Abstract
Autologous nerve grafting is the gold standard method for peripheral nerve injury with defects. Artificial nerve conduits have been developed to prevent morbidity at the harvest site. However, the artificial conduit regeneration capacity is not sufficient. A Bio 3D printer is technology that creates three-dimensional tissue using only cells. Using this technology, a three-dimensional nerve conduit (Bio 3D nerve conduit) was created from several cell spheroids. We reported the first application of the Bio 3D nerve conduit for peripheral nerve injury. A Bio 3D nerve conduit that was created from several cells promotes peripheral nerve regeneration. The Bio 3D nerve conduit may be useful clinically to treat peripheral nerve defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Direct nerve suture is performed if possible to treat peripheral nerve laceration. If direct sutures are not possible due to a nerve defect, an autologous nerve graft is generally performed to bridge this defect. Autologous nerve segments include Schwann cells, fibroblasts, macrophages, and extracellular matrix, which are essential for peripheral nerve regeneration. Therefore, autologous nerve grafting is recognized as the gold standard for peripheral nerve injury with defects [1]. Autologous nerve segments are harvested from the sural nerve or the medial antebrachial cutaneous nerve, leading to donor site morbidity such as loss of feeling and a painful nerve stump in the leg or arm. To prevent these donor site morbidities, artificial nerve conduits have been developed to bridge nerve defects. Artificial nerve conduits are composed of absorbable artificial materials such as collagen, polyglycolic acid, polylactic acid, or polylactic-co-glycolic acid [1]. Artificial nerve conduits contain only extracellular matrix without cellular elements such as Schwann cells. Therefore, nerve regeneration through artificial nerve conduits is not as good as regeneration using an autologous nerve segment. Several basic studies were conducted to add cellular elements such as Schwann cells and mesenchymal stem cells to the artificial conduits, and the added cellular elements resulted in somewhat better nerve regeneration [2,3,4,5,6,7,8,9,10]. However, the survival rate of the added cells is poor, which means that this technique is difficult to use clinically [11, 12]. The problem is how to add cells effectively to peripheral nerve-injured areas. Thus, we focused on Bio 3D printing technology that can produce a three-dimensional tissue structure using only cells [13].
Bio 3D printing
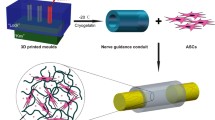
When cells are cultured, a large number of cells spontaneously aggregate, which is called a spheroid. Bio 3D printers (Regenova, Cyfuse, Tokyo) shape these spheroids three-dimensionally by placing them onto thin needles according to a pre-designed three-dimensional data (Fig. 1). This method is called the Kenzan method [13]. Approximately 1 week after 3D printing, adjacent spheroids were fused to construct a single tubular shape in the Kenzan, and the Kenzan was removed. Next, the conduit was transferred to an intravenous catheter, and the spheroids were then cultured in a perfusion bioreactor to promote self-organization of the living cells until the desired tissue function and strength were achieved (https://www.cyfusebio.com/product/regenova). This finished three-dimensional tissue consists of only cells, with no artificial scaffold. The tubular design allows large tissues to mature while supplying oxygen and nutrients to the inside of the structure. It is also possible to combine several types of cells to create a three-dimensional tissue. It can be applied to various cells and can be applied to various three-dimensional organs. An advantage of this system is that the structure (e.g., size, shape, and length) can be designed according to the clinical application using a computer-controlled system. Another advantage is that the constructed tissue does not contain foreign materials, which may induce foreign body reactions, infection, or allergy. Additionally, the strength of the structure can be controlled by the duration of cell culture. Bio 3D printing technology has been used for blood vessels, cartilage, bone, and trachea [14,15,16]. The structure is strong enough that suturing can be performed using 10–0 nylon.
