Abstract
High and variable levels of salinity were investigated in an intermittent stream in a high-rainfall area (∼800 mm/year) of the Mt. Lofty Ranges of South Australia. The groundwater system was found to have a local, upslope saline lens, referred to here as a groundwater salinity ‘hotspot’. Environmental tracer analyses (δ18O, δ2H, 87/86Sr, and major elements) of water from the intermittent stream, a nearby permanent stream, shallow and deep groundwater, and soil-water/runoff demonstrate seasonal groundwater input of very saline composition into the intermittent stream. This input results in large salinity increases of the stream water because the winter wet-season stream flow decreases during spring in this Mediterranean climate. Furthermore, strontium and water isotope analyses demonstrate: (1) the upslope-saline-groundwater zone (hotspot) mixes with the dominant groundwater system, (2) the intermittent-stream water is a mixture of soil-water/runoff and the upslope saline groundwater, and (3) the upslope-saline-groundwater zone results from the flushing of unsaturated-zone salts from the thick clayey regolith and soil which overlie the metamorphosed shale bedrock. The preferred theory on the origin of the upslope-saline-groundwater hotspot is land clearing of native deep-rooted woodland, followed by flushing of accumulated salts from the unsaturated zone due to increased recharge. This cause of elevated groundwater and surface-water salinity, if correct, could be widespread in Mt. Lofty Ranges areas, as well as other climatically and geologically similar areas with comparable hydrogeologic conditions.
Résumé
Des niveaux élevés et variables de salinité ont été étudiés dans un cours d’eau intermittent dans une zone à forte pluviométrie (∼800 mm/an) des chaînes montagneuses du Mont Lofty dans le Sud de l’Australie. On a constaté que le système des eaux souterraines possédait une lentille saline locale, inclinée vers le haut, appelée ici « hotspot » de salinité des eaux souterraines. Des analyses des traceurs environnementaux (δ18O, δ2H, 87/86Sr, et ions majeurs) de l’eau du cours d’eau intermittent, d’un cours d’eau permanent situé à proximité, des eaux souterraines de surface et profondes, et de l’eau de sol/eau de ruissellement sur le sol mettent en évidence un apport saisonnier des eaux souterraines d’une composition très saline au cours d’eau intermittent. Cet apport a pour conséquence d’importantes augmentations de la salinité dans le cours d’eau parce que le flux hivernal de saison humide du cours d’eau diminue au printemps dans ce climat méditerranéen. En outre, les analyses d’isotopes du strontium et de l’eau démontrent: (1) la zone d’eau souterraine saline ascendante (hotspot) se mélange au système d’eau souterraine dominant, (2) l’eau du cours d’eau intermittent est. un mélange d’eau de sol/ruissellement et d’eau souterraine salée ascendante, et (3) la zone ascendante d’eau souterraine salée résulte du lessivage des sels de la zone non saturée du régolithe argileux épais et du sol qui recouvrent le substrat rocheux de schiste métamorphique. La théorie préférée concernant l’origine de ce hotspot d’eau souterraine saline ascendante est. le défrichage des terrains indigènes boisés à racines profondes, suivi du lessivage des sels accumulés dans la zone non saturée en raison d’une recharge accrue. La cause de l’élévation des eaux souterraines et de la salinité des eaux de surface, si elle est. correcte, pourrait s’étendre dans les zones du Mont Lofty, ainsi que dans d’autres régions climatiques et géologiques similaires avec des conditions hydrogéologiques comparables.
