathogenic mutation was identified in 122 patients. CCM1 was mutated in 80 patients (65 %), CCM2 in 23 (19 %), and CCM3 in 19 (16 %). One hundred patients harbored a loss of function point mutation (82 %) and 22 had a large deletion (18 %). Novel unclassified variants were detected in the patients among whom six led to a splicing defect. The causality of three missense variants that did not modify the splicing could not be established. These findings expand the CCM mutation spectrum and highlight the importance of screening the three CCM genes with both direct sequencing and a quantitative method. In addition, six new unclassified variants were shown to be deleterious because they led to a splicing defect. This underlines the necessity of the cDNA analysis when an unknown variant is detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cavernous angioma, also known as cerebral cavernous malformations (CCM-OMIM# 116860) are vascular malformations mostly located within the CNS and characterized by abnormal enlarged capillary cavities without intervening brain parenchyma [1]. CCM can occur sporadically or in an autosomal dominant fashion, with variable expression and incomplete penetrance. The incidence in the general population has been evaluated to roughly 0.5 % [2]. Clinical symptoms typically appear between 20 and 30 years of age. They include recurrent headaches, focal neurological deficits, hemorrhagic stroke, and seizures [3, 4], but CCM can also be asymptomatic. Familial forms of CCM have been attributed to mutations in three genes, CCM1/KRIT1 (krev interaction trapped 1) [MIM# 604214] [5, 6], CCM2/MGC4607 (encoding a protein named malcavernin) [MIM# 607929] [7, 8], and CCM3/PDCD10 (programmed cell death 10) [MIM# 609118] [9]. Over 90 % of familial CCM patients harbor a mutation in one of the three known CCM genes [10, 11]. Large rearrangement screening methods allow now to find mutations that are not detected by sequencing [12–16]. Our objective was to establish in more detail the mutation spectrum associated with cerebral cavernous malformations in a large cohort of consecutive patients referred for molecular diagnosis and screened for both point mutations and large rearrangements.
Material and methods
Patients
Two hundred seventy-nine unrelated patients were referred to our laboratory by French hospitals between January 2006 and December 2010 for CCM genes screening. Molecular diagnosis was requested either for genetic counseling in patients showing typical CCM lesions on cerebral MRI, or for patients in whom the diagnosis of CCM was discussed, based on clinical history of cerebral hemorrhages of unknown etiology. Written informed consent was provided by the patients themselves when aged above 18 and by both parents for patients younger than 18 years. One hundred forty-four healthy controls were included for the testing of unknown variants.
Sequencing
Sequencing of the three CCM genes was performed using specific primer pairs amplifying the 16 coding exons of CCM1 (transcript reference NM_004912.3), the 10 exons of CCM2 (transcript reference NM_031443.3) and the 7 coding exons of CCM3 (transcript reference NM_007217.3). Primers are provided in Electronic supplementary material (ESM) Table 1. Sequence products were run on an automated sequencer (ABI 3130, Applied Biosystems, Foster City, CA, USA) and data were analyzed with Seqscape V.2.6 software (Applied Biosystems).
QMPSF conditions
The quantitative multiplex PCR of short fluorescent fragments (QMPSF) method is described in detail elsewhere [17]. Oligonucleotide primer pairs for amplification of short fluorescent fragments corresponding to the 19 exons of CCM1, the 10 exons of CCM2 and the 10 exons of CCM3 were designed using the Primer Premier Software (Primer Biosoft International, Palo Alto, CA, USA). Primers are provided in ESM Table 1. Four multiplex PCR were set up to check the copy number of all exons of CCM genes. Forward primers were 5′-labelled with the 6-FAM fluorochrome. Each multiplex PCR set contained a control primer set that amplified a short sequence of hydroxymethylbilane synthase gene (HMBS), not involved in CCM. Amplicon sizes ranged between 120 and 250 bp. Reactions were done as described previously [18].
PCR products were separated by capillary electrophoresis on an ABI 3130 Genetic Analyzer (Applied Biosystems). Quantification of the area of peaks corresponding to the tested exons and to the internal HMBS control was determined using GeneMapper analysis software version 4.0 (Applied Biosystems). The copy number of each tested exon was expressed as the ratio: (area of the peak corresponding to a tested exon for the patient/area of the peak corresponding to HMBS for the patient)/(area of the peak corresponding to a tested exon for the control DNA/area of the peak corresponding to HMBS for the control DNA). Calculation of ratios was done by the software. A ratio close to 1 is obtained when two copies of the exon are present and a ratio close to 0.5 when only one copy of the tested exon is present (hemizygous DNA).