Bio 3D printer. The method is called the KENZAN method (https://en.cyfusebio.com/product/regenova/)
Bio 3D nerve conduit
A three-dimensional nerve conduit (Bio 3D nerve conduit; length, 8 mm and diameter, 3 mm) was fabricated from human fibroblasts using a Bio 3D printer [17]. A 5-mm nerve defect was created at the right mid-thigh level of the sciatic nerve in immunodeficient rats, and the nerve gap was bridged using 8-mm Bio 3D nerve conduits. Nerve regeneration was evaluated kinematically, electrophysiologically, histologically, and morphologically 8 weeks after surgery. In the control group, a silicone tube was used to bridge the nerve gap. In rats, walking kinematic analysis 8 weeks after surgery showed that the angle of the toes to the metatarsal bone at the end of the swing phase in the right hind limb was significantly improved compared with the control group. In the toe-spread test, which evaluates the recovery of muscle strength in the foot, there was a significant improvement compared with the control group. Electrophysiological examination showed that the compound muscle action potential in the pedal adductor muscle was significantly higher than that in the control group. The nerve gap was bridged successfully in all rats, and Bio 3D nerve conduit degradation was confirmed macroscopically 8 weeks after surgery. No neuroma formation was observed. However, in the control group, a small amount of regenerated nerve was observed, and one rat showed no evidence of neural tissue formation. Histologically and morphologically, good axonal regeneration was observed. Significantly more myelinated nerve axons were observed compared with the control group. The tibialis anterior wet muscle weight was significantly higher compared with the control group. Immunohistological staining showed abundant S-100-positive cells compared with the control group, which indicated that Schwann cell expression was promoted by the Bio 3D nerve conduit. Better nerve regeneration using Bio 3D nerve conduits than that in the control group was confirmed.
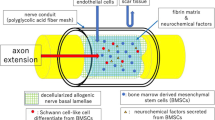
Schwann cells, macrophages, and fibroblasts are required for nerve regeneration, and fibroblasts in particular promote Schwann cell migration (Fig. 2) [18, 19]. Additionally, fibroblasts are easy to culture and proliferate well in vitro, and good mechanical strength of the Bio 3D nerve conduit is relatively easy to obtain.
The process of regeneration of nerve injury by Bio 3D nerve conduit. A The nerve gap is bridged with Bio 3D nerve conduits. B Induction of angiogenesis (long arrows) occurs from both of the nerve stumps. C Schwann cells (✹) migrate from both of the nerve stumps with support of the transplanted fibroblasts. D Nerve axons (long arrows) extend from proximal nerve stumps
This study is the first to demonstrate the efficacy of a completely biological, tissue-engineered, scaffold-free Bio 3D nerve conduit on peripheral nerve regeneration (Table 1). This technology may be useful for several neurological disorders, including brachial plexus injuries and severe trauma, in which nerve sources are needed for nerve grafts to treat peripheral nerve defects.
Mechanism of nerve regeneration through Bio 3D nerve conduit
Bio 3D nerve conduit development from normal human dermal fibroblasts and peripheral nerve regeneration through the Bio 3D nerve conduit have been previously described [17]. In this section, the regenerative mechanism of peripheral nerves using a Bio 3D nerve conduit in a rat sciatic nerve defect model will be described [20]. Human fibroblasts were labeled using PKH26, and Bio 3D nerve conduits were fabricated from the labeled fibroblasts. Bio 3D nerve conduits were transplanted into immunodeficient rats with a 5-mm-long sciatic nerve defect in the same manner as described above, and cell tracked analysis was performed. The PKH26-labeled cells transplanted into the regenerative nerve remained, and the cells survived in the regenerative nerve in a tube-like structure. Most regenerated axons were located within the stable tube-like cell structure. Some PKH26-labeled cells were positive for S-100, and they differentiated into Schwann-like cells. The proportion of PKH26 and S-100-positive cells to PKH26-positive cells was 40.76 ± 6.21%. This was consistent with previous results, showing that fibroblasts that were induced differentiated into Schwann cells in vitro [21]. Modified Masson’s trichrome staining of the Bio 3D nerve conduit revealed the formation of a prominent extracellular matrix in between the cells, which appeared to connect and spread into the spaces among the cells to maintain the tube-like structure. The Bio 3D nerve conduit cell administration method and tube-like structure are both considered to be stable. Macroscopic observation revealed the induction of angiogenesis in the resected regenerated nerve, which is characterized by an increase in the number of blood vessels. A RECA-1-positive, blood vessel-like construction in both longitudinal and transverse sections was observed, which revealed the vascularity of the regenerated nerve through Bio 3D nerve conduits (Fig. 2). Cell viability in the Bio 3D nerve conduit immediately before transplantation and 1 week after transplantation was evaluated using the LIVE/DEAD cell viability assay. LIVE/DEAD staining showed 88.56 ± 1.70% live cells in the transverse sections immediately before transplantation and 87.58 ± 9.11% live cells in the transverse sections 1 week after surgery. This indicates that the cell survival rate in the Bio 3D nerve conduit was high immediately before transplantation and 1 week after transplantation.
