Abstract
A bilayered artificial dermis (AD) composed of an upper silicone sheet and a lower collagen sponge has been widely applied for skin defects. After application, fibroblasts and capillaries infiltrate the AD and the collagen sponge is replaced by host dermal tissue within a few weeks. However, this delay and the high incidence of infection are concerns regarding the use of AD in the treatment of chronic ulcers. In this study, we compared the neovascularization of conventional AD seeded with autologous fibroblasts (cultured dermis: CD) and collagen/gelatin sponge (CGS), which is a novel artificial dermis capable of sustained release of basic fibroblast growth factor (bFGF) after application using laser Doppler imaging (LDI). CD (n = 5) and CGS impregnated with bFGF (n = 6) were applied to diabetic foot ulcers after debridement. Perfusion units (PUs) were measured just after, and 1, 2 and 3 weeks after application, and complete healing rates within 16 weeks were compared. No significant differences in PUs were seen 1, 2 and 3 weeks after application and in healing rates within 16 weeks between the two groups. This study suggested that CD and CGS treatments were effective, but there were no significant differences between them in the treatment of diabetic ulcers .
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A bilayered artificial dermis (AD) composed of an upper silicone sheet and a lower collagen sponge has been used in severe burn injuries since the 1990s [1, 2]. Recently, AD has been widely applied for skin defects after traumatic injury or tumor resection and also for chronic skin ulcers [3, 4]. When AD is implanted into a full-thickness skin defect, fibroblasts and capillaries infiltrate it and the collagen sponge is biodegraded and replaced by host dermis-like tissue within a few weeks before secondary split-thickness skin grafting [1–3]. However, this long waiting time before secondary grafting and the relatively high incidence of infection when applied to chronic skin ulcers such as diabetic ulcers are concerns in the treatment using conventional AD [4].
Recently, with the development of regenerative medicine using cell culturing techniques and genetic technology, skin substitutes containing living cells or growth factors have been applied clinically and their effectiveness has been reported [5–7]. It is important to accelerate neovascularization into AD to overcome the clinical issues associated with it because well-vascularized tissue can more easily receive a skin graft and is also resistant to infection. To promote neovascularization into AD, we have developed an autologous fibroblast-seeded artificial dermis (cultured dermis: CD) [8, 9] and a novel artificial dermis (collagen/gelatin sponge: CGS) [10, 11] that can maintain the release of recombinant basic fibroblast growth factor (bFGF), which is the most potent angiogenic factor and approved for the treatment of chronic ulcers in Japan [7]. Clinical trials of these new materials have already been completed in the treatment of chronic skin ulcers and their safety and efficacy have been reported [12].
Living cells and growth factors are effective for wound healing; however, it is not easy to compare their effectiveness under the same conditions in clinical cases. We have reported that laser Doppler imaging (LDI) can detect the neovascularization in AD as a perfusion signal and predict the take rate of skin grafts implanted on dermis-like tissue [13]. LDI is a technique used to evaluate skin microcirculation that is based on the principle of a Doppler frequency shift of a laser beam reflected by moving red blood cells in the cutaneous microcirculation [13, 14]. In this study, we compared the neovascularization of CD and CGS applied for diabetic foot ulcers enrolled in our clinical trials at 1, 2 and 3 weeks after application and compared the healing rate 16 weeks after application. Then, we discuss the efficacy of cell therapy and growth factors in combination with AD.
Materials and methods
Cultured dermis (CD) and collagen/gelatin sponge (CGS)
Cultured dermis (CD) is an autologous fibroblast-seeded artificial dermis (AD). We used AD (Pelnac®; Gunze Co. Ltd., Kyoto, Japan) and seeded a patient’s autologous fibroblasts cultured using animal product-free medium [11].
CGS is a modified version of the conventional AD and consists of an upper silicone sheet that is reinforced with nylon mesh and a lower CGS containing a 10 wt% concentration of acidic gelatin [10]. CGS works as a scaffold for dermal regeneration in the same manner as the conventional AD. In addition, CGS can exhibit sustained release of positively charged growth factors such as bFGF for more than 10 days. We used bFGF spray (Fibrast spray®; Kaken Pharmaceutical, Tokyo, Japan) and showed that the dermal regeneration of CGS impregnated with bFGF was accelerated in our clinical trial [12].
