Abstract
Migraine is a common neurological disorder with a significant genetic component. Although a number of linkage and association studies have been undertaken, the number and identity of all migraine susceptibility genes has yet to be defined. The existence of dopaminergic hypersensitivity in migraine has been recognised on a pharmacological basis and some studies have reported genetic association between migraine and dopamine-related gene variants. Our laboratory has previously reported association of migraine with a promoter STR marker in the dopamine beta hydroxylase (DBH) gene. In the present study, we analysed two additional DBH markers in two independent migraine case–control cohorts. These two markers are putative functional SNPs, one within the promoter (−1021C→T) and another SNP (+1603C→T) in exon 11 of the DBH gene. The results showed a significant association for allelic and genotypic frequency distribution between the DBH marker in the promoter and migraine in the first (P = 0.004 and P = 0.012, respectively) and the second (P = 0.013 and P = 0.031, respectively) tested cohorts. There was no association observed between either genotype and/or allelic frequencies for the DBH marker located in exon 11 and migraine (P ≥ 0.05). The promoter DBH marker, reported associated with migraine in this study, has been shown to affect up to 52% of plasma DBH activity. Varying DBH activity levels have been postulated to be involved in migraine process with an increase of dopamine, resulting from a lower DBH activity shown positively correlated with migraine severity. It is plausible that the functional promoter variant of DBH may play a role in the migraine disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a common neurological disorder that affects up to 25% of females and 8% of males in a western population [26]. Migraine symptoms range from severe headache to nausea, vomiting, photophobia, phonophobia and variations of the visual field. The most common forms of this disorder have been classified as migraine with aura (MA) and migraine without aura (MO) [21]. Although definitive guidelines are available to help diagnose migraine, the aetiology of the disorder is less clear. Cerebral blood flow changes, specifically a decrease corresponding to the clinically affected area, have been noted as occurring before or at the onset of aura symptoms, in a number of sub-types of MA. In MO, however, regional cerebral blood flow remains normal or slightly increased. Several formative and perceptive sensory systems are part of these modulations, such as the autonomous nervous system, comprising mostly of the sympathetic division (acting via noradrenaline, NA neurotransmitter), but also the diffuse modulatory system (with its transmitters serotonin, dopamine (DA), NA and acetylcholine) and/or the trigeminal sensory system [17, 36, 47]. An imbalance in any of these neurological systems either at the transmitter or on the receptor side may lead to a higher susceptibility for migraine.
Numerous studies have implicated the catecholaminergic system in migraine [38]. Several migraineurs have a hypersensitised dopaminergic system resulting in an increased dopamine receptor density on T cells [3, 5]. A central dopaminergic hyperfunction, and possible coexisting noradrenergic dysfunction, may lead to migraine attacks with severity positively correlated to dopamine concentration [4]. Cerebral blood flow and somatosensory evoked potentials can also be changed by this dopamine hyperactivation [14].
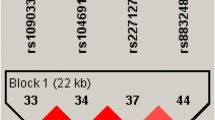
The dopaminergic system has also been explored for a potential role in susceptibility to this complex neurological disorder. Several dopaminergic candidate genes have been investigated in different migraine case–control cohorts with varying results [11, 40, 33]. Thirty years ago, Weinshilboum and collaborators observed a low level of dopamine beta hydroxylase (DBH), an intracellular enzyme catalysing the conversion of DA to NA, in 3% (in adults) to 4% (in children) of the European population [52]. The variation in both plasma DBH activity [6, 8, 51] and cerebrospinal fluid levels of immunoreactive DBH protein [8] has then been shown to be associated with several molecular markers at the DBH locus. More recently, a new functional variation located at −1,021 bp to the translational start site of the DBH gene (−1021C→T, rs 1611115) has been reported to be responsible for affecting up to 52% of the DBH activity in plasma [53]. Interestingly, this polymorphism is part of a 10-kb haplotype block at the DBH locus (spreading from a 19-bp deletion located in the promoter region (−4784–4803) to a single variation in the coding region (A444G) [54]. Investigation of the variants spreading over 50 kb at the DBH gene revealed that the highest association with the DBH activity phenotype were for markers within the 10-kb haplotype block, suggesting the presence of a potential quantitative trait locus for plasmatic DBH activity [54]. Previously, we examined two genetic markers (a 19-bp insertion/deletion (indel) and a short tandem repeat, STR) located in the promoter of the DBH gene (approximately 4.5-kb upstream of the transcriptional start site) and part of the 10-kb block in an unrelated case–control population (150 cases vs. 150 controls) and in 263 patients from 82 families of migraineurs [27] (cf Fig. 1). The results showed a distortion of allele transmission of STR marker in individuals suffering from both migraine with or without aura [27]. The first association between DBH alleles of this STR and DBH plasma concentration was previously reported in a unrelated British population [51], observation confirmed by Cubells et al. [8], who also investigated the promoter indel polymorphism. They showed that the indel was also functionally linked, reporting that an individual with the deletion of both alleles had only half of the mean plasma enzyme activity compared with a homozygote with the insertion/insertion genotype [6]. Our subsequent investigations studying the indel DBH marker in a larger Caucasian case–control population (275 cases vs. 275 controls), reported a positive association between this 19-bp insertion/deletion (−4784–4803), and migraine (χ2 = 8.92, P = 0.011) and more specifically with MA (χ2 = 11.48, P = 0.003) (cf. Fig. 1) [15].
