Abstract
Tapered optical fibers coated with graphene and graphene oxide (GO) as the active layer for ethanol sensing were reported. The multimode optical fiber with 125 µm diameter was tapered to 40 µm diameter to enhance the sensitivity. Graphene and GO thin films were characterized using a scanning electron microscopy, Raman spectroscopy and ultraviolet–visible (UV–Vis) spectroscopy. The absorbance properties of the developed sensors increased when exposed to ethanol due to the change of light in the evanescent field. The sensing results indicated that the GO-coated sensor showed better performance with absorbance change of 80 % towards ethanol concentration of 5 % when compared to graphene-coated sensor with 40 % absorbance change towards ethanol with similar concentrations. The reliable response of the graphene and GO-coated on tapered fibers for detecting ethanol concentrations was achieved at room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent times, graphene has attracted much attention in many fields due to its unique characteristics such as chemical stability, flexibility, high mechanical strength and unique optical properties [1]. The nano-scale structure of graphene provides immense surface-to-volume ratio. As a result, it can detect the lowest level of a target gas (single molecule) [2], making it an attractive candidate for chemical sensing applications. As an alternative, GO which is a graphene precursor, also possesses promising properties for sensing applications. The structure of GO is described as a graphene sheet bonded to oxygen in the form of carboxyl, hydroxyl or epoxy groups [3], which can significantly improve the hydrophilicity of GO and subsequently enhances its sensitivity. GO has been used to detect humidity [3], heavy metal [4], and NO2 [5]. Therefore, graphene based materials are highly potential to be deployed in optical fiber for ethanol sensing applications.
Optical fibers are playing an increasingly important transduction platform in the field of sensing. It is unaffected by electromagnetic interference (EMI), making it possible to be used in any environment with EMI [6]. Recently, fiber optic sensors based on tapered fiber have received wide attention because of its unique properties [7, 8]. Basically, in a tapered fiber, evanescent fields in the core-exposed region interact directly with the surrounding medium and hence increase the sensitivity. In a standard optical fiber, the intensity of the evanescent wave decays exponentially to zero at core cladding interface. The exponential decay is given by [6],
where d p is the penetration depth given by;
where λ is the wavelength of the transmitted light, θ is the incident angle of the light at core/cladding interface, and n 1 and n 2 are the refractive indexes of core and cladding, respectively.In a tapered optical fiber coated with an active layer, interaction occurs within the evanescent field region of the optical fiber. Therefore, the output of the sensor will depend on the absorption coefficient of the active material and the fraction of the total power that is carried in the evanescent field. In this case, the optical fiber plays a major role in the sensing mechanism. The characteristic equation of the tapered optical fiber sensors is given as [9],
where I and I 0 are the input and output light of the sensor, respectively. C is the concentration of the analyte, l is the length of the tapered fiber and α is the absorption coefficient. The value, r represents the reduction factor in attenuation with an optical fiber.
In general terms, r can be written for an optical fiber as [9],
where n r is the refractive index of the analyte, n e is the effective refractive index of the guided mode and f is the fraction of the total evanescent wave power that interacts with analyte. The value of r depends on the quantity of the evanescent field that interacts with the analyte. It is important in the design of evanescent wave sensors to maximize the value of r.
Tapered optical fibers are used with high efficiency in evanescent wave and absorption based sensors. Villatoro et al. [10] reported hydrogen sensing using single mode tapered optical fiber coated with thin palladium layer. Their tapered fiber enhances the evanescent waves of the propagated light that interact with the palladium coating exposed to hydrogen. This results in fast response of approximately 10 s towards 3.6 % of hydrogen. Zhang et al. [7] reported humidity sensor using a sub-wavelength diameter fiber coated with gelatine film. The sensor exhibited high sensitivity, reversibility and response time of approximately 70 ms when relative humidity (RH) jumps from 75 to 88 %. Tapered plastic optical fiber for measurement of uric acid concentrations in deionised water was recently reported by Harun et al. [8]. The tapered plastic fiber was coated with single walled carbon nanotubes polyethylene oxide composite. The output voltage of the sensor photodetector increase linearly from 6.13 to 7.35 mV as uric acid concentration increases from 0 to 500 ppm. The sensor shows sensitivity of 0.0023 mV/% and linearity of more than 97.20 %.