Laser Doppler imaging (LDI)
We used an LDI instrument (Moor LDI2-IR; Moor Instruments Ltd., Axminster, Devon, UK) in this study and measured the perfusion units (PUs) of CD and CGS. LDI is a technique to determine the local distribution of PUs over a skin area without direct contact with the skin surface using a laser beam (785 nm) [13, 14]. PUs are a relative measure of blood flow that represents the average speed and number of blood cells at a depth of about 1–1.5 mm [15]. LDI provides color-coded blood-flow images in which dark blue represents the lowest perfusion and the highest perfusion is shown in red [13–15]. In our previous study, we evaluated the blood flow of AD and reported that dermis-like tissue of more than 165 PUs could receive skin grafts [13].
Patients
In this study, we retrospectively compared PUs measured by LDI of diabetic foot ulcers enrolled in our two clinical trials with conventional CD and CGS [9, 12]. All patients were treated at Kyoto University Hospital. An outline of the treatment groups and outcome is provided in Table 1. In the cultured dermis group, five patients with diabetic ulcers were treated with autologous fibroblast-seeded AD from September 2008 to December 2009 [9]. Its main inclusion criteria were diabetic foot ulcers as follows: not healing for at least 1 month; without granulation tissue and skin graft not expected to take; adequate arterial blood supply assessed by measuring skin perfusion pressure (SPP) of 25 mmHg or greater at a site 1 cm proximal or distal to those ulcers [9]. In the CGS impregnated with bFGF group, 17 patients with chronic ulcers were enrolled and treated with CGS impregnated with bFGF from May 2010 to June 2011 [12]. The main inclusion criteria were the presence of chronic skin ulcers as follows: not healing for at least 4 weeks with conventional treatments; skin graft not expected to take; adequate arterial blood supply assessed by measuring skin perfusion pressure (SPP) of 30 mmHg or greater at a site 1 cm proximal or distal to those ulcers if chronic skin ulcers were present on the lower extremities [12]. Five diabetic ulcers enrolled in the CD trial and 6 diabetic ulcers enrolled in the CGS trial out of 17 chronic ulcers were analyzed in this study.
Evaluation of neovascularization using LDI
At first, we evaluated the effects of the silicone sheet of CD and the reinforced silicone sheet of CGS on PUs. PUs of existing AD (Pelnac®) and CGS in 1 × 1 cm were evaluated from the silicone side and the collagen side (n = 5). Then, PUs of the identical forearm region in 1 × 1 cm (n = 5) from 2 volunteers were evaluated without a silicone sheet, with a silicone sheet of AD and with a reinforced silicone sheet of CGS.
CD or CGS was applied to the ulcer after debridement and sutured to the marginal skin. The PUs were measured just after, and 1, 2 and 3 weeks after application. The silicone sheet of CD was removed 3 weeks before evaluating PUs and the reinforced silicone sheet of CGS was removed 2 weeks before evaluating PUs. The average PUs of the whole area of each CD or CGS were used for analysis. In the CD group, the usage of bFGF was prohibited for 3 weeks after application and, in the CGS group, 7 µg/cm2 bFGF was impregnated with CGS before application and the additional dosing of bFGF was prohibited for 4 weeks. After the prohibition period, bFGF was used when needed. Subsequent therapy including skin graft and any topical agent except for bFGF was started 3 weeks after application in both groups. Skin graft was performed when it was expected to take on the formed dermis-like tissue. Patients were followed up until 16 weeks after application and the rate of complete wound healing was evaluated.
Statistical analysis
The age, wound area, duration of ulcer and SPP were compared between the CD group and the CGS group using Student’s t test. Fisher’s exact probability test was used for the analysis of complete wound healing in each treatment group. The statistical significance of differences in PU was evaluated using one-way analysis of variance (ANOVA) and Scheffe’s test as a post hoc test. All data of PU are expressed as the mean ± standard error (SE). p < 0.05 was accepted as significant.