In the present study, we investigated the putative functional single nucleotide polymorphism (SNP) (–1021C→T) within the promoter of DBH gene and another SNP (+1603C→T, rs6271) in exon 11 (see Fig. 1) of the same gene, encoding a non-conservative difference in primary amino acid sequence (arg535 cys), in two independent large case–control populations. This exonic SNP (+1603C→T) has been shown to have a small effect on DBH activity being responsible for ~2% of the enzymatic activity of DBH and may be involved in protein structural changes [9]. It is believed that carriers of the 1603 T (cys encoding) allele may have disulfide bridge formation between the four units constituting the DBH holoenzyme (tetramer) and this may affect homospecific activity.
Materials and methods
Subjects
Before commencing the study, ethical clearance was sought and approved by Griffith University’s Ethics Committee for Experimentation on Humans. Individuals for the study were recruited from the local general population using advertising via notices at doctors surgeries and in pharmacies, as well as through media release on local radio, television and in press articles. Potential participants contacted the Genomics Research Centre and suitability for inclusion in the study was determined using a detailed questionnaire completed by all participants, providing demographic parameters, ancestry information and family medical history. The control group consisted of individuals with no personal and family history of migraine. Volunteers who did not meet these criteria were not included in the study. All recruited individuals for the study gave informed consent and were adult (18 years or older) Caucasians of European descent living in Australia, having emigrating ancestors within the last 160 years from various locations within the British Isles and other parts of Europe. In total, ~600 cases and an equivalent number of controls were collected over several years, with 275 cases and 275 matched controls collected first and used routinely for our genotyping studies, and other samples collected later and DNA prepared as a second independent population of 300 cases and 300 controls for replication studies. Samples used for the genotyping studies were all individuals, not families, with care taken not to include any related individuals in the case–control population. Case and control individuals were recruited from in and around the South Eastern Australia Region, with collections undertaken in the Genomics Research Centre Clinic at the Gold Coast, Queensland, Australia. To minimise potential bias from population stratification, the control group was matched for sex, age (±5 years) and ethnicity. Migraine patients were clinically defined and suitably matched with non-migraine individuals who made up the control population. The subjects were diagnosed for migraine by a clinical neurologist from responses provided on a detailed questionnaire in accordance with the International Headache Society criteria (3). Questions used to define migraineurs included length and frequency of attack; pain location, type and intensity; associated symptoms such as nausea, vomiting, phonophobia, photophobia and other visual disturbances, and other neurological symptoms. All individuals were grouped together and phenotyped as being affected with typical migraine (MA + MO = migraine), as well as being diagnosed separately as MA or MO subgroups. The blood samples obtained from patients were collected through the Genomics Research Centre patient clinic and purified DNA from these samples was obtained using standard extraction methods. Around 90% of the examined DNA samples gave good genotyping results for the four selected genetic markers. We excluded the samples with unclear genotyping results. The study protocol was approved by Griffith University’s Ethics Committee for Experimentation on Humans.
The first study population was comprised of 200 migraineurs (115 MA/ 85 MO) and 200 unrelated control individuals and the second one contains 300 migraineurs and 300 controls. To minimise potential bias from population stratification, the control group was matched for sex, age (±5 years) and ethnicity (as previously described [15]. The second population was collected in the same way as the first population.
Markers/genotyping
The study investigated two different polymorphisms at the DBH gene locus, one within the promoter and the other one in the coding region (see Fig. 1). The first marker was SNP located at −1,021 bp to the translational start site of the DBH gene (ref SNP database, rs 1611115), named DBHpr. The polymerase chain reaction (PCR) analysis was carried out using a modification of a previously described method [49, 54]. The PCR reactions (10 μl final volume) contained 2 mmol/L MgCl2, 0.8 mol/L of each primer, 200 mol/L dNTPs, 1 U of Taq polymerase and approximately 20 μg of genomic DNA.
Primers were:
-
Sense: 5′-GGAGGGACAGCT TCT AGTCC-3′
-
Anti sense: 5′-CACCTCTCCCTCCTGTCCTCTCGC-3′
Thermal cycling was performed with an initial denaturation of 5 min at 94°C, followed by 35 cycles of 30 s at 94°C, 30 s at 60°C, 30 s at 72°C and a terminal extension of 10 min at 72°C. The PCR products were digested with HhaI and analysed by electrophoresis on 3% agarose gels. Ethidium bromide-stained gels were digitally imaged and manually scored for genotypes. The PCR products were 131 bp in size. The T alleles did not digest with HhaI, whereas C alleles digested to give 109 bp and 22 bp fragments.