Long-range PCR and sequencing
Long-range PCR amplifications were performed with the TripleMaster® PCR System (Eppendorf AG, Hamburg, Germany) according to the manufacturer recommendations. Sequencing was performed using standard protocols, on an ABI 3130 DNA analyzer (Applied Biosystems).
cDNA analysis
Total RNAs were extracted from patients’ peripheral blood leukocytes either with TRIzol (Invitrogen) or with the PreAnalytix system (Qiagen) according to the manufacturer’s procedures. RT-PCR was done using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). Sequencing of RT-PCR amplification products was performed using standard protocols on an ABI 3130 DNA analyzer (Applied Biosystems). Primers are provided in ESM Table 1.
In silico analysis
The prediction of the pathogenicity of the mutations was assessed using Human Splicing Finder and MaxEntScan at http://www.umd.be/HSF [19]. The MaxEntScan framework is based on the maximum entropy principle and uses large datasets of human splice sites and takes into account adjacent and non-adjacent dependencies. Human Splicing Finder includes several matrices to analyze splice sites and splicing silencers and enhancers.
Results
Point mutations detected by direct sequencing
Eighty-three different heterozygous variants were identified (Table 1): 20 nonsense mutations, 31 insertions/deletions leading to a frameshift, 12 AG/GT consensus splice site mutations, 5 missense variants, 4 synonymous variants, and 11 intronic variants located outside the invariant AG/GT splice sites. Loss of function mutations (nonsense mutations, frameshift mutations, and AG/GT consensus splice site mutations) are considered as typical pathogenic variants. The synonymous mutation CCM2/c.30G>A, the missense mutation CCM2/L198R and the three intronic variants CCM1/c.1730 + 4_1730 + 7del, CCM1/c.2026-12A>G, and CCM2/c.30 + 5_30 + 6delinsTT had previously been reported in CCM patients [7, 8, 20, 21]. The other variants were novel. The missense mutation CCM2/p.L113P was present in three relatives harboring multiple CCM lesions. The three other missense mutations (CCM1/p.L308F, CCM2/M439T, and CCM3/p.M20V) were identified in sporadic CCM patients with multiple lesions for whom parents were not available. The missense variants were absent in 288 control chromosomes.
Large rearrangements detected with QMPSF
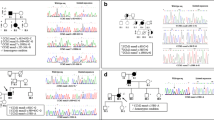
QMPSF analysis detected a large deletion in 22 patients, 12 in CCM1, 7 in CCM2, and 3 in CCM3. Deletions varied in size, ranging from a single exon to the whole gene (Table 2 and Fig. 1). Whole gene deletion was found in three patients. CCM2/exon 2 deletions had already been reported [13]. It leads to a shorter in frame alternative normal transcript, but the absence of the full length transcript has been shown to be deleterious by preventing the formation of a CCM1/CCM2/CCM3 protein complex [11]. Two patients had a deletion of CCM2/exon 5; long-range PCR products sequencing showed that the breakpoints were different in these two cases. Two deletions encompassed the ATG start codon (CCM1/deletion of exons 1–5 and CCM3/deletion of exons 1B-3) and two patients harbored a deletion of the three noncoding exons of CCM1. This deletion was not present in 240 control chromosomes and no other mutation was found in the three CCM genes for these two patients. Both of them had typical CCM lesions on cerebral MRI. Clinical data on relatives were not contributory. These deletions are likely responsible of the CCM phenotype in these patients.
cDNA analysis
The consequences on cDNA have been investigated for 61 of the 83 distinct genomic variants and for 11 intragenic large deletions (Tables 1 and 2). Surprisingly, the cDNA of the patient showing an apparently nonsense CCM3 mutation c.418G>T/p.E140X located in exon 7 was deleted of whole exon 7. The CCM3/c.394_395delinsGATT cDNA allele was nearly undetectable by sequencing. All the other nonsense and ins/del exonic mutations identified in genomic DNA were detectable in the cDNA sequence and did not lead to abnormal splicing.