Resumen
Se investigaron niveles altos y variables de salinidad en un arroyo intermitente en una zona de alta precipitación (∼ 800 mm/año) de los Mt. Lofty Ranges en Australia del Sur. Se encontró que el sistema de agua subterránea tenía una lente salina local, ascendente, a la que se hace referencia aquí como un “hotspot” de salinidad del agua subterránea. Los análisis de trazadores ambientales (δ18O, δ2H, 87/86Sr, y elementos mayoritarios) del agua de la corriente intermitente, una corriente permanente cercana, aguas subterráneas someras y profundas y el agua del suelo/escurrimiento demuestran la entrada estacional de agua salada en la corriente intermitente. Esta entrada da lugar a grandes aumentos en la salinidad del agua de la corriente porque el flujo de la corriente en la estación húmeda del invierno disminuye durante la primavera en este clima mediterráneo. Además, los análisis de isótopos de estroncio y agua demuestran: (1) la zona de agua subterránea salina (hotspot) se mezcla con el sistema de agua subterránea dominante, (2) el agua de flujo intermitente es una mezcla de agua de suelo/escurrimiento y la solución salina ascendente, y (3) la zona de agua subterránea salina ascendente resulta de la descarga de las sales de la zona no saturada del espeso regolito arcilloso y del suelo que se superponen a la roca base de esquistos metamórficos. La teoría preferida sobre el origen en la zona húmeda de agua subterránea salina ascendente es el desmonte de bosques autóctonos de raíces profundas, seguido por el lavado de sales acumuladas de la zona no saturada debido al aumento de la recarga. Esta causa de elevación del agua subterránea y la salinidad del agua superficial, si es correcta, podría extenderse a las áreas del Mt. Lofty Ranges, así como a otras áreas climáticamente y geológicamente similares con condiciones hidrogeológicas comparables.
摘要
调查了澳大利亚Lofty Ranges山脉强降雨区(800 mm/year)一个间歇河的很高的并且不断变化的含盐度水平。发现地下水系统具有局部的、向上的含盐透镜体,这里称为地下水含盐度“热点区”。间歇河水、附近的永久河水、浅层和深层地下水以及土壤水/径流的环境示踪剂分析((δ18O、 δ2H、 87/86Sr及主要元素)证明,有非常咸的组分的地下水进入间歇河。由于春季期间在地中海气候条件下,冬季雨季河流量降低,地下水流入间歇河导致河水的含盐量大幅增加。此外,锶和水同位素分析显示:(1)向上趋势的地下水咸水带(热点区)与占主导的地下水系统混合;(2)间歇河水是土壤水/径流和盐度向上的地下水的混合水;(3)盐度向上的地下咸水带是覆盖变质页岩基岩的厚层黏土风化层中非饱和带盐类的冲刷造成的。盐度向上的地下咸水热点区成因的首选理论就是本地根深蒂固的林地开荒,其次就是由于补给增加致使非饱和带积累的盐分受到冲刷。如果地下水和地表水的盐度增加的这个原因正确的话,那就可能在Lofty Ranges山脉地区以及具有可比较的水文地质条件的气候上和地质上类似的区域非常普遍。
Resumo
Níveis altos e variáveis de salinidade foram investigados em um fluxo intermitente em área com muita pluviosidade (∼800 mm/ano) nas Cadeias do Monte Lofty no Sul da Austrália. Foi descoberto que o sistema de águas subterrâneas apresentava no gradiente superior do declive, lentes salinas, referido aqui como ponto crítico salinidade das águas subterrâneas. Análises de traçadores ambientais de água (δ18O, δ2H, 87/86Sr, e elementos maiores) do fluxo intermitente, do fluxo permanente próximo, águas subterrâneas rasas e profundas, e escoamento de água/solo demonstram entradas de águas subterrâneas de composição muito salina no fluxo intermitente. Essa entrada resulta em grandes aumentos de salinidade na água do fluxo porque o fluxo da estação-úmida do inverno diminui durante a primavera no clima mediterrâneo. Além disso, as análises de água e estrôncio demonstram: (1) a zona de águas subterrâneas na parte superior do declive (ponto crítico) se mistura com o sistema de águas subterrâneas dominante, (2) a água do fluxo intermitente é uma mistura do escoamento de água/solo e das águas subterrâneas salinas da parte superior do declive, e (3) a zona das águas subterrâneas salinas na parte superior do declive resultam das descargas de sal da zona não-saturada de um regolito argiloso grosso e solo que sobrepõe uma rocha base de xisto metamorfoseado. A teoria preferida para a origem do ponto crítico de águas subterrâneas salina na parte superior do declive é uma clareira de floresta nativa de raízes longas, seguida de descarga de sais acumulados da zona não-saturada pelo aumento da recarga. Essa causa de águas subterrâneas e águas superficiais de elevada salinidade, se correta, pode ser disseminada nas áreas das Cadeias do Monte Lofty, assim como em outras áreas similares geologicamente e em clima com condições hidrogeológicas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding the extent and cause of groundwater ‘salinity hotspots’ (>8,000 μS/cm) as well as assessing their impact, both present and future, on associated groundwater and surface-water systems, is of critical concern to overall water quality and resource management in sub-humid to dry areas—for example, saline groundwater input into the Murray River (Australia) has been documented, highlighted, and incorporated into management plans for overall river health (Allison et al. 1990; Jolly et al. 2001; Lamontagne et al. 2005). In the Eastern Mt. Lofty Ranges (MLR) of South Australia, areas of high-salinity surface water and groundwater have been spatially evaluated in terms of climatic and hydrogeologic factors (Poulsen et al. 2006). Poulsen et al. (2006) found groundwater salinity hotspots were associated with geologic units comprising marine shales and generally occurred in the drier eastern parts of the Mt. Lofty Ranges. In the study presented here, this hydrologic association between metamorphosed shale bedrock and high groundwater salinity is extended to a high-rainfall area of the Mt. Lofty Ranges. The example documented here extends Mt. Lofty Ranges groundwater understanding by connecting the hotspot directly to an intermittent stream by identifying seasonal groundwater input to the stream.