The second marker was SNP located at +1,603 bp in the coding region of the DBH gene (ref SNP database, rs6271), named DBHex. The PCR analysis was also performed using a modification of a previously described study [49, 54]. The PCR reactions (10 μl final volume) contained 2 mmol/L MgCl2, 0.8 mol/L of each primer, 200 mol/L dNTPs, 1 U of Taq polymerase and approximately 20 μg of genomic DNA.
Primers were:
-
Sense: 5′-CCAGGGACAGGACTCGAGTTG-3′
-
Anti sense: 5′-AGCAGTTTGGAGTGCAGACCC-3′
Thermal cycling was performed with an initial denaturation of 5 min at 94°C, followed by 35 cycles of 30 s at 94°C, 30 s at 62°C, 30 s at 72°C and a final extension of 10 min at 72°C. The PCR products were digested with Bst UI and analyzed by electrophoresis on 3% agarose gels. Ethidium bromide-stained gels were digitally imaged and manually scored for genotypes. The PCR products were 352 bp in size. The T alleles did not digest with Bst UI, whereas C alleles digested to give three fragments of 184, 139 and 29 bp.
The genotyping for the DBHpr marker was also been performed in some samples from the first population for corroboration of obtained RFLP genotyping results using the high resolution melt (HRM) method. HRM was carried out using a modification of previous reports of this technique HRM [13, 25, 37], using Rotor-Gene 6000 (HRM)™ (Corbett Research). The same forward and reverse primers for DBHpr that were used above (RLFP method) were also used for HRM as they efficiently amplified a short size PCR product of 131 bp. PCR reactions contained 1 μl of genomic DNA, 0.2 mmol/L MgCl2, 0.2 mmol/L dNTPs, 1.25 U of Platinum Taq DNA polymerase 300 nM of each primer and 1.5 μM of SYTO 9 (Invitrogen) made up to 25 μl with filter sterilised water. Samples were run on a Rotor-Gene 6000 (HRM)™ (Corbett Research) using temperature cycling conditions of: 10 min at 95°C followed by 40 cycles of 95°C for 5 s and 60°C for 10 s. This was followed by a melt step of 65–85°C in 0.2°C increments pausing for 2 s per step. The increase in SYTO 9 fluorescence was monitored in real time during the PCR and the subsequent decrease during the melt phase by acquiring each cycle/step to the green channel (470-nm excitation and 510-nm emission) of the Rotor-Gene. Genotypes were scored by examining normalised and difference melt plots using the Rotor-Gene Software.
Statistical analysis
To detect association between each marker and migraine, we performed chi-square (χ2) analysis to test for significant differences in allele and genotype frequencies between case and control groups [34]. χ2 provides the likelihood of a deviation in the distribution of the same attributes in different classes (e.g. allelic frequencies in controls versus affected subjects). If the probability (P-value) of a χ2 statistic is below the pre-determined α-level of 0.05, this is evidence of an association.
We performed χ2 analysis for migraineurs MA, MO and combined migraine groups versus control subjects for the DBHpr and DBHex polymorphisms. We also tested for linkage disequilibrium (LD) between biallelic tested markers using the Graphical Overview of Linkage Disequilibrium (GOLD) program, a new bioinformatic software to analyse dense genetic maps. In addition, the GOLD program provides a distinct graphical representation of disequilibrium patterns [1]. For this analysis, we included data found in our previous genotyping of the insertion/deletion marker (19 bp), localised at −4,784 bp within the promoter of DBH gene and reported to be significantly associated with migraine [15].
Results were also tested for Hardy–Weinberg Equilibrium (HWE) investigating genotype frequencies of the DBHpr and DBHex markers to detect a deviation from the normal genotype distribution in the population and odds ratios (ORs) were calculated to assess the magnitude of associations. We also performed endophenotype analysis, investigating the distribution of several migraine-associated symptoms, including nausea, vomiting and diarrhoea, according to genotype.
Power calculations indicate that for the smaller of the two cohorts, a sample size of 200 cases (400 alleles) and a rarer allele frequency of 0.25 this study has >80% a priori power to detect a significant allelic association conferring an odds ratio of 1.5 or greater.
Results
Two markers located one at 1.02-kb upstream of the starting point and the other one at +1.6 kb in the coding of the DBH gene, were analysed for association with migraine in two independent cohorts (200 migraineurs versus 200 healthy individuals; 300 migraineurs versus 300 healthy individuals, respectively) of Australian Caucasians. Genotypes for both DBH markers were determined in the migraine case and control populations. The distribution of DBHpr and DBHex genotypes in the studied populations did not deviate significantly from Hardy–Weinberg Equilibrium (P > 0.05).
Table 1 represents the results of the allelic and genotypic frequency distribution of DBHpr in the two studied populations. Results showed a significant association of DBHpr alleles with migraine in the first (Table 1) and the second independent (Table 1) analysed populations (χ2 = 8.24, P = 0.004; χ2 = 6.17, P = 0.013). This positive result was also found for the genotypic frequencies of DBHpr marker in both studied populations (χ2 = 8.73, 2 df, P = 0.012and χ2 = 6.91, 2 df, P = 0.031, respectively) (Table 1). In addition, this significant association was also observed in the MA group in the first and the second studied populations for both genotypic (χ2 = 6.57, 2 df P = 0.037; χ2 = 7.58, 2 df, P = 0.022, respectively; Table 2) and allelic frequencies (χ2 = 6.26, 1 df, P = 0.011; χ2 = 7.19, 1 df, P = 0.007, respectively; Table 3).