The tube-like distribution of human dermal fibroblasts comprising the Bio 3D nerve conduit was stable even 8 weeks after surgery because of the extensive extracellular matrix formation. It is essential for the material that bridges the defect between the peripheral nerve stumps to maintain the luminal structure that provides a place for nerve regeneration and prevents scar formation. It is also essential to allow blood flow and permeability for growth factors, neurotrophic factors, and cytokines. The extracellular matrix also contributed to cell survival. Human dermal fibroblasts induced Schwann cell proliferation and differentiation into functional Schwann-like cells for axonal elongation. Most of the regenerated axons penetrated the stable tube-like structure. High levels of angiogenesis were observed throughout the regeneration process. Extracellular matrix formation, increased cell viability, and the differentiation of human dermal fibroblasts into Schwann-like cells during the regenerative process promoted peripheral nerve regeneration using a Bio 3D nerve conduit.
Long-term outcome of nerve regeneration using the Bio 3D nerve conduit
Evaluation of the regenerated nerve through the Bio 3D nerve conduit 8 weeks after transplantation was described above. The 8-week period is relatively short to evaluate nerve regeneration, and the long-term safety and nerve regeneration of the Bio 3D nerve conduit must be investigated [22]. Bio 3D nerve conduits are fabricated similarly from human dermal fibroblasts using a Bio 3D printer and transplanted into a 5-mm nerve defect at the mid-thigh level of the right sciatic nerve in an immunodeficient rat. Nerve regeneration was evaluated 24 weeks after surgery. The control group had a silicone tube that bridged the nerve gap. In the toe-spread test, significant improvement 24 weeks after surgery was observed compared with the control group, which was similar to that of the autologous nerve transplantation group. In the rat walking kinematic analysis 24 weeks after surgery, the angle of the toes compared to the metatarsal bone at the end of the swing phase in the right hind limb and the ratio of the number of steps taken with the lower limb dragging without lifting to the total number of steps were significantly improved compared with the control group and were similar to the autologous nerve graft group. In the electrophysiological study, the value of the compound muscle action potential in the Bio 3D nerve conduit group was similar to that in the autologous nerve graft group. For the tibialis anterior muscle weight, the value was significantly higher in the Bio 3D nerve conduit group than that in the control group, and it was almost the same as that in the autologous nerve graft group. Macroscopic observation of the regenerated nerve after 24 weeks showed that thick nerve tissue, and no tumor formation including neuromas, was observed in any of the rats. However, in the control group, all regenerated nerves were thinner than the normal sciatic nerves. Morphologically, the number of myelinated nerve axons, mean myelin nerve axon diameter, mean myelin thickness, and G-ratio were significantly better than those of the control group, and there was no significant difference compared to the autologous nerve graft group. Immunohistochemical examination showed S-100 and NF-200 expression in the middle portion of the regenerated nerve, which revealed the existence of Schwann cells and neural fibers at the defect sites between the proximal and distal stumps of the dissected sciatic nerves that were bridged by the Bio 3D nerve conduits.
The number of regenerated axons in the Bio 3D nerve conduit group was significantly larger than that of the control group, and the myelinated axon diameter, myelin thickness, and G-ratio also showed significantly greater results in the Bio 3D nerve conduit group compared with the control group. These outcomes were not significantly different than those of the autologous nerve graft group. This means that maturation and myelination of the regenerated nerves in the Bio 3D nerve conduit group showed more progression than those in the control group and were similar to those in the autologous nerve graft group over the long-term period of 24 weeks. These results are consistent with those of the wet muscle weight of the tibialis anterior muscle and the muscles used in the walking kinematic analysis. Thus, target muscle atrophy was prevented because of sciatic nerve regeneration and re-innervation into the target muscle. The three-dimensional nerve conduit showed good nerve regeneration, which was comparable to that of autologous nerve transplantation, and the regenerated nerve between 8 and 24 weeks was considered to be mature.
Nerve regeneration through the Bio 3D nerve conduit with a longer nerve gap
We performed Bio 3D nerve conduit transplantation into a longer nerve defect [23]. A 12-mm Bio 3D nerve conduit was fabricated from human dermal fibroblasts and transplanted into a 10-mm right sciatic nerve defect in immunodeficient rats. Nerve regeneration was evaluated 8 weeks after surgery. A silicone tube was used to bridge the nerve gap in the control group. Nerve regeneration was confirmed in all rats with a transplanted Bio 3D nerve conduit 8 weeks after transplantation. Some of the rats in the control group showed no nerve continuity. An electrophysiological examination showed that the nerve conduction velocity of the regenerated sciatic nerve and the compound muscle action potential in the pedal adductor muscle were significantly higher than those in the control group. In the morphological study, the number of myelinated axons, the mean diameter of myelinated axons, and mean myelin thickness in the regenerative nerve were significantly higher than those in the control group. There was no significant difference between the Bio 3D nerve conduit group and the autologous nerve graft group.