The five missense variants (CCM1/p.L308F, CCM2/p.L198R, p.L113P, p.M439T, and CCM3/p.M20V) were found in the corresponding cDNA. Among the four synonymous variants, the CCM1/c.2025G>C variant led to the abnormal splicing of exon 17. The CCM2/c.30G>A variant was suspected to lead to an aberrant splicing since sequencing of the cDNA obtained with primers located in exon 1 and 10 did not show the mutation, as well as the other polymorphisms that were detectable on genomic DNA sequences. Nevertheless, the RT-PCR product 5–10 (obtained with a forward primer within exon 5 and a reverse primer within exon 10) showed heterozygosity in exon 8 for one of the patients, in favor of the presence of an alternative transcript. The two other variants (CCM1/c.1782A>G and CCM3/c.213C>T) are likely polymorphisms since no aberrant transcript was detected. ESM Table 2 gives a meta analysis data on consequences on splicing of the exonic apparently missense or silent mutations reported in CCM patients.
The cDNA analysis of nine patients carrying an intronic variant located outside the invariant AG/GT showed the presence of a shorter transcript caused by an abnormal splicing (Table 3); these mutations were considered as pathogenic. Two CCM2 intronic variants localized close to exon 1 donor splice site (c.30 + 5_30 + 6delinsTT and c.30 + 5G>A found twice) were suspected to prevent classical splicing because the RT-PCR product showed lost of heterozygosity for different polymorphisms detected in the genomic DNA. These two variants were not present in 280 control chromosomes. At last, the variant CCM2/c.804-5C>T and the variant CCM3/c.97-5T>C (found in a patient harboring also a deletion of CCM2 exon 5) were considered as probable polymorphisms since no aberrant transcript was detected. Data are summarized in Table 3. cDNA sequencing was also performed for eight additional patients harboring AG/GT splice site mutations. Among the whole splicing defects (eight in AG/GT and six outside AG/GT), 11 lead to the deletion of the proximal exon and 3 lead to the use of a cryptic splice site. Result of the in silico analyses are given in ESM Table 3.
Discussion
This study reports a large series of patients referred in a diagnostic context for CCM molecular analysis. A mutation considered as pathogenic was found in 122 of 279 of the patients. This mutation rate is lower than the one reported in research CCM series. Indeed, many patients were referred because of cerebral hemorrhages of unknown etiology and did not have typical CCM lesions on cerebral MRI. They are therefore expected to be affected by other conditions.
Among the 122 patients harboring a mutation considered as deleterious, CCM1 was involved in 80 cases (65.6 %), CCM2 in 23 cases (18.8 %), and CCM3 in 19 cases (15.6 %). One hundred patients had a point mutation (82 %) and 22 had a large deletion (18 %), limited to one or a few exons in 19 cases and complete in three cases. Twelve deletions concerned CCM1 (54 %), seven CCM2 (32 %), and three in CCM3 (14 %; Fig. 2). The distribution of the deletions is different from the one reported in the American and in the Italian series [13, 14] and no recurrent deletion has been detected in our cohort.
The majority of mutations introduce premature termination codon into the mRNA, most likely leading to mRNA decay. Seven CCM1 mutations were in frame deletions that result from a splicing defect or a large deletion. It may point out important functional domains of the protein; however, in-frame deletions are also known to lead to protein instability and degradation. The in-frame deletion of CCM2 exon 2 found in two patients in our series and already reported in two others CCM patients [13] emphasizes the importance of the full length transcript for vascular integrity.
Fourteen new unclassified variants (intronic variants outside the consensus AG/GT splice site or exonic missense or silent variants) were identified in patients for whom cDNA analysis was performed. Six of them led to an abnormal splicing and were considered as pathogenic; four variants did not modify the splicing, did not change any amino acid and were considered as probable polymorphisms. Four unreported variants led to an amino acid change but splicing was unchanged. They all involve a conserved amino acid and were absent in 280 control chromosomes. The missense mutation CCM2 L198R previously reported in a family in which it co-segregated with the presence of CCM was shown to abolish the interaction between CCM1 and CCM2 [22] and is up to now the only proven pathogenic missense mutation that does not induce aberrant splicing. The clinical significance of the missense variants found in our series remains to be established.
Although in silico analysis is not sufficient to definitely conclude on the effect of an unknown variant on RNA splicing, results obtained in our cohort using the combination of MaxEntScan and Splice Site finder show that they most often provide good splicing predictions. Houdayer et al. proposed an analysis pipeline to check the unknown variants with a cutoff ratio of 15 % for MaxEntScan and 5 % for Splice Site finder like [23]. Using those cutoff, predictions were exact in 8/10 variants leading to an abnormal splicing, and to 8/8 variants not leading to an abnormal splicing. Experimental analysis of the effect on splicing is however needed in a diagnosis context.