Ethanol sensors based on optical fiber transducing platforms has also been reported previously. Elousa et al. [11] presented the method of ethanol concentration sensing based on reflectance principles. Multimode cleaved ended fiber coated with vapochromic materials was used. To measure the response, the intensity of the reflected signal was monitored. In the reflectance based sensors, the power of the evanescent wave is quite low and the output power of the reflected signal is also low. This is due to the poor ambient fiber coupling efficiency of the evanescent waves [12]. Since the biconical taper structure has overcome these limitations, it has become the preferred choice in application. King et al. [13] reported three point optical fiber sensor based on U-shaped fiber for ethanol sensing. In their study, the U-shaped fiber was used to enhance the evanescent fields for stronger interaction with the analyte. However, the used U-shaped fiber structure is complex as compared to biconical taper which is simpler and efficient.
Herein, we report the absorbance response of tapered optical fiber coated with graphene based nanomaterials (graphene and GO) towards ethanol in a range of concentration between 5 and 40 % in water. The sensitivity, response and recovery time as well reversibility of the developed sensors were studied and compared.
2 Experimental section
2.1 Preparation of thin film of graphene and GO
The fabrication of the tapered optical fiber is carried out according to our previous work [14]. The preparation of graphene was done by electrochemical exfoliation of graphite rod using sodium dodecyl benzene solfonate (SDBS) [15] and GO was synthesized using the simplified Hummers method [3]. Graphene and GO were deposited on glass substrate and on tapered optical fiber using drop-casting technique. Graphene and GO suspended in water with concentration of 1 mg/ml were separately drop-casted on glass substrates for micro-characterization purposes. The solutions were also drop-casted on the tapered area of the multimode optical fiber for ethanol testing purpose. Subsequently, the glass substrate and tapered optical fiber were annealed at 70 °C for 1 h and left overnight to further enhance the formation of thin film on the surface.
2.2 Materials characterization
Surface morphology of the graphene and GO thin film was studied using images obtained from Scanning Electron Microscopy (SEM, S-3400) operated at 15 kV. Raman spectroscopy was performed at room temperature using UTHS Raman spectrometer system (WITec alpha 300 M) with 532 nm excitation source. Optical absorption properties of the graphene and GO were assessed using a UV–Visible spectrophotometer (Thermoscientific Evolution 300).
2.3 Experimental setup
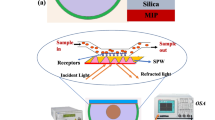
The experimental setup for the absorbance measurement is shown in Fig. 1. A tungsten-halogen lamp (Ocean Optics HL2000) with a wavelength range of 360–2400 nm was used to transmit light into the optical fiber. The tapered fiber coated with the active layer was fixed in the ethanol chamber on a holder. A spectrometer (Ocean Optics USB4000) with a spectral range of 200–1100 nm was used as the light detector to measure the output light intensity of the sensor. The spectrometer was connected to the computer via a USB port. The spectra were characterized using SpectraSuite software.