Similar to previous reports in 5-mm defect models, transplantation of the Bio 3D nerve conduit achieved good nerve regeneration even in the 10-mm defect model. Bio 3D nerve conduits were cultured in a perfusion culture system for several weeks after being molded into a tubular shape. This extended culturing promoted the production of collagen matrix and other extracellular matrix components. This extracellular matrix formation and maturation contributed to the Bio 3D nerve conduits obtaining adequate mechanical strength to endure surgical handling and compressive forces from surrounding tissues after transplantation. All Bio 3D nerve conduits successfully bridged the nerve gap with regenerated axons, which suggests that the strength of these conduits is sufficient to maintain their shape and central lumen throughout the nerve regeneration period. The Bio 3D nerve conduit successfully bridged a 10-mm sciatic nerve defect, and this nerve regeneration was promoted by the Bio 3D nerve conduit even in the 10-mm nerve defect model.
Nerve regeneration through a Bio 3D nerve conduit with larger diameter
Large-diameter Bio 3D nerve conduit transplantation was also evaluated [24]. Dermal fibroblasts were isolated and cultured from canine inguinal-region skin to fabricate a Bio 3D nerve conduit with a 5-mm diameter for 8 weeks. The ulnar nerve in the forelimb was exposed under general anesthesia and cut to create a 5-mm inter-stump gap. An 8-mm autologous Bio 3D nerve conduit was transplanted to bridge the created ulnar nerve gap. Ten weeks after surgery, nerve regeneration was evaluated. The pinprick test was performed to evaluate sensory recovery. A pinching stimulus was applied from the tip of the fifth digit to the ulnar side of the wrist using standardized forceps. All affected forearms subjected to this test showed a withdrawal response due to the stimulus at the tip of the fifth digit. The compound muscle action potentials of the hypothenar muscles were detected at 10 weeks after surgery. The motor nerve conduction velocity was also calculated through the re-innervated ulnar nerve. The ulnar nerves were successfully bridged using the Bio 3D nerve conduit in all canines. The hypothenar muscle mean wet weight indicated that there was little muscle atrophy in Bio 3D nerve conduit group because of early re-innervation. Immunohistochemical examination showed NF-200 and S-100 expression in a transverse section of the middle portion of the Bio 3D nerve conduit, and this indicates the existence of neurofilaments, Schwann cells, and nerve regeneration throughout the Bio 3D nerve conduit. Histological evaluation showed that transverse sections of both the middle and distal portions of the regenerated nerves contained many well-myelinated axons in the Bio 3D nerve conduit group. No adverse events were observed.
This study involved a larger animal and used each animal’s cells to generate that animal’s conduit. The efficacy of the Bio 3D nerve conduit was demonstrated and a protocol was established for its use in peripheral nerve regeneration for future clinical application. Proof-of-concept for a biological scaffold-free conduit transplantation treatment composed of autologous dermal fibroblasts was confirmed as a preclinical study. Bio 3D nerve conduits were biological tissues that were fabricated from autologous cells, so that they would be compatible with surrounding tissues and reduce the risk of foreign body reaction, infection, local fibrosis, and allergy by foreign materials. Bio 3D nerve conduits can be applied in clinical settings, and this technology is expected to resolve several problems in peripheral nerve injury treatment, including cases with trauma or nerve sacrifice during tumor resection.
Bio 3D nerve conduit created from bone marrow mesenchymal stem cells
Because Bio 3D printing technology can produce three-dimensional tissue in any cell type, a Bio 3D nerve conduit was fabricated from bone marrow mesenchymal stem cells (BMSCs) [25]. Primary bone marrow mesenchymal stem cells were isolated from femur bone marrow of Lewis rats, and 8-mm long Bio 3D nerve conduits with a 2-mm internal diameter were fabricated from those cells using a Bio 3D printer. Bio 3D conduits were transplanted into other Lewis rats to bridge a 5-mm right sciatic nerve gap. A silicone tube was used to bridge the gap in the control group. Nerve regeneration was evaluated 8 weeks after transplantation in all rats that received a Bio 3D nerve conduit transplant. In the rat walking kinematic analysis, the angle of the toes to the metatarsal bone at the end of the swing phase in the right hind limb in Bio 3D nerve conduit rats was significantly larger than that in the control group. The compound muscle action potentials in the pedal adductor muscle amplitude were significantly larger in the Bio 3D nerve conduit group compared to the control group. Macroscopic observation in the Bio 3D nerve conduit group confirmed nerve regeneration, and no neuroma formation was found. However, thin regenerated nerves were observed in the control group. The wet muscle weight was significantly higher in the Bio 3D nerve conduit group than that in the control group, which indicated that the re-innervated muscles showed less muscle atrophy in the Bio 3D nerve conduit group than that in the control groups. The histological study revealed significantly more myelinated axons in transverse sections of the regenerated nerves in the Bio 3D nerve conduit group than in the control group. Myelin thickness was also significantly greater in the Bio 3D nerve conduit group than in the control group. In the immunohistochemical study, both S-100 and NF-200-positive cells were abundant in the Bio 3D nerve conduit group, which showed the existence of Schwann cells and neural fibers in regenerated nerves using the Bio 3D nerve conduit conduits. PKH26-labeled bone marrow mesenchymal stem cells were transplanted. Eight weeks after transplantation, some of the PKH26-positive cells also expressed the Schwann cell markers S-100, p75-NTR, and GFAP.