The systematic use of a semi quantitative technique as QMPSF or MLPA, in addition to DNA sequencing, has substantially increased the detection rate of CCM mutations. Nevertheless, cDNA sequencing is required to test the consequences on splicing of unknown variants and is also recommended to confirm large intragenic deletions, especially when they involve only one exon.
Significant progress has been made in the understanding of the mechanisms leading to CCM lesions in transgenic mouse models and preclinical trials are already ongoing. It is therefore important to identify precisely the molecular anomaly in the CCM patients for the constitution of homogeneous cohorts for future clinical trials and this study shows that both genomic DNA and cDNA analyses are needed to reach a good sensitivity. In addition, molecular diagnosis remains helpful in atypical cases showing cerebral hemorrhage but no typical CCM lesions on MRI.
References
Russell DS, Rubinstein LJ (1989) Pathology of tumors of the nervous system, 5th edn. Williams and Wilkins, Baltimore, pp 730–36
Rigamonti D, Hadley MN, Drayer BP, Johnson PC, Hoenig-Rigamonti K, Knight JT et al (1988) Cerebral cavernous malformations. Incidence and familial occurrence. N Engl J Med 319:343–347
Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP et al (1994) The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 80:422–432
Zabramski JM, Henn JS, Coons S (1999) Pathology of cerebral vascular malformations. Neurosurg Clin N Am 10:395–410
Laberge-le CS, Jung HH, Labauge P, Houtteville JP, Lescoat C, Cecillon M et al (1999) Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat Genet 23:189–193
Sahoo T, Johnson EW, Thomas JW, Kuehl PM, Jones TL, Dokken CG et al (1999) Mutations in the gene encoding KRIT1, a Krev-1/rap1a binding protein, cause cerebral cavernous malformations (CCM1). Hum Mol Genet 8:2325–2333
Liquori CL, Berg MJ, Siegel AM, Huang E, Zawistowski JS, Stoffer T et al (2003) Mutations in a gene encoding a novel protein containing a phosphotyrosine-binding domain cause type 2 cerebral cavernous malformations. Am J Hum Genet 73:1459–1464
Denier C, Goutagny S, Labauge P, Krivosic V, Arnoult M, Cousin A et al (2004) Mutations within the MGC4607 gene cause cerebral cavernous malformations. Am J Hum Genet 74:326–337
Bergametti F, Denier C, Labauge P, Arnoult M, Boetto S, Clanet M et al (2005) Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet 76:42–51
Denier C, Labauge P, Bergametti F, Marchelli F, Riant F, Arnoult M et al (2006) Genotype–phenotype correlations in cerebral cavernous malformations patients. Ann Neurol 60:550–556
Stahl S, Gaetzner S, Voss K, Brackertz B, Schleider E, Surucu O et al (2008) Novel CCM1, CCM2, and CCM3 mutations in patients with cerebral cavernous malformations: in-frame deletion in CCM2 prevents formation of a CCM1/CCM2/CCM3 protein complex. Hum Mutat 29:709–717
Gaetzner S, Stahl S, Surucu O, Schaafhausen A, Halliger-Keller B, Bertalanffy H et al (2007) CCM1 gene deletion identified by MLPA in cerebral cavernous malformation. Neurosurg Rev 30:155–159, discussion 159–60
Liquori CL, Berg MJ, Squitieri F, Leedom TP, Ptacek L, Johnson EW et al (2007) Deletions in CCM2 are a common cause of cerebral cavernous malformations. Am J Hum Genet 80:69–75
Liquori CL, Penco S, Gault J, Leedom TP, Tassi L, Esposito T et al (2008) Different spectra of genomic deletions within the CCM genes between Italian and American CCM patient cohorts. Neurogenetics 9:25–31
Felbor U, Gaetzner S, Verlaan DJ, Vijzelaar R, Rouleau GA, Siegel AM (2007) Large germline deletions and duplication in isolated cerebral cavernous malformation patients. Neurogenetics 8:149–153