3 Results and discussion
3.1 Raman analysis
Raman spectroscopy has been widely used to characterize carbon based materials such as graphene. In our work, Raman spectroscopy was utilized to prove the presence of graphene and GO. Figure 2a, b shows the Raman spectrum of graphene and GO, respectively. The most striking peaks in the Raman spectra of graphene materials are D and G band. The Raman spectrum for the graphene and GO shows intense peak of D and G band. The D peak for both graphene and GO is located at 1348 cm−1 [1]. The D band is assigned to local defects and disorder of graphene that is caused by the attachment of hydroxyl and epoxide groups on the carbon basal plane and edges [16]. The G peak for the graphene is located at 1580 cm−1 and at 1593 cm−1 for GO. The G peak corresponds to first order scattering of the E 2g mode [16]. Graphene has lower I D/I G ratio (~0.6) compared to GO (~1.0) suggesting that graphene has less defects. Graphene and GO Raman spectra also show a 2D band around 2678 and 2685 cm−1, respectively. Because the 2D band is the second order of the D band, it is always stronger in graphene [17]. The Raman spectrum of the prepared graphene and GO are in good agreement with the reports of previous studies [16, 17].
3.2 Characterization of graphene and GO by UV–Vis spectroscopy
Ultraviolet–visible (UV–Vis) spectroscopy is a characterization technique based on interaction of light in the ultraviolet and visible region with the material of interest. Figure 3 shows the UV–Vis absorption spectrum of graphene and GO. It can be seen from the figure that GO exhibits peak at 227 nm and shoulder around 300 nm. The peak at 227 nm is assigned to p → p* transitions of the aromatic C–C bonds and the shoulder around 300 nm corresponds to n → p transitions of C=O bonds [17]. These observations suggest that GO contains oxygen functional groups. For the graphene spectrum, peak at 270 nm is observed and this indicates the restoration of the electronic conjugation of C–C bonds within the graphene sheet [18]. The absorbance spectra of graphene and GO are low for the entire visible region, suggesting high transparency in the visible wavelength range.
3.3 Morphology and structure
Figure 4a, b shows the SEM picture of non-tapered and tapered fiber, respectively. The SEM image of the tapered fiber shows smooth tapers with good diameter uniformity. In addition, the surface of the tapers exhibited no visible irregularities. Figure 4c shows the transition region (down taper) of the tapered fiber. The transition region has sufficiently long length with a linear taper profile and it also exhibited good surface smoothness. The fabricated taper has a waist diameter of 40 µm and waist length of 20 mm. Tapers with uniform waist diameters enhanced the performance of optical fiber sensor by optimizing the interaction between the evanescent wave and the analyte [19].
Figure 5a, b shows the SEM images of graphene and GO films, respectively, prepared on glass substrate. Both, graphene and GO thin films exhibit corrugated and wrinkled structures overlapping at the edges. The structures are identified in the graphene thin films for providing high surface area [20, 21]. However, the wrinkled structure and overlapping edges are more obvious on the GO film. Figure 5c, d illustrates the SEM image of the tapered optical fiber coated with graphene and GO, respectively. The images clearly show that the smooth surface of the uncoated tapered fiber (Fig. 4b) transformed into a rough surface upon graphene and GO deposition.
3.4 Ethanol sensing performance
The absorbance response for graphene and GO-coated and tapered fiber is shown in Fig. 6a, b. The absorbance trend is observed across the visible wavelength range.
From Fig. 6a, b, it is observed that the sensor’s initial absorbance in air is high due to the graphene and GO layers. When exposed to ethanol, the absorbance response increases with the increase in the ethanol concentrations. This is possibly due to the high surface area of the graphene and GO layer increases the ethanol molecules interaction with the sensing layer and thus, significantly changes the evanescent field. It was reported that volatile organic compounds (VOCs) such as ethanol and acetone can interact strongly with graphene based surfaces because graphene films are more sensitive to VOCs with OH− or carbonyl/carboxyl groups [22]. However, the magnitude of the response reduces with the increase of ethanol concentrations. The peak around 660 nm was identified to be induced by absorption of water [23]. The peak diminishes as the concentration of ethanol increases because the surface of the graphene and GO-coated fiber gradually interacts more with ethanol instead of water. The GO-coated and tapered fiber exhibited larger absorbance magnitude changes as compared to that of tapered fiber coated with graphene. The larger absorbance magnitude changes observed for GO-coated sensor might be due to higher surface area in the film as compared to the graphene-coated sensor.