BMSCs differentiate into Schwann cell-like cells both in vitro and in vivo [4, 6, 26,27,28]. In the current study, it was confirmed that transplanted BMSCs differentiated into Schwann cell-like cells. The regenerated nerves included both differentiated Schwann cell-like cells and migrated Schwann cells from the nerve stumps. The Bio 3D nerve conduit group had more S-100-positive cells in the transverse and longitudinal sections, indicating the existence of more functional Schwann cells, which led to better nerve regeneration in this group than in the control group. The Bio 3D nerve conduit can be prepared using bone marrow mesenchymal stem cells, and this Bio 3D nerve conduit can efficiently support regeneration using bone marrow mesenchymal stem cells, which then successfully differentiate into Schwann cell-like cells. The Bio 3D nerve conduit derived from bone marrow mesenchymal stem cells promotes peripheral nerve regeneration and should be considered as a treatment option for peripheral nerve surgery.
Bio 3D nerve conduit created from human-induced pluripotent stem cell‑derived mesenchymal stem cells
Because mesenchymal stem cells promote nerve regeneration, the Bio 3D nerve conduit was also fabricated from human-induced pluripotent stem (iPS) cell-derived mesenchymal stem cells (iMSC) [29]. iMSCs induced differentiation of mesenchymal stem cells from iPS cells. The 8-mm long Bio 3D nerve conduits were fabricated from xeno-free iMSCs. The 5-mm gap in the sciatic nerve in immunodeficient rats was bridged using the Bio 3D nerve conduit in same manner as described above. A silicone tube was used to bridge the 5-mm nerve gap in the control group. Nerve regeneration was evaluated in all rats that underwent Bio 3D nerve conduit transplantation 8 weeks after transplantation. Macroscopic observation revealed that the nerve gap was successfully bridged in the Bio 3D nerve conduit group, and neovascularization was markedly observed in the superficial layer of the Bio 3D nerve conduit. However, only a thin regenerated nerve was observed in the control group. The pinprick test was performed to evaluate sensory recovery. All rats in the Bio 3D nerve conduit group revealed a withdrawal response in response to the toe-pinching stimulus. In the control group, almost all of the rats revealed a withdrawal response only to a pinching stimulus on the dorsum of the foot. This indicates that the Bio 3D nerve conduit group exhibited more functional recovery of the sensory nerve. The toe-spread test was performed to evaluate motor recovery. Almost all of the rats in the Bio 3D nerve conduit group showed toe abduction with extension, indicating that the Bio 3D nerve conduit group exhibited more functional recovery of the motor nerve compared to rats in the control group. In the rat walking kinematic analysis, the Bio 3D nerve conduit group showed a significantly greater angle of the toes to the metatarsal bone at the end of the swing phase in the right lower limb compared to the control group. In the electrophysiological study of the pedal adductor muscle amplitude, the Bio 3D nerve conduit group exhibited significantly greater compound muscle action potentials than that in the control group. The tibialis anterior muscle wet weight was significantly greater in the Bio 3D nerve conduit group than the control group, indicating that the target muscle was re-innervated in the early stage of recovery in the Bio 3D nerve conduit group. Morphological analysis of the regenerated nerve showed that the Bio 3D nerve conduit group exhibited many well-myelinated axons compared to the control group. Significantly more myelinated axons and a significantly larger myelinated axon diameter were detected in the Bio 3D nerve conduit group compared to the control group. Global gene expression profiles revealed iMSCs in Bio 3D nerve conduit-generating step including a large subset of upregulated neurogenesis and angiogenesis-related genes in the Gene Ontology categories.