The occurrences of upslope-saline-groundwater zones in landscapes with otherwise good quality groundwater and moderate precipitation, as documented here, have not been characterised in detail in the scientific literature. They may be a common occurrence in areas such as the Mount Lofty Ranges (MLR) and elsewhere (Greeff 1994). The hydrogeologic parameters of the Scott Creek site described in this paper are representative of significant portions of the MLR (Taylor et al. 1974; Preiss 1987; Poulsen et al. 2006; Bestland et al. 2016). Similar groundwater salinity/chloride profiles (lenses of saline groundwater) have been noted in the Clare Valley, South Australia (Stewart 2005; Love et al. 2002), and the Burra Creek catchment, South Australia (Banks et al. 2007). However, the salinity of the upslope groundwater zone at the Scott Creek site is much greater than these other two examples. It may be the case that the comprehensive shallow-to-moderate depth piezometer nests at the Scott Creek site have allowed for the discovery of very saline water at the top of the aquifer. The documentation of this phenomenon will alert land managers to the existence of groundwater salinity hotspots in high-rainfall areas of the MLR and an understanding of their cause will aid in land management decisions.
The objectives of this study were to: (1) understand the interactions between the intermittent stream and the groundwater system including the salinity hotspot and the dominant groundwater system, and (2) identify the extent of the groundwater salinity hotspot and understand its cause. The study utilises environmental tracer data (δ18O, δ2H, 87/86Sr, and major elements) from the intermittent stream, a nearby permanent stream, soil-water/runoff, the shallow saline groundwater lens or hotspot, and the dominant groundwater system.
Study site
The Scott Bottom site in the Scott Creek catchment (SCC; Fig. 1) has been an experimental site for a number of hydrogeologic investigations (Chittleborough et al. 1992; Stevens et al. 1999; James-Smith and Harrington 2002; Harrington 2004a, b; Ranville et al. 2005; Banks et al. 2009; Bestland and Stainer 2013; Bestland et al. 2009, 2016). These studies document the soil hydrology of the dominant Xeralf (red brown earths) soils of the area as well as outlining the site’s hydrogeological heterogeneity and geological-geomorphic complexity. A series of soil-water studies (Chittleborough et al. 1992; Stevens et al. 1999; Bestland et al. 2009) documented throughflow hydrochemical characteristics. Banks et al. (2009) and Bestland and Stainer (2013) documented the importance of the fractured bedrock zone and deep clayey regolith for the groundwater/surface-water interactions in relation to the permanent stream Scott Creek. These groundwater studies built upon the investigations of James-Smith and Harrington (2002) and Harrington (2004a, b) that documented the basic hydrology and hydrogeology of the Scott Creek catchment.