In regard to the analyses by gender for DBHpr, a significant association was found for all combined migraine compared to controls in females for both genotypic (χ2 = 8.56, 2 df, P = 0.013) and allelic frequencies (χ2 = 7.88, 1 df, P = 0.005), but was not significant in male groups (χ2 = 0.79, 2 df, P = 0.67; χ2 = 0.8, 1 df, P = 0.37, respectively) (Tables 2–3) in the first population. The identical pattern was observed for the genotypic frequencies (χ2 = 10.32, 2 df, P = 0.006; Table 2) and allelic frequencies (χ2 = 8.29, 1 df, P = 0.004; Table 3) in the female group of the second studied population. However, no significant association was reported in the male group of the second population for both genotypic (χ2 = 0.57, 2 df, P = 0.75) and allelic frequencies (χ2 = 0.26, 1 df, P = 0.6) (Table 2–3).
As is shown in Table 4, there was no significant association between either genotype or allelic frequencies for DBHex and migraine (χ2 = 2.95, 2 df, P = 0.229; χ2 = 2.44, 1 df, P = 0.118), respectively in the first analysed population (Table 4) but also in the second population (χ2 = 2.31, 2 df, P = 0.315; χ2 = 0.93, 1 df, P = 0.335; Table 4). When we analysed by gender and by subtype of migraine, no significant association was similarly observed for DBHex genotype and allelic distribution (P > 0.05) in both studied populations.
LD was calculated for the present studied DBH genetic markers, including the insertion/deletion (indel) reportedly associated with both migraine (χ2 = 8.92, 2 df, P = 0.011) and more specifically with MA (χ2 = 11.48, 2 df, P = 0.003) groups in our previous study [15] (Fig. 1). The analysis of LD between the studied genetic markers revealed moderate but significant linkage disequilibrium between DBHpr and indel (D’ = 0.42, P = 0.00001). However, this LD was found to be non-significant (P < 0.05), when LD was measured between DBHex and DBHpr, and DBHex and indel. Our previous association between DBH markers located in the promoter and migraine (and more specifically MA) has been confirmed in this study and extended to the DBHpr, marker in linkage with the indel marker [15].
Endophenotype analysis of the positively associated DBHpr marker was also undertaken. We were particularly interested in nausea and emesis as dopamine receptor antagonists are an established class of anti-emetic agent [44] and diarrhoea, as dopaminergic defects have been associated with enteric dysfunction in humans [45] (cf Fig. 2). As illustrated in Fig. 2 results of this analysis showed that 8% of individuals with the CC genotype suffered from diarrhoea compared to 23% of individuals with the CT/TT genotype. Migraineurs with at least one T allele were three times more likely to suffer diarrhoea. Another interesting finding shown in Fig. 2 was that individuals with at least one T allele were also more likely to suffer from emesis (60% CC genotype compared to 78% with CT/TT genotype). Hence, it appears that possession of the CC genotype may confer a protective effect for both emesis and diarrhoea associated with migraine.
Endophenotypes analysed in the studied populations in comparison with DBHpr genotypes. a 8% CC with diarrhoea, 23% CT/TT with diarrhoea; P = 0.04 OR 3.14 (95% CI 0.9908 to 9.9286). Those with CT/TT genotype ~3 times more likely to suffer diarrhoea; b 60% CC suffered emesis, 78% CT/TT emesis; P = 0.08 OR 2.3 (95% CI 0.886 to 5.792)
Discussion
During the last three decades, the dopaminergic system has been considered as playing a part in the pathogenesis of migraine and DBH enzyme plays an important role in the regulation of DA levels in the synapse. Interestingly a significant decrease in serum DBH has been observed in several reports in migraine patients compared with healthy control subjects [10, 18, 41] and during a migraine attack [2], although a previous study reported ~30 years ago, in a group of 17 migraineurs showed a contradictory result [20].
Several functional polymorphisms have been reported for the DBH gene. Lea and colleagues have showed a distortion of allele transmission of the STR marker in individuals suffering from both migraine with or without aura [27]. Our previous study undertaken in a larger case–control population also reported a positive association between the deletion genotype and migraine (P ≤ 0.05) and also migraine with aura (P ≤ 0.01) [15].
In the present study, we examined the distribution of genotype and allelic frequencies of two functional polymorphisms of the DBH gene, DBHpr located at 1.02-kb upstream of the starting point and DBHex in exon 11 of the DBH gene in two independent and unrelated case–control populations. The analysis for both allelic and genotypic frequency distribution showed a significant association between the DBHpr marker and migraine in the first (P = 0.004 and P = 0.012, respectively) and the second (P = 0.013 and P = 0.031, respectively) independent tested populations. These positive results seem to be attributable to the MA subtype in both studied populations (P < 0.05).