D’Angelo R, Marini V, Rinaldi C, Origone P, Dorcaratto A, Avolio M et al (2011) Mutation analysis of CCM1, CCM2 and CCM3 genes in a cohort of italian patients with cerebral cavernous malformation. Brain Pathol 21:215–224
Casilli F, Di Rocco ZC, Gad S, Tournier I, Stoppa-Lyonnet D, Frebourg T et al (2002) Rapid detection of novel BRCA1 rearrangements in high-risk breast-ovarian cancer families using multiplex PCR of short fluorescent fragments. Hum Mutat 20:218–226
Saugier-Veber P, Goldenberg A, Drouin-Garraud V, de La Rochebrochard C, Layet V, Drouot N et al (2006) Simple detection of genomic microdeletions and microduplications using QMPSF in patients with idiopathic mental retardation. Eur J Hum Genet 14:1009–1017
Desmet FO, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C (2009) Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res 37:e67
Cave-Riant F, Denier C, Labauge P, Cecillon M, Maciazek J, Joutel A et al (2002) Spectrum and expression analysis of KRIT1 mutations in 121 consecutive and unrelated patients with cerebral cavernous malformations. Eur J Hum Genet 10:733–740
Gallione CJ, Solatycki A, Awad IA, Weber JL, Marchuk DA (2011) A founder mutation in the Ashkenazi Jewish population affecting messenger RNA splicing of the CCM2 gene causes cerebral cavernous malformations. Genet Med 13:662–666
Zawistowski JS, Stalheim L, Uhlik MT, Abell AN, Ancrile BB, Johnson GL et al (2005) CCM1 and CCM2 protein interactions in cell signaling: implications for cerebral cavernous malformations pathogenesis. Hum Mol Genet 14:2521–2531
Houdayer C, Caux-Moncoutier V, Krieger S, Barrois M, Bonnet F, Bourdon V et al (2012) Guidelines for splicing analysis in molecular diagnosis derived from a set of 327 combined in silico/in vitro studies on BRCA1 and BRCA2 variants. Hum Mutat 33:1228–1238
Verlaan DJ, Siegel AM, Rouleau GA (2002) Krit1 missense mutations lead to splicing errors in cerebral cavernous malformation. Am J Hum Genet 70:1564–1567
Gault J, Sain S, Hu LJ, Awad IA (2006) Spectrum of genotype and clinical manifestations in cerebral cavernous malformations. Neurosurgery 59:1278–1284, discussion 1284–5
Tonelli A, Lanfranconi S, Bersano A, Corti S, Bassi MT, Bresolin N (2009) Aberrant splicing due to a silent nucleotide change in CCM2 gene in a family with cerebral cavernous malformation. Clin Genet 75:494–497
Acknowledgments
We thank the patients and the clinicians who referred the patients: K. Abel Lablanche, Y. Benkaci, S. Berroir, J.Y. Bignon, P. Bitoun, M.P. Brechard, B. Bussel, F. Cartault, JF. Cassarini, H. Chabriat, S. Chapuis, M. Clanet, P. Corcia, C. Coubes, F. Darcel, A. David, C. de la Rochebrochard, I. Delalande, X. Deplanque, L. Derex, I. Desguerre, O. Detante, F. Di Rocco, A. Dieux, B. Doray, V. Drouin-Garraud, C. Férec, H. Flodrops, C. Francannet, , E. Ginglinger, I. Gobron, O. Godefroy, B. Godet, C. Goizet, V. Golfier., L. Guyant Marechal, D. Hervé, M. Holder Espinasse, H. Hosseini, E. Jeandidier, P. Labauge, A. Landais, C. Laroche, B. Leheup, P. Lejeune, G. Lesca, D. Leys, C. Marescaux, D. Martin Coignard, S. Mercier, J.M. Mussini, G. Nadeau, J.P. Neau, S. Odent, E. Ollagnon, P. Parent, G. Plessis, S. Puget, G. Ruvanot, D. Saibi, C. Sainte Rose, C. Scherer-Gagou, I. Sibon, S. Sigaudy, S. Spagnolo, C. Stapf, C. Thauvin, A. Toutain, C. Verny, J. Vigneron, M. Voicu, F. Voimant, and M. Zerah.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
DOC 124 KB
ESM Table 2
DOC 86.0 KB
ESM Table 3
DOC 53.5 KB
Rights and permissions
About this article
Cite this article
Riant, F., Cecillon, M., Saugier-Veber, P. et al. CCM molecular screening in a diagnosis context: novel unclassified variants leading to abnormal splicing and importance of large deletions. Neurogenetics 14, 133–141 (2013). https://doi.org/10.1007/s10048-013-0362-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-013-0362-0