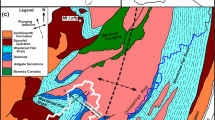
a–b Maps showing the location of South Australia and the Scott Creek catchment. c Scott Creek catchment geology (the box outlines the Scott Bottom study site shown in d). d Location of the nested piezometers sites A–F, Scott Creek and Sam’s Creek, and the distribution of soil types which follow closely the bedrock geology. Scott Creek in d and other streams in the Scott Creek catchment are depicted by solid blue lines. Intermittent streams in d are depicted with dashed blue line. The catchment boundary of Sam’s Creek is depicted with blue dashed line and is labelled. Blue areas are farm dams and a pond at the weir on Scott Creek. Elevation contour lines are in meters AHD
The Scott Creek catchment (Fig. 1) spans an area of 27 km2 and is located approximately 30 km south-east of Adelaide. Most of the aforementioned studies on groundwater/surface-water interactions within the Scott Creek catchment were focused on the perennial Scott Creek with the Scott Bottom area as a primary site (Fig. 2). The intermittent stream, which is the focus of this study and informally named Sam’s Creek, is a tributary to Scott Creek entering Scott Creek at the Scott Bottom site. Stream water in Scott Creek is sustained in the creek throughout the hot, dry summers via groundwater discharge. During severe drought conditions (e.g. as occurred in the summer of 2006–2007), however, the stream was reduced to a series of disconnected groundwater-fed pools (Kretchmer 2007). The salinity in Scott Creek during both low flow and high flow regimes, is lower than in Sam’s Creek. Salinities in Scott Creek vary from 500 μS/cm in winter to above 2,000 μS/cm during the low-flow period from January to April. The seasonal salinity variations were explained by evaporative enrichment and groundwater input during the summer contrasting with runoff dilution during the winter wet season (Harrington 2004b).
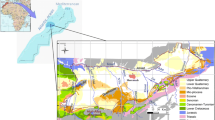
Aerial image from Google Earth (2015) of the Scott Bottom area showing surface-water sampling sites and piezometer nest locations, as well as cross-sections lines A and B which are shown in Fig. 10
Sam’s Creek drains a small catchment of 0.9 km2 which has 150 m of relief and several small farm dams, only one of which still fills with water (Fig. 1). When the stream flows during the winter wet season, it consists of a series of pools and small streamlets which flow for several months to over half the year depending on the year’s rainfall. Sam’s Creek was first observed to have substantial salinity variations during its 3-month flow in 2009. Salinity levels of Sam’s Creek contrast markedly and are much higher than the nearby ephemeral Mackreath Creek (Milgate 2007; Bestland et al. 2009; Pichler 2009). These investigations revealed Mackreath Creek to only flow after significant Autumn–Winter rainfall. It has very low salinity (200–500 μS/cm) and little salinity variation throughout its flow season. Preliminary data from 2009 suggested that the salinity in Sam’s Creek vastly exceeded the general variation of salinity in Scott Creek (Harrington 2004b; Cranswick 2005; Banks et al. 2009; Anderson 2013). This was the initial impetus of this investigation.
The Scott Creek catchment as well as most of the Mt. Lofty Ranges (MLR) has a Mediterranean climate with cool, moist winters and warm, dry summers. Average daily temperature ranges from 8 to 14 °C in winter, and 14 to 27 °C in summer. The MLR receives varying seasonal precipitation (almost all as rainfall) with yearly averages ranging from 400 to over 1,000 mm/year and with 85% of the rainfall occurring between May and September (BOM 2007). The large annual variability in rainfall in this area impacts the groundwater/surface-water systems as evidenced by the before-mentioned very low flow and disconnected flow during droughts in Scott Creek. Winter wet season recharge causes the water table to rise between 1 and 2 m (Banks et al. 2009). The Scott Bottom site has a rainfall collector at an elevation of 210 m above Australian Height Datum and an average annual rainfall of 804 mm/year (Bestland et al. 2009). The site’s closest Class A evaporation pan, Mt. Bold, has an average evaporation of 1,555 mm/year and therefore evaporation exceeds rainfall from October to May (spring, summer and autumn).
Geology and hydrogeology
Due to the geology of the Adelaide Geosyncline and the folding and faulting in the area, the geology of the catchment is complex but well understood (Preiss 1987; Banks et al. 2009; Bestland et al. 2016). It consists of various metamorphosed sedimentary formations of Late Precambrian age, including the Woolshed Flat Shale, Stonyfell Quartzite, Skillogalee Dolomite, Saddleworth Formation (including Glen Osmond Slate) and Emeroo Subgroup (including Aldgate Sandstone and Bungaree Quartzite), as well as the recent alluvial deposits (Drexel et al. 1993). Woolshed Flat Shale dominates the lithology of the Scott Bottom site (Banks et al. 2009).