Results
Patients and outcome 16 weeks after treatment
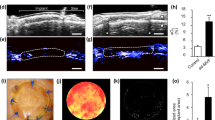
There was no significant difference in age, HbA1c, wound area, duration of ulcer, SPP and the complete healing rates within 16 weeks between the CD group and the CGS group (Table 1). Skin graft was performed for one patient in the CD group and two patients in the CGS group. One patient in the CGS group dropped out before 16 weeks. Time courses of gross photographs of wounds and color-coded images of LDI of each treatment group are shown (Figs. 1, 2). No perfusion signal was detected just after the application of CD and CGS (Figs. 1b, 2b). A perfusion signal was confirmed 1 week after application in both groups (Figs. 1c, 2c). Three weeks after application, mature dermis-like tissue was formed and perfusion signal was also increased in both groups (Figs. 1d, 2d). In this CD case, the ulcer was epithelialized 9 weeks after application and there was no recurrence 16 weeks after it (Fig. 1e). In CGS cases, the ulcer was large and split-thickness skin graft was applied on the formed dermis-like tissue 4 weeks after application, and no recurrence was observed 16 weeks after application (Fig. 2e).
Time course of a diabetic ulcer in the CD group. a A 65-year-old male patient with diabetes had a non-healing ulcer for 24 months on the plantar surface of the first metatarsophalangeal joint. b After debridement, CD was applied. c Color-coded image of LDI; the mean PU was 46.4 in the applied area indicated by the white circle. d Mature dermis-like tissue was formed and epithelialization from the margin started at 3 weeks after application. e Color-coded image of LDI at 3 weeks after application; the mean PU in the circle was 244.1. f Four months after application. The ulcer was epithelialized 9 weeks after application
Time course of a diabetic ulcer in the CGS impregnated with bFGF group. a A 61-year-old female patient had a diabetic ulcer over her stump after amputation of the fourth and fifth toes of the left foot. b After debridement, CGS impregnated with bFGF was applied. c Color-coded image of LDI; the mean PU was 24.0 in the applied area indicated by the white circle. d Mature dermis-like tissue was formed on most of the ulcer surface at 3 weeks after application. e Color-coded image of LDI; the mean PU in the circle was 149.4. f Four months after application. Split-thickness skin graft was applied on the formed dermis-like tissue 4 weeks after application
Neovascularization in CD and CGS
The PU value of AD from the silicone side was 20.0 ± 0.28 and that from the collagen side was 18.3 ± 0.56. The PU value of CGS from the reinforced silicone side was 18.0 ± 0.68 and that of CGS from the collagen side was 17.9 ± 0.7. There was no significant difference among PUs of these four groups. The PU value of the forearm region was 54.3 ± 9.63. The PU value of the forearm region with a silicone sheet was 56.52 ± 8.83 and that with a reinforced silicone sheet was 50.7 ± 8.18. There was no significant difference in PUs with or without silicone or reinforced silicone sheets.
The time course and comparison of PUs in the CD- and CGS-applied groups are shown in Fig. 3. There was no significant difference among PUs at Day 0 and 1 week. Two weeks after application, PUs in the CD and CGS groups were significantly larger than that in the CGS group on Day 0 (p < 0.05). There was no significant difference between PUs in the CD and CGS groups at 1 week and 2 weeks. Three weeks after application, the PU value in the CD group was significantly larger than those in the CD and CGS groups on Day 0 (p < 0.01).
Comparison of PU in applied CD and CGS groups. There was no significant difference at Day 0 and 1 week. Two weeks after application, PUs in the CD and CGS groups were significantly larger than that in the CGS group on Day 0 (p < 0.05). Three weeks after application, PU in the CD group was significantly larger than those in the CD and CGS groups on Day 0 (p < 0.01)
Discussion
The healing of chronic skin ulcers such as diabetic ulcers and venous leg ulcers is difficult despite the advance of treatments and the fact that their number is still increasing [16, 17]. A bilayered living human skin equivalent (Apligraf; Organogenesis Inc., Canton, MA) and a human fibroblast-derived dermal substitute (Dermagraft; Advanced BioHealing Inc., La Jolla, CA) are representative tissue-engineered skin substitutes containing living cells; these products have been reported to be effective in the treatment of chronic skin ulcers [5, 18, 19]. In addition to cell therapy, recombinant or genetically engineered growth factors such as platelet-derived growth factor BB (PDGF-BB), epidermal growth factor (EGF) and bFGF have also been reported to be effective in the treatment of chronic ulcers [7, 20, 21]. These novel products are generally accepted to promote wound healing strongly, whereas there is still discussion about whether living cells or growth factors are more effective for wound healing. Universal agreement has not been reached on the mechanism of action of living skin equivalents, but their release of several growth factors essential for wound healing while implanted cells are viable is thought to be important [22].