Subjects with two copies of the allele T genotype had a decreased risk of migraine compared to controls in both tested populations (OR = 0.55, 95% Cl 0.37–0.83 and OR = 0.67, 95% Cl 0.48–0.92). The percentage of patients with allele T was significantly lower in migraine (13.5% and 15.5 %, respectively) and MA (12.8% and 14.7%, respectively) groups compared to controls (21.9% and 21.5%, respectively) in both of the two studied populations. Interestingly, the MO subgroup had an average ~20% different allele frequency than the MA group, supporting the possibility that there may be MA/MO heterogeneous differences. Two Danish population-based studies have also provided evidence to suggest that MA and MO may be two distinct disorders with an independent genetic identity [43]. Nevertheless, the percentage of MA subgroup in the first (52.9%) and second (83.2%) populations is significantly higher to what is reported in the general population (20%) [30], which might introduce a limitation to the results reported in our study. With regards to the size of our two populations and the high percentage of MA compared to the general population, we will consider in our study the group “migraine” (as MA and MO subgroups altogether) for our discussion.
Observed genotypic frequencies for the DBHpr marker (genotypes, TT = 3–6%, TC = 32–36% and CC = 60–62%) in our control groups for both tested populations gave results similar to previous studies [12, 22, 50]. Healy and collaborators investigating the role of −C1021T polymorphism in Parkinson Disease sufferers compared to two large independent cohorts of controls (n = 637 for cohort A and n = 450 for cohort B), showed a genotypic profile comparable to our control population results (genotypes, TT = 6.3%, TC = 32.3% and CC = 61.2% for cohort A and TT = 5.8%, TC = 31.3% and CC = 62.8% for cohort B) [22]. The same group of researchers has also reported no association for the DBHpr polymorphism between a population suffering for epilepsy compared to the identical control cohorts (A and B) [12]. Tang and collaborators have recently examined the relationship between DBH polymorphisms (including the DBHpr and DBHex polymorphisms) and plasma DBH activity in an African-American population [50]. Genotypic frequencies (DBHpr and DBHex) reported in this study (genotypes, TT = 7.3%, TC = 25.7% and CC = 67% and TT–, TC = 5.5% and CC = 94.5%, respectively) again showed a similar pattern to that observed in our first (genotypes, TT = 5.7%, TC = 32.4% and CC = 61.9% for DBHpr and TT–, TC = 12.4% and CC = 87.6% for DBHex markers) and second (genotypes, TT = 3.6%, TC = 35.8% and CC = 60.6% for DBHpr and TT–, TC = 12.4% and CC = 87.6% for DBHex markers) tested populations. As shown in our study, DBHex was not significantly associated with migraine or its subtypes (MA, MO) with chi-square results producing P values greater than 0.05 for most analyses in both studied populations. It should be noted that we observed in our study no substantial LD between DBHpr and DBHex polymorphisms as measured by r 2 (r 2 < 0.001). This result has been reported previously by [53] and more recently by Tang and collaborators [50].
The activity of the DBH enzyme can be measured in serum (or the plasma) due to the release of this enzyme from the central and peripheral adrenergic and noradrenergic neurons as well as adrenomedullar cells during an excitation of the sympathetic system [35]. Several polymorphisms of the DBH gene have been reported in previous studies as being associated with the activity of plasma DBH [6–9, 49, 54]. The DBH promoter variant DBHpr, is responsible for 31–52 % of the variance of the plasma DBH enzyme activity in Caucasian populations [54]. Interestingly, the DBHex polymorphism also tested in our study seems to independently account for additional variance in plasma DBH activity. Recently, Tang et al. have evaluated the effect of four DBH polymorphisms (DBHpr, indel, rs 2519152, DBHex) on the activity of plasma DBH in African American populations [50]. This report showed that a low activity DBH profile is significantly associated with haplotypes T–C–C (for DBHpr–rs 2519152–DBHex, respectively) (P = 0.0036), but also with haplotype C–T–C (P = 0.0025) [50]. Significant decrease serum DBH activity have been observed in migraine (MO and MA) patients compared with healthy control subjects [18]. A lower percentage of migraineurs with the T allele (1.2%) for the DBHpr marker compared to the controls (3.5–5.7%) was observed in our examined populations. It may be judicious to test the rs2519152 polymorphism in our samples to confirm that migraine sufferers with the C allele for DBHpr and DBHex also express the T allele for rs2519152, verifying the C–T–C haplotype, reported in the Tang et al. study [50].