The benefits of iMSCs on tissue regeneration have been associated with the ability to produce a broad variety of cytokines and paracrine factors. Newly formed blood vessels within the nerve gap play a key role in providing a scaffold for Schwann to cross the nerve gap, thereby offering a more conductive environment for axonal regrowth. Thus, the Bio 3D nerve conduit composed of xeno-free human iMSCs contributes to peripheral nerve regeneration. If the role of iPS cells in regenerative medicine is established by HLA type in the future, Bio 3D nerve conduits for each HLA type could be prepared and stocked in advance. Thus, Bio 3D nerve conduits could be available to transplant for peripheral nerve injuries during the acute phase in the field of emergency medical care. Additionally, using an iPS strain with the HLA knocked out suggests that this strain could be used universally.
Investigator-initiated clinical trial
We have started the investigator-initiated clinical trial of the Bio 3D nerve conduit transplantation fabricated using the Bio 3D printer for traumatic peripheral nerve defect in the hand. The Bio 3D nerve conduit transplantation fabricated using the Bio 3D printer for clinical use. It is conducted mainly by Department of Orthopaedic Surgery, Kyoto University, with the support of Institute for Advancement of Clinical and Translational Science (iACT) and Center for Research and Application of Cellular Therapy (C-RACT), Kyoto University Hospital. The clinical Bio 3D printer (Cyfuse, Tokyo) was installed in Center for Cell and Molecular Therapy (CCMT) in C-RACT to create the Bio 3D nerve conduit. Inclusion criteria is patients aged 20 to 60 years who are traumatic peripheral nerve defects distal to the wrist within 6 months of the injury. Skin is harvested from the patient's inguinal region 6 weeks before the transplantation, fibroblasts are expanded and cultured by CCMT in C-RACT, and a Bio 3D nerve conduit is fabricated by a clinical Bio 3D printer. The 3D nerve conduit is transplanted into the nerve defect 6 weeks after the skin harvest.
Conclusion
Cells play a key role in regenerative medicine. We focused on the Bio 3D printer to efficiently use cells in peripheral nerve regeneration. This technology prints cell spheroids on Kenzan on the basis of three-dimensional data to fabricate three-dimensional tissue that only consists of cells. We confirmed peripheral nerve regeneration using Bio 3D nerve conduits that were fabricated from various cells using a Bio 3D printer. The Bio 3D nerve conduit derived from several cell types promotes peripheral nerve regeneration and should be considered as a treatment option for peripheral nerve surgery.
References
Griffin JW, Hogan MV, Chhabra AB, Deal DN. Peripheral nerve repair and reconstruction. J Bone Jt Surg. 2013;95:2144–51. https://doi.org/10.2106/JBJS.L.00704.
Cuevas P, Carceller F, Dujovny M, Garcia-Gómez I, Cuevas B, González-Corrochano R, Diaz-González D, Reimers D. Peripheral nerve regeneration by bone marrow stromal cells. Neurol Res. 2002;24:634–8. https://doi.org/10.1179/016164102101200564.
Cuevas P, Carceller F, Garcia-Gómez I, Yan M, Dujovny M. Bone marrow stromal cell implantation for peripheral nerve repair. Neurol Res. 2004;26:230–2. https://doi.org/10.1179/016164104225013897.
Chen X, Wang XD, Chen G, Lin WW, Yao J, Gu XS. Study of in vivo differentiation of rat bone marrow stromal cells into schwann cell-like cells. Microsurgery. 2006;26:111–5. https://doi.org/10.1002/micr.20184.
Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, Wang CC, Wang WY, Huang YS, Hsu SH. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–53. https://doi.org/10.1016/j.expneurol.2006.12.004.
Yamakawa T, Kakinoki R, Ikeguchi R, Nakayama K, Morimoto Y, Nakamura T. Nerve regeneration promoted in a tube with vascularity containing bone marrow-derived cells. Cell Transplant. 2007;16:811–22. https://doi.org/10.3727/000000007783465226.
Wang D, Liu XL, Zhu JK, Jiang L, Hu J, Zhang Y, Yang LM, Wang HG, Yi JH. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008;1188:44–53. https://doi.org/10.1016/j.brainres.2007.09.098.
Nijhuis TH, Brzezicki G, Klimczak A, Siemionow M. Isogenic venous graft supported with bone marrow stromal cells as a natural conduit for bridging a 20 mm nerve gap. Microsurgery. 2010;30:639–45. https://doi.org/10.1002/micr.20818.
Ding F, Wu J, Yang Y, Hu W, Zhu Q, Tang X, Liu J, Gu X. Use of tissue-engineered nerve grafts consisting of a chitosan/poly (lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng Part A. 2010;16:3779–90. https://doi.org/10.1089/ten.TEA.2010.0299.