Figure 7a, b exhibits the dynamic responses of the tapered fiber sensor coated with graphene and GO towards different concentrations of ethanol in water at room temperature. The dynamic response of the sensor coated with graphene and GO exhibit a reverse absorbance changes. Both sensors show distinct sensitivity towards different concentrations of ethanol with stable baseline. This indicates that both graphene and GO layers are sensitive to aqueous ethanol and have a significant role in the sensing of ethanol. The change in the magnitude of response reduces with the increase of ethanol concentrations. The sign of saturation in the sensor response upon increasing of the ethanol concentrations is in good agreement with the response in Fig. 6a, b. For the GO-coated fiber, a maximum absorbance decrease of 78 % can be observed when the developed sensor was exposed to ethanol with 5 % concentrations. The graphene-coated fiber shows a maximum response of 40 % when it was exposed to 5 % ethanol. When compared to the response of the graphene-coated fiber, the GO-coated fiber shows significant improvement in the sensitivity, by a factor of ~2. The GO-coated fiber shows higher sensitivity to ethanol than the graphene-coated fiber. This could be attributed not only by the high GO surface area due to the wrinkles but also the fact that GO contains polar functional groups (hydroxyl, carboxyl, and epoxy) which can interact strongly with various VOCs due to the formation of intermolecular polar interactions [24].
The response and recovery times for the GO-coated fiber when exposed to 5 % ethanol concentration was calculated to be 15 and 18 s, respectively and that for the graphene-coated fiber was found to be 25 and 12 s, respectively. It was found that the response time for both GO and graphene-coated fiber is between 15 and 40 s. Both sensors show almost similar response times. However, there is a distinctive difference for the recovery time of the sensor. The GO-coated fiber recovered faster than that of graphene-coated fiber sensor. The recovery time for the GO-coated fiber sensor is less than 20 s for all concentrations while the graphene-coated one recovered to the baseline within 80 s. The excellent response and recovery times for the GO-coated fiber is attributed to the presence of larger interlayer space in the GO films, which improves ethanol–water mixture desorption [3, 25].
The repeatability and reversibility of the graphene and GO-coated fiber sensor are presented in Fig. 8a, b. The sensor was immersed to three consecutive cycles in the presence of 5 % ethanol concentration and air at room temperature. The stable result implies that both graphene and GO adhered well onto the surface of the optical fiber. The fabricated ethanol sensor showed very fast response and recovery with high stability. The rapid response, recovery and high reversibility of the developed optical fiber sensor made it highly potential for industrial applications.
3.5 Sensitivity of the sensors
Figure 9 shows the calibration curve for the graphene-and GO-coated sensor. The absorbance for the GO-coated fiber sensor was observed to be higher than that of graphene-coated fiber sensor. Both, water and ethanol molecules can be easily interpolated into the external surface of carbon through the carboxyl groups present at the edge of GO, leading to the swelling of GO film [25]. The swelling effect causes higher increase in the light absorption of the GO layers. The sensitivity of graphene-coated fiber sensor is 0.829/vol % ethanol concentration. The sensitivity of GO-coated fiber sensor is shown to be higher at 1.330/vol % ethanol concentration. Therefore, overall, the interactions of different functional groups in the ethanol with the hydrophilic GO film make the sensor a preferred candidate for aqueous ethanol detection.
3.6 Ethanol sensing mechanism
The ethanol sensing mechanism that involves chemical interaction between graphene surface and ethanol is proposed in ref [26]. When air interacted with the graphene layer, oxygen molecules were adsorbed onto the graphene leading to the transfer of electrons from the molecules into graphene [27]. When graphene surface was exposed to ethanol, the ethanol molecules reacted with the oxygen molecules to form CO2 and H2O. The interaction of ethanol molecules with oxygen might lead to the transfer of electron back to graphene [22, 28].