Dopamine beta hydroxylase plays a key part in the balance of NA/DA circulating levels in the synaptic space and is available to act on post-synaptic neurons. A wide variety of clinical signs indicate that dysfunction of the sympathetic system exists in migraine sufferers, in both in the interictal and attacks periods [39]. Many independent investigations have reported lower levels of supine plasma NA (51% to 53%) compared to controls, indicating a hypoactivity of the sympathetic nervous system [20, 31, 32, 48]. Opposed to the hypofunction of the sympathetic nervous system, many studies reported a hyperactivity of the dopaminergic system as indicated by a high levels of dopamine in plasma measured during attacks [41], but also between attacks in both plasma and platelets in migraineurs compared to controls [10]. In addition, clinical trials involving treatment with dopamine receptor antagonists [16, 23] confirms the involvement of dopamine in migraine. In fact, administration of dopaminergic agonists can induce the same symptoms seen during a migraine attack and dopamine antagonist treatment can be used effectively in migraineurs during an attack [16, 23]. Hypersensitivity of the dopaminergic system can also lead to vegetative symptoms such as nausea, sweating and yawning, observed also during migraine attacks. In the present study, we investigated the association of specific endophenotypes (as emesis and diarrhoea) in relation to the genotypes of DBHpr analysed in our populations. Interestingly, the CT/TT combined genotype group showed significantly more than two times the risk to develop emesis (OR 2.3 (95% CI 0.886 to 5.792)) and three times the risk to present with diarrhea related symptoms (OR 3.14 (95% CI 0.9908 to 9.9286)) compared to the CC genotype group for the DBHpr marker.
This study has limitations. We acknowledge that reliability of migraine diagnosis based on questionnaire data has been under question [42] and further, that distinction of migraine without aura and migraine with aura can be challenging. ICHD 2 criteria has been described as gold standard as a diagnostic tool in a clinical interview, however a clinical interview is not always feasible or appropriate for a large population-based study. Therefore, questionnaires based on ICHD 2 criteria have been developed, validated and used in many published studies [24, 43]. A simple questionnaire is an effective tool screening for migraine in the general population [19, 28]. Furthermore, a simple three-question tool has been described as a valid tool for migraine diagnosis both within and outside of the clinical setting [29, 46]. Therefore we believe that diagnosis by a clinical neurologist according to responses to a detailed questionnaire in accordance with ICHD 2 criteria is a reliable method in the absence of a clinical interview.
This study showed a significant association between a DBHpr polymorphism and migraine in two independent populations. Although the DBHpr polymorphism has a functional role and has been shown to be responsible for 31% to 52% of the variance of plasmatic DBH, it accounts for only half of the total variability of the enzyme and other factors may be involved in this variation. Further analysis regarding other functional polymorphisms of this gene and how they affect the DBH enzyme and more specifically, impact on migraine, are warranted.
References
Abecasis GR, Cookson WO (2000) GOLD–graphical overview of linkage disequilibrium. Bioinformatics 16:182–183. doi:10.1093/bioinformatics/16.2.182
Anthony M (1981) Biochemical indices of sympathetic activity in migraine. Cephalalgia 1:83–89. doi:10.1111/j.1468-2982.1981.tb00014.x
Barbanti P, Fabbrini G, Ricci A, Bruno G, Cerbo R, Bronzetti E, Amenta F, Luigi Lenzi G (2000) Reduced density of dopamine D2-like receptors on peripheral blood lymphocytes in Alzheimer’s disease. Mech Ageing Dev 120:65–75. doi:10.1016/S0047-6374(00)00183-4
Castillo J, Martinez F, Suarez C, Naveiro J, Lema M, Noya M (1996) Cerebrospinal fluid tyrosine and 3,4-dihydroxyphenylacetic acid levels in migraine patients. Cephalalgia 16:56–61. doi:10.1046/j.1468-2982.1996.1601056.x
Cerbo R, Barbanti P, Buzzi MG, Fabbrini G, Brusa L, Roberti C, Zanette E, Lenzi GL (1997) Dopamine hypersensitivity in migraine: role of the apomorphine test. Clin Neuropharmacol 20:36–41. doi:10.1097/00002826-199702000-00004
Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, Gelernter J (2000) A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry 5:56–63. doi:10.1038/sj.mp.4000657
Cubells JF, Price LH, Meyers BS, Anderson GM, Zabetian CP, Alexopoulos GS, Nelson JC, Sanacora G, Kirwin P, Carpenter L, Malison RT, Gelernter J (2002) Genotype-controlled analysis of plasma dopamine beta-hydroxylase activity in psychotic unipolar major depression. Biol Psychiatry 51:358–364. doi:10.1016/S0006-3223(01)01349-X
Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O’Connor DT, Price LH, Malison R, Rao PA, Kobayashi K, Nagatsu T, Gelernter J (1998) Dopamine beta-hydroxylase: two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Hum Genet 102:533–540. doi:10.1007/s004390050736
Cubells JF, Zabetian CP (2004) Human genetics of plasma dopamine beta-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology (Berl) 174:463–476. doi:10.1007/s00213-004-1840-8
D’Andrea G, Granella F, Perini F, Farruggio A, Leone M, Bussone G (2006) Platelet levels of dopamine are increased in migraine and cluster headache. Headache 46:585–591. doi:10.1111/j.1526-4610.2006.00407.x
Del Zompo M, Cherchi A, Palmas MA, Ponti M, Bocchetta A, Gessa GL, Piccardi MP (1998) Association between dopamine receptor genes and migraine without aura in a Sardinian sample. Neurology 51:781–786
Depondt C, Cock HR, Healy DG, Burley MW, Weinshenker D, Wood NW, Goldstein DB, Sisodiya SM (2004) The -1021C->T DBH gene variant is not associated with epilepsy or antiepileptic drug response. Neurology 63:1497–1499
Dobrowolski SF, Ellingson C, Coyne T, Grey J, Martin R, Naylor EW, Koch R, Levy HL (2007) Mutations in the phenylalanine hydroxylase gene identified in 95 patients with phenylketonuria using novel systems of mutation scanning and specific genotyping based upon thermal melt profiles. Mol Genet Metab 91:218–227. doi:10.1016/j.ymgme.2007.03.010
Fanciullacci M, Alessandri M, Del Rosso A (2000) Dopamine involvement in the migraine attack. Funct Neurol 15(Suppl 3):171–181
Fernandez F, Lea RA, Colson NJ, Bellis C, Quinlan S, Griffiths LR (2006) Association between a 19 bp deletion polymorphism at the dopamine beta-hydroxylase (DBH) locus and migraine with aura. J Neurol Sci 251:118–123. doi:10.1016/j.jns.2006.09.013
Fisher H (1997) Migraine research methods. CMAJ 157:1015–1016, 1018
Friberg L, Olesen J, Iversen HK, Sperling B (1991) Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet 338:13–17. doi:10.1016/0140-6736(91)90005-A
Gallai V, Gaiti A, Sarchielli P, Coata G, Trequattrini A, Paciaroni M (1992) Evidence for an altered dopamine beta-hydroxylase activity in migraine and tension-type headache. Acta Neurol Scand 86:403–406
Gervil M, Ulrich V, Olesen J, Russell MB (1998) Screening for migraine in the general population: validation of a simple questionnaire. Cephalalgia 18:342–348. doi:10.1046/j.1468-2982.1998.1806342.x
Gotoh F, Kanda T, Sakai F, Yamamoto M, Takeoka T (1976) Serum dopamine-beta-hydroxylase activity in migraine. Arch Neurol 33:656–657
HCCIHS (2004) Headache Classification Committee for the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. 2nd edn. Cephalgia 24(Suppl 1):1–60
Healy DG, Abou-Sleiman PM, Ozawa T, Lees AJ, Bhatia K, Ahmadi KR, Wullner U, Berciano J, Moller JC, Kamm C, Burk K, Barone P, Tolosa E, Quinn N, Goldstein DB, Wood NW (2004) A functional polymorphism regulating dopamine beta-hydroxylase influences against Parkinson’s disease. Ann Neurol 55:443–446. doi:10.1002/ana.20063
Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S (2006) Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache 46:781–787. doi:10.1111/j.1526-4610.2006.00438.x
Kirchmann M, Seven E, Bjornsson A, Bjornssdottir G, Gulcher JR, Stefansson K, Olesen J (2006) Validation of the deCODE Migraine Questionnaire (DMQ3) for use in genetic studies. Eur J Neurol 13:1239–1244. doi:10.1111/j.1468-1331.2006.01491.x
Krypuy M, Ahmed AA, Etemadmoghadam D, Hyland SJ, DeFazio A, Fox SB, Brenton JD, Bowtell DD, Dobrovic A (2007) High resolution melting for mutation scanning of TP53 exons 5-8. BMC Cancer 7:168. doi:10.1186/1471-2407-7-168
Launer LJ, Terwindt GM, Ferrari MD (1999) The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology 53:537–542
Lea RA, Dohy A, Jordan K, Quinlan S, Brimage PJ, Griffiths LR (2000) Evidence for allelic association of the dopamine beta-hydroxylase gene (DBH) with susceptibility to typical migraine. Neurogenetics 3:35–40
Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68:343–349. doi:doi:10.1212/01.wnl.0000252808.97649.21
Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J, Harrison W (2003) A self-administered screener for migraine in primary care: the ID migraine validation study. Neurology 61:375–382
Lipton RB, Stewart WF (1993) Migraine in the United States: a review of epidemiology and health care use. Neurology 43:S6–S10
Martinez F, Castillo J, Rodriguez JR, Leira R, Noya M (1993) Neuroexcitatory amino acid levels in plasma and cerebrospinal fluid during migraine attacks. Cephalalgia 13:89–93. doi:10.1046/j.1468-2982.1993.1302089.x
Mikamo K, Takeshima T, Takahashi K (1989) Cardiovascular sympathetic hypofunction in muscle contraction headache and migraine. Headache 29:86–89. doi:10.1111/j.1526-4610.1989.hed2902086.x