On the other hand, the periods of bioactivity of recombinant growth factors in vivo are very short and they must be administered frequently (at least once a day) or some controlled release systems for growth factors are required [10–12].
In this study, we compared the effect of autologous fibroblasts and a sustained release system of bFGF in the dermal regeneration process after application to diabetic ulcers. There was no significant difference in wound healing within 16 weeks and in neovascularization as indicated by LDI between the two treatments. Our previous study indicated that AD with PU >165 had sufficient blood flow to receive secondary skin grafting and it takes 1 or 2 weeks after application to skin defects in which the wound healing process is not disturbed [13]. The PU values of diabetic ulcers in the CD and CGS groups reached a sufficient level to receive secondary skin grafting 2 weeks after application, as in normal skin defects; therefore, it is suggested that both autologous fibroblasts and controlled release of bFGF improve neovascularization into a dermal substitute from the wound bed.
As for the efficacy of fibroblasts or bFGF, there was no significant difference in PU values and wound healing within 16 weeks. However, PU values in the CD group were relatively high compared with those in the CGS group 3 weeks after application, albeit with no significant difference. On this point, CGS can maintain the release of bFGF for around 10 days [10] and the additional application of CGS impregnated with bFGF might be necessary to improve the perfusion signal. However, the sample size of this study was small and further study will be needed to clarify this issue.
With regard to the study design, we do not have PU data of AD applied to diabetic ulcers as a control and we cannot show the efficacy of CD and CGS treatments statistically. We do not use only conventional AD without additional procedures in the treatment of chronic ulcers because infection can easily occur and dermis-like tissue cannot form [9, 23]. According to previous reports of studies using skin equivalent products or growth factors in the treatment of diabetic ulcers, the healing rates within 12 weeks are reported to be 24–56 % and the rates within 20 weeks are 43–50 % [5, 12, 18, 23]. The healing rates within 16 weeks of CD and CGS groups were comparable to those of previous reports and CD and CGS treatments seem to promote wound healing to some degree.
Although skin equivalents containing living cells have been reported to be effective in the treatment of chronic ulcers, the high cost and the storage or transportation of living cell products are key issues in clinical practice [7]. CGS and bFGF can be stored at room temperature and their transportation is easy. The impregnation of bFGF to CGS is simple, in that bFGF is sprayed onto it just before its application. By using CGS impregnated with bFGF, it will be possible to reduce the number of treatments compared with daily administration of bFGF. This study suggests that CGS impregnated with bFGF might be superior to living cell products in terms of cost and versatility.
CD and CGS have not yet been approved in Japan, so more patients should be available after their approval, and further investigation should then be performed.
Conclusion
We compared the neovascularization and efficacy of autologous fibroblast-seeded artificial dermis (CD) and CGS that can exhibit sustained release of bFGF in the treatment of diabetic ulcers. This study suggested that CD and CGS treatments were effective but there were no significant differences between them in the treatment of diabetic ulcers.
References
Yannas IV. Emerging rules for inducing organ regeneration. Biomaterials. 2013;34:321–30.
Suzuki S, Matsuda K, Nishimura Y, et al. Review of acellular and cellular artificial skins. Tissue Eng. 1996;2:267–75.
Momoh AO, Lypka MA, Echo A, et al. Reconstruction of full-thickness calvarial defect: a role for artificial dermis. Ann Plast Surg. 2009;62:656–9.
Ito K, Ito S, Sekine M, et al. Reconstruction of the soft tissue of a deep diabetic foot wound with artificial dermis and recombinant basic fibroblast growth factor. Plast Reconstr Surg. 2005;115:567–72.
Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407–24 (Review).
Langer A, Rogowski W. Systematic review of economic evaluations of human cell-derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res. 2009;9:115.