A set of shallow to moderate depth piezometer nests were drilled in 2005 and their locations are shown in Fig. 2. The piezometer nests A–F enabled an analysis of the hydrogeological structure and flow dynamics of the site (Banks et al. 2009); details of the drilling are available in Cranswick (2005). In addition, six 2–3-m-deep backhoe trenches were excavated and sampled for soil and regolith hydrochemical properties (Bestland and Stainer 2013). The regolith is categorised by a soil zone and a deep saprolite (weathered bedrock) zone of up to 10–15 m (Cranswick 2005). Spatially variable fractured bedrock underlies this zone. The degree and depth of weathering of the saprolite is important in controlling groundwater flow and recharge. Banks et al. (2009) described groundwater flow in the catchment as occurring in three zones: the soil zone, saprolite zone and fractured bedrock zone. The vastly different hydraulic conductivities of each zone has resulted in large heterogeneity of the groundwater system (Harrington 2004a; Banks et al. 2009). The soil zone is characterised as a duplex soil with a sandy-silty A horizon (10–20 cm depth) over a clayey B horizon (Chittleborough et al. 1992).
The saprolite zone changes progressively from a very clayey poorly structured material to the composition of the unweathered bedrock (Cranswick 2005; Bestland and Stainer 2013). Banks et al. (2009) found that the hydraulic conductivity of this zone ranged from 0.04 to 2.5 m/day and was generally less than the hydraulic conductivity of the fractured bedrock zone. The unweathered bedrock (Woolshed Flat Shale) was a grey siliceous slate. Hydraulic conductivity here ranged from 1.5 to 14 m/day. Banks et al. (2009) also found the fracture density to be on average 0.21 m. The fractured-rock aquifer was determined to be the most active part of the dominant groundwater system with some valley-bottom deep bores being artesian.
Methods
At the Scott Bottom site, six shallow to moderate depth piezometer nests were installed in July 2005 in order to monitor the groundwater processes in the soil, saprolite and fractured-bedrock zones (Figs. 1 and 2; Banks et al. 2009). In addition, water samples were obtained from eight open pre-existing bores, installed in March–April 2002 and reaching up to 96 m in depth (James-Smith and Harrington 2002). The six nests are positioned along a transect perpendicular to the creek valley, on an inferred groundwater flow path covering a distance of about 330 m (Figs. 1 and 2). Nests A to F have depths varying from 1.5 to 28.5 m. The construction details of each piezometer are depicted in Banks et al. (2009). The ground elevation and piezometers were surveyed and the water table elevations were corrected to a standing water level relative to the Australian Height Datum (Cranswick 2005).
Stream and groundwater sampling
Water samples of Sam’s Creek at two locations (SMC1 and SMC 2) and the two piezometers from nest A (A1 and A2) were collected on a weekly basis for environmental tracer analysis (Fig. 2). Weekly sampling was undertaken to document the gradual salinity change in the creek during the change from winter wet season to spring and early summer (Fig. 3). The sampling period occurred from early August to late October 2012 when surface flow ceased in Sam’s Creek. A sample was taken, where possible, from each site. Grab samples of water were taken from the creek and farm dam, whereas a submersible 12 V battery pump was used to collect the groundwater samples.
A handheld YSI multi-parameter meter was used at each sample location to measure the pH, electrical conductivity (EC), dissolved oxygen (DO%), redox and temperature. A flow-through cell was utilised during groundwater measurements. Water samples were placed into clean, plastic and airtight sample bottles and filtered through a 0.45-μm filter in the laboratory a few hours after sampling. Samples for ICP analysis were acidified to a pH less than 2 using nitric acid (HNO3). Water samples were transferred the same day as sampling and stored in a 30-ml McCartney bottle for water isotope (2H/1H and 18O/16O) analysis. To prevent exchange of water vapour with the air, McCartney bottles were filled with no headspace and sealed with electrical tape.
For the analysis of the major and trace elements, 16 samples were sent for a 72-element Group 2C, inductively coupled plasma mass spectrometry analysis at ACME analytical laboratories (ACME Labs 2012), Vancouver, Canada. Analysis of 18O/16O and 2H/1H isotope ratios from water samples were done at the Stable Isotope Facility, University of California – Davis (UC Davis), USA, using a Laser Water Isotope Analyser