Siemionow M, Duggan W, Brzezicki G, Klimczak A, Grykien C, Gatherwright J, Nair D. Peripheral nerve defect repair with epineural tubes supported with bone marrow stromal cells: a preliminary report. Ann Plast Surg. 2011;67:73–84. https://doi.org/10.1097/SAP.0b013e318223c2db.
Jesuraj NJ, Santosa KB, Newton P, Liu Z, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE, Johnson PJ. A systematic evaluation of Schwann cell injection into acellular cold-preserved nerve grafts. J Neurosci Methods. 2011;197:209–15. https://doi.org/10.1016/j.jneumeth.2011.02.015.
Walsh SK, Kumar R, Grochmal JK, Kemp SW, Forden J, Midha R. Fate of stem cell transplants in peripheral nerves. Stem Cell Res. 2012;8:226–38. https://doi.org/10.1016/j.scr.2011.11.004.
Nakayama K. Development of a scaffold-free 3D Biofabrication system "Kenzan method". In: Kenzan method for scaffold-free biofabrication. Springer Nature Switzerland AG; 2021. p. 1–15.
Itoh M, Nakayama K, Noguchi R, Kamohara K, Furukawa K, Uchihashi K, Toda S, Oyama J, Node K, Morita S. Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS ONE. 2015;10: e0136681. https://doi.org/10.1371/journal.pone.0136681.
Ishihara K, Nakayama K, Akieda S, Matsuda S, Iwamoto Y. Simultaneous regeneration of full-thickness cartilage and subchondral bone defects in vivo using a three-dimensional scaffold-free autologous construct derived from high-density bone marrow-derived mesenchymal stem cells. J Orthop Surg Res. 2014;9:98. https://doi.org/10.1186/s13018-014-0098-z.
Machino R, Matsumoto K, Taniguchi D, Tsuchiya T, Takeoka Y, Taura Y, Moriyama M, Tetsuo T, Oyama S, Takagi K, Miyazaki T, Hatachi G, Doi R, Shimoyama K, Matsuo N, Yamasaki N, Nakayama K, Nagayasu T. Replacement of rat tracheas by layered, trachea-like, scaffold-free structures of human cells using a Bio-3D printing system. Adv Healthcare Mater. 2019;8: e1800983. https://doi.org/10.1002/adhm.201800983.
Yurie H, Ikeguchi R, Aoyama T, Kaizawa Y, Tajino J, Ito A, Ohta S, Oda H, Takeuchi H, Akieda S, Tsuji M, Nakayama K, Matsuda S. The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model. PLoS ONE. 2017;12: e0171448. https://doi.org/10.1371/journal.pone.0171448.
Pan D, Mackinnon SE, Wood MD. Advances in the repair of segmental nerve injuries and trends in reconstruction. Muscle Nerve. 2020;61:726–39. https://doi.org/10.1002/mus.26797.
Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–55. https://doi.org/10.1016/j.cell.2010.08.039.
Yurie H, Ikeguchi R, Aoyama T, Ito A, Tanaka M, Noguchi T, Oda H, Takeuchi H, Mitsuzawa S, Ando M, Yoshimoto K, Akieda S, Nakayama K, Matsuda S. Mechanism of peripheral nerve regeneration using a Bio 3D conduit derived from normal human dermal fibroblasts. J Reconstr Microsurg. 2021;37:357–64. https://doi.org/10.1055/s-0040-1716855.
Thoma EC, Merkl C, Heckel T, Haab R, Knoflach F, Nowaczyk C, Flint N, Jagasia R, Jensen Zoffmann S, Truong HH, Petitjean P, Jessberger S, Graf M, Iacone R. Chemical conversion of human fibroblasts into functional Schwann cells. Stem Cell Rep. 2014;3:539–47. https://doi.org/10.1016/j.stemcr.2014.07.014.
Ando M, Ikeguchi R, Aoyama T, Tanaka M, Noguchi T, Miyazaki Y, Akieda S, Nakayama K, Matsuda S. Long-term outcome of sciatic nerve regeneration using Bio3D conduit fabricated from human fibroblasts in a rat sciatic nerve model. Cell Transplant. 2021;30:9636897211021357. https://doi.org/10.1177/09636897211021357.
Takeuchi H, Ikeguchi R, Aoyama T, Oda H, Yurie H, Mitsuzawa S, Tanaka M, Ohta S, Akieda S, Miyazaki Y, Nakayama K, Matsuda S. A scaffold-free Bio 3D nerve conduit for repair of a 10-mm peripheral nerve defect in the rats. Microsurgery. 2020;40:207–16. https://doi.org/10.1002/micr.30533.