4 Conclusions
A tapered fiber optic absorbance sensor was successfully developed to detect various concentrations of ethanol in water. Graphene and GO thin film were coated on the surface of the optical fiber to enhance the evanescent field interactions with the analyte. The behavior of the sensor has been investigated for graphene and GO for various concentrations of ethanol. As the solution concentration of ethanol in water varied from 5 to 40 %, the absorbance of the sensors increased accordingly. The GO-coated sensor exhibited better sensing performance than the graphene-coated sensor with sensitivity of 1.330/ %vol ethanol concentration with fast response and recovery. The higher sensitivity of GO-coated sensor was due to the presence of different functional groups and its higher volume to surface area, which led to faster adsorption of ethanol molecules. The simplicity of the sensor and its fast operation makes it a prospect for applications in pharmaceutical, biomedical and food industries.
References
Zhu, Y., Murali, S., Cai, W., Li, X., Suk, J.W., Potts, J.R., Ruoff, R.S.: Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 22(35), 3906–3924 (2010)
Schedin, F., Geim, A.K., Morozov, S.V., Hill, E.W., Blake, P., Katsnelson, M.I., Novoselov, K.S.: Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 6(9), 652–655 (2007)
Bi, H., Yin, K., Xie, X., Ji, J., Wan, S., Sun, L., Terrones, M., Dresselhaus, M.S.: Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 3(5), 2714 (2013)
Lokman, N.F., Bakar, A.A.A., Suja, F., Abdullah, H., Rahman, W.B.W.A., Huang, N.-M., Yaacob, M.H.: Highly sensitive SPR response of Au/chitosan/graphene oxide nanostructured thin films toward Pb(II) ions. Sens. Actuators B: Chem. 195, 459–466 (2014)
Prezioso, S., Perrozzi, F., Giancaterini, L., Cantalini, C., Treossi, E., Palermo, V., Nardone, M., Santucci, S., Ottaviano, L.: Graphene oxide as a practical solution to high sensitivity gas sensing. J. Phys. Chem. C. 117(20), 10683–10690 (2013)
Grattan, K.T.V., Meggitt B.T.: Optical Fiber Sensor Technology. Springer Science, Dordrecht (1999)
Zhang, L., Gu, F., Lou, J., Yin, X., Tong, L.: Fast detection of humidity with a subwavelength-diameter fiber taper coated with gelatin film. Opt. Express 16(17), 13349–13353 (2008)
Harun, S., Batumalay, M., Ahmad, F.: Tapered plastic optical fiber coated with single wall carbon nanotubes polyethylene oxide composite for measurement of uric acid concentration. Sensor 34, 75–79 (2014)
Jin, W., Ho, H., Cao, Y., Ju, J., Qi, L.: Gas detection with micro-and nano-engineered optical fibers. Opt. Fiber Technol. 19, 741–759 (2013)
Villatoro, J., Monzón-hernández, D.: Fast detection of hydrogen with nano fiber tapers coated with ultra thin palladium layers. Opt. Express 13(13), 5087–5092 (2005)
Elosúa, C., Bariáin, C., Matías, I.R., Arregui, F.J., Luquin, A., Laguna, M.: Volatile alcoholic compounds fibre optic nanosensor. Sens. Actuators B: Chem. 115(1), 444–449 (2006)
Golden, J., Anderson, G.: An evanescent wave biosensor. II. Fluorescent signal acquisition from tapered fiber optic probes. IEEE Trans. Biomed. Eng. 41(6), 585–591 (1994)
King, D., Lyons, W.B., Flanagan, C., Lewis, E.: Interpreting complex data from a three-sensor multipoint optical fibre ethanol concentration sensor system using artificial neural network pattern recognition. Meas. Sci. Technol. 15(8), 1560–1567 (2004)