Mochi M, Cevoli S, Cortelli P, Pierangeli G, Soriani S, Scapoli C, Montagna P (2003) A genetic association study of migraine with dopamine receptor 4, dopamine transporter and dopamine-beta-hydroxylase genes. Neurol Sci 23:301–305. doi:10.1007/s100720300005
Nahmias J, Burley MW, Povey S, Porter C, Craig I, Wolfe J (1992) A 19 bp deletion polymorphism adjacent to a dinucleotide repeat polymorphism at the human dopamine beta-hydroxylase locus. Hum Mol Genet 1:286. doi:10.1093/hmg/1.4.286
O’Connor DT, Cervenka JH, Stone RA, Levine GL, Parmer RJ, Franco-Bourland RE, Madrazo I, Langlais PJ, Robertson D, Biaggioni I (1994) Dopamine beta-hydroxylase immunoreactivity in human cerebrospinal fluid: properties, relationship to central noradrenergic neuronal activity and variation in Parkinson’s disease and congenital dopamine beta-hydroxylase deficiency. Clin Sci (Lond) 86:149–158
O’Connor TP, van der Kooy D (1988) Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J Neurosci 8:2468–2476
Pasay C, Arlian L, Morgan M, Vyszenski-Moher D, Rose A, Holt D, Walton S, McCarthy J (2008) High-resolution melt analysis for the detection of a mutation associated with permethrin resistance in a population of scabies mites. Med Vet Entomol 22:82–88. doi:10.1111/j.1365-2915.2008.00716.x
Peroutka SJ (1997) Dopamine and migraine. Neurology 49:650–656
Peroutka SJ (2004) Migraine: a chronic sympathetic nervous system disorder. Headache 44:53–64. doi:10.1111/j.1526-4610.2004.04011.x
Peroutka SJ, Wilhoit T, Jones K (1997) Clinical susceptibility to migraine with aura is modified by dopamine D2 receptor (DRD2) NcoI alleles. Neurology 49:201–206
Pradalier A, Launay JM, Soliman M, Dreux C, Guittard M, Hanna KM, Dry J (1987) Platelet release of dopamine in the common migraine attack. Presse Med 16:1321–1323
Rasmussen BK, Jensen R, Olesen J (1991) Questionnaire versus clinical interview in the diagnosis of headache. Headache 31:290–295. doi:10.1111/j.1526-4610.1991.hed3105290.x
Russell MB, Ulrich V, Gervil M, Olesen J (2002) Migraine without aura and migraine with aura are distinct disorders. A population-based twin survey. Headache 42:332–336. doi:10.1046/j.1526-4610.2002.02102.x
Sanger GJ, Andrews PL (2006) Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci 129:3–16. doi:10.1016/j.autneu.2006.07.009
Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, Quigley EM (1995) Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 346:861–864. doi:10.1016/S0140-6736(95)92707-7
Siva A, Zarifoglu M, Ertas M, Saip S, Karli HN, Baykan B, Keskinaslan A, Senocak M (2008) Validity of the ID-migraine screener in the workplace. Neurology 70:1337–1345. doi:10.1212/01.wnl.0000309221.85545.0d
Suzuki N, Hardebo JE (1993) The cerebrovascular parasympathetic innervation. Cerebrovasc Brain Metab Rev 5:33–46
Takeshima T, Takao Y, Urakami K, Nishikawa S, Takahashi K (1989) Muscle contraction headache and migraine. Platelet activation and plasma norepinephrine during the cold pressor test. Cephalalgia 9:7–13. doi:10.1046/j.1468-2982.1989.0901007.x
Tang Y, Anderson GM, Zabetian CP, Kohnke MD, Cubells JF (2005) Haplotype-controlled analysis of the association of a non-synonymous single nucleotide polymorphism at DBH (+ 1603C –> T) with plasma dopamine beta-hydroxylase activity. Am J Med Genet B Neuropsychiatr Genet 139:88–90. doi:10.1002/ajmg.b.30220
Tang YL, Epstein MP, Anderson GM, Zabetian CP, Cubells JF (2007) Genotypic and haplotypic associations of the DBH gene with plasma dopamine beta-hydroxylase activity in African Americans. Eur J Hum Genet 15:878–883. doi:10.1038/sj.ejhg.5201838
Wei J, Ramchand CN, Hemmings GP (1997) Possible control of dopamine beta-hydroxylase via a codominant mechanism associated with the polymorphic (GT)n repeat at its gene locus in healthy individuals. Hum Genet 99:52–55. doi:10.1007/s004390050310
Weinshilboum RM (1978) Serum dopamine beta-hydroxylase. Pharmacol Rev 30:133–166
Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, Kim KS, Kim CH, Malison RT, Gelernter J, Cubells JF (2001) A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet 68:515–522. doi:10.1086/318198
Zabetian CP, Buxbaum SG, Elston RC, Kohnke MD, Anderson GM, Gelernter J, Cubells JF (2003) The structure of linkage disequilibrium at the DBH locus strongly influences the magnitude of association between diallelic markers and plasma dopamine beta-hydroxylase activity. Am J Hum Genet 72:1389–1400. doi:10.1086/375499
Acknowledgements
The authors would like to thank Dr. Yi-lang Tang and Prof. Joseph Cubells for the helpful information regarding the studied polymorphisms. This work was supported by funding from an ARC Linkage Grant and Corbett Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandez, F., Colson, N., Quinlan, S. et al. Association between migraine and a functional polymorphism at the dopamine β-hydroxylase locus. Neurogenetics 10, 199–208 (2009). https://doi.org/10.1007/s10048-009-0176-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-009-0176-2