Uchi H, Igarashi A, Urabe K, Koga T, Nalayama J, Kawamori R, Tamaki K, Hirakata H, Ohura T, Furue M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol. 2009;19:461–8.
Morimoto N, Takemoto S, Kanda N, Ayvazyan A, Taira MT, Suzuki S. The utilization of animal product-free media and autologous serum in an autologous dermal substitute culture. J Surg Res. 2011;171:339–46.
Morimoto N, Ito T, Takemoto S, Katakami M, Kanda N, Tada H, Tanaka S, Teramukai S, Kawai K, Nakamura Y, Kasai Y, Masayuki Y, Maekawa T, Shimizu A, Suzuki S. An exploratory clinical study on the safety and efficacy of an autologous fibroblast-seeded artificial skin cultured with animal product-free medium in patients with diabetic foot ulcers. Int Wound J. 2014;11:183–9.
Takemoto S, Morimoto N, Kimura Y, Taira T, Kitagawa T, Tomihata K, Tabata Y, Suzuki S. Preparation of collagen/gelatin sponge scaffold for sustained release of bFGF. Tissue Eng Part A. 2008;14:1629–38.
Kanda N, Morimoto N, Ayvazyan AA, Takemoto S, Kawai K, Nakamura Y, Sakamoto Y, Taira T, Suzuki S. Evaluation of a novel collagen-gelatin scaffold for achieving the sustained release of basic fibroblast growth factor in a diabetic mouse model. J Tissue Eng Regen Med. 2014;8:29–40.
Morimoto N, Yoshimura K, Niimi M, Ito T, Aya R, Fujitaka J, Tada H, Teramukai S, Murayama T, Toyooka C, Miura K, Takemoto S, Kanda N, Kawai K, Yokode M, Shimizu A, Suzuki S. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue Eng Part A. 2013;19:1931–40.
Morimoto N, Takemoto S, Kawai K, Aya R, Ishisaka T, Suzuki S. Immediate evaluation of neovascularization in a grafted bilayered artificial dermis using laser Doppler imaging. Ann Plast Surg. 2014;72:84–8.
Monstrey SM, Hoeksema H, Baker RD, et al. Burn wound healing time assessed by laser Doppler imaging. Part 2: validation of a dedicated colour code for image interpretation. Burns. 2011;37:249–56.
Riordan CL, McDonough M, Davidson JM, et al. Noncontact laser Doppler imaging in burn depth analysis of the extremities. J Burn Care Rehabil. 2003;24:177–86.
Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg. 2009;50:275–91 (Review).
Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–28.
Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701–5.
Cavorsi J, Vicari F, Wirthlin DJ, Ennis W, Kirsner R, O’Connell SM, Steinberg J, Falanga V. Best-practice algorithms for the use of a bilayered living cell therapy (Apligraf) in the treatment of lower-extremity ulcers. Wound Repair Regen. 2006;14:102–9 (Review).
Tuyet HL, Quynh TTN, Minh HVH, Bich DNT, Dinh TD, Tan DL, Van HL, Huy TL, Huu HD, Trong TNT. The efficacy and safety of epidermal growth factor in treatment of diabetic foot ulcers: the preliminary results. Int Wound J. 2009;6:159–66.
Senet P, Vicaut E, Beneton N, Debure C, Lok C, Chosidow O. Topical treatment of hypertensive leg ulcers with platelet-derived growth factor-BB: a randomized controlled trial. Arch Dermatol. 2011;147:926–30.
Yamada N, Uchinuma E, Kuroyanagi Y. Clinical trial of allogeneic cultured dermal substitutes for intractable skin ulcers of the lower leg. J Artif Organs. 2008;11:100–3.
Dinh TL, Veves A. The efficacy of Apligraf in the treatment of diabetic foot ulcers. Plast Reconstr Surg. 2006;117:152S–7S (Review).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Morimoto, N., Kakudo, N., Valentin Notodihardjo, P. et al. Comparison of neovascularization in dermal substitutes seeded with autologous fibroblasts or impregnated with bFGF applied to diabetic foot ulcers using laser Doppler imaging. J Artif Organs 17, 352–357 (2014). https://doi.org/10.1007/s10047-014-0782-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-014-0782-0