Girei, S., Shabaneh, A.: Tapered multimode fiber sensor for ethanol sensing application. In: IEEE 4th International conference on photonics. Malecca, Malysia., p. 275–277 (2013)
Song, Y., Lee, H., Ko, J., Ryu, J.: Preparation and characterization of surfactant-exfoliated graphene. Bull. Korean Chem. 35(7), 2009–2012 (2014)
Yang, D., Velamakanni, A., Bozoklu, G., Park, S., Stoller, M., Piner, R.D., Stankovich, S., Jung, I., Field, D.A., Ventrice, C.A., et al.: Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 47(1), 145–152 (2009)
Gurunathan, S., Han, J.W., Eppakayala, V., Kim, J.-H.: Biocompatibility of microbially reduced graphene oxide in primary mouse embryonic fibroblast cells. Colloids Surf. B, Biointerfaces 105, 58–66 (2013)
Marlinda, A.R., Huang, N.M., Muhamad, M.R., An’amt, M.N., Chang, B.Y.S., Yusoff, N., Harrison, I., Lim, H.N., Chia, C.H., Kumar, S.V.: Highly efficient preparation of ZnO nanorods decorated reduced graphene oxide nanocomposites. Mater. Lett. 80, 9–12 (2012)
Villatoro, J., Monzón-Hernández, D., Luna-Moreno, D.: In-line optical fiber sensors based on cladded multimode tapered fibers. Appl. Opt. 43(32), 5933–5938 (2004)
Wang, C., Zhang, L., Guo, Z., Xu, J., Wang, H., Zhai, K., Zhuo, X.: A novel hydrazine electrochemical sensor based on the high specific surface area graphene. Microchim. Acta 169(1–2), 1–6 (2010)
Shen, Y., Lua, A.C.: A facile method for the large-scale continuous synthesis of graphene sheets using a novel catalyst. Sci. Rep. 3, 3037 (2013)
Gautam, M., Jayatissa, A.H.: Detection of organic vapors by graphene films functionalized with metallic nanoparticles. J. Appl. Phys. 112(11), 114326 (2012)
Langford, V.S., Mckinley, A.J., Quickenden, T.I.: Temperature dependence of the visible-near-infrared absorption spectrum of liquid water. J. Phys. Chem. A. 105, 8916–8921 (2001)
Paredes, J.I., Villar-Rodil, S., Martínez-Alonso, A., Tascón, J.M.D.: Graphene oxide dispersions in organic solvents. Langmuir 24(19), 10560–10564 (2008)
Some, S., Xu, Y., Kim, Y., Yoon, Y., Qin, H., Kulkarni, A., Kim, T., Lee, H.: Highly sensitive and selective gas sensor using hydrophilic and hydrophobic graphenes. Sci. Rep. 3, 1868 (2013)
Aziz, A., Lim, H.N., Girei, S.H., Yaacob, M.H., Mahdi, M.A., Huang, N.M., Pandikumar, A.: Silver/graphene nanocomposite-modified optical fiber sensor platform for ethanol detection in water medium. Sens. Actuators B: Chem. 206, 119–125 (2015)
Khun Khun, K., Mahajan, A., Bedi, R.K.: SnO thick films for room temperature gas sensing applications. J. Appl. Phys. 106(12), 124509 (2009)
Chang, Y., Yao, Y., Wang, B., Luo, H., Li, T., Zhi, L.: Reduced graphene oxide mediated SnO2 nanocrystals for enhanced gas-sensing properties. J. Mater. Sci. Technol. 29(2), 157–160 (2013)
Acknowledgments
The authors would like to acknowledge Universiti Putra Malaysia for the project fund under Research University Grant Scheme (RUGS) No. 05-02-12-2015RU and 05-02-12-1882RU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Girei, S.H., Shabaneh, A.A., Ngee-Lim, H. et al. Tapered optical fiber coated with graphene based nanomaterials for measurement of ethanol concentrations in water. Opt Rev 22, 385–392 (2015). https://doi.org/10.1007/s10043-015-0075-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10043-015-0075-8