Mitsuzawa S, Ikeguchi R, Aoyama T, Takeuchi H, Yurie H, Oda H, Ohta S, Ushimaru M, Ito T, Tanaka M, Kunitomi Y, Tsuji M, Akieda S, Nakayama K, Matsuda S. The efficacy of a scaffold-free Bio 3D conduit developed from autologous dermal fibroblasts on peripheral nerve regeneration in a canine ulnar nerve injury model: a preclinical proof-of-concept study. Cell Transplant. 2019;28:1231–41. https://doi.org/10.1177/0963689719855346.
Yurie H, Ikeguchi R, Aoyama T, Tanaka M, Oda H, Takeuchi H, Mitsuzawa S, Ando M, Yoshimoto K, Noguchi T, Akieda S, Nakayama K, Matsuda S. Bio 3D conduits derived from bone marrow stromal cells promote peripheral nerve regeneration. Cell Transplant. 2020;29:963689720951551. https://doi.org/10.1177/0963689720951551.
Kaizawa Y, Kakinoki R, Ikeguchi R, Ohta S, Noguchi T, Takeuchi H, Oda H, Yurie H, Matsuda S. A Nerve conduit containing a vascular bundle and implanted with bone marrow stromal cells and decellularized allogenic nerve matrix. Cell Transplant. 2017;26:215–28. https://doi.org/10.3727/096368916X692951.
Tanaka H, Kakinoki R, Kaizawa Y, Yurie H, Ikeguchi R, Akagi M. Bone marrow-derived mesenchymal stem cells transplanted into a vascularized biodegradable tube containing decellularized allogenic nerve basal laminae promoted peripheral nerve regeneration; can it be an alternative of autologous nerve graft? PLoS ONE. 2021;16: e0254968. https://doi.org/10.1371/journal.pone.0254968.
Kashani IR, Golipoor Z, Akbari M, Mahmoudi R, Azari S, Shirazi R, Bayat M, Ghasemi S. Schwann-like cell differentiation from rat bone marrow stem cells. Arch Med Sci. 2011;7:45–52. https://doi.org/10.5114/aoms.2011.20603.
Mitsuzawa S, Zhao C, Ikeguchi R, Aoyama T, Kamiya D, Ando M, Takeuchi H, Akieda S, Nakayama K, Matsuda S, Ikeya M. Pro-angiogenic scaffold-free Bio three-dimensional conduit developed from human induced pluripotent stem cell-derived mesenchymal stem cells promotes peripheral nerve regeneration. Sci Rep. 2020;10:12034. https://doi.org/10.1038/s41598-020-68745-1.
Ikeguchi R, Aoyama T, Yurie H, Takeuchi H, Mitsuzawa S, Zhao C, Ando M, Yoshimoto K, Miyazaki Y, Noguchi T, Akieda S, Ikeya M, Nakayama K, Matsuda S. Nerve regeneration using Bio 3D printer. Jpn J Artif Organs. 2021;50:94–7.
Acknowledgements
R.I would like to acknowledge funding from the Japan Agency for Medical Research and Development under Grant Numbers 17lm0203034h0001, 18lm0203053h0001, and 19lm0203053h0002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Koichi Nakayama is the co-founder and shareholder of Cyfuse Biomedical K.K., Tokyo, Japan (Cyfuse). Shizuka Akieda, who are the president of Cyfuse, contributed to the manufacturing of 3D nerve conduits and Cyfuse provided the bioprinter to manufacture the conduit. Cyfuse also provided support in the form of a salary for Shizuka Akieda and provided research grants to Ryosuke Ikeguchi, Tomoki Aoyama, Koichi Nakayama, and Shuichi Matsuda. Cyfuse did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. These competing interests do not alter the authors’ adherence to Journal of Artificial Organs’ policies on sharing data and materials.
Review of Japanese Journal of Artificial Organs
This paper is the review based on the paper published in Japanese Journal of Artificial Organs [30].
Ethical approval
The experimental protocols were approved by the Animal Research Committee, Kyoto University Graduate School of Medicine.
Statement of human and animal rights
All animal studies were approved by the Animal Research Committee, Kyoto University Graduate School of Medicine and were performed according to the guidelines of the Animal Research Committee, Kyoto University Graduate School of Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikeguchi, R., Aoyama, T., Tanaka, M. et al. Nerve regeneration using the Bio 3D nerve conduit fabricated with spheroids. J Artif Organs 25, 289–297 (2022). https://doi.org/10.1007/s10047-022-01358-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-022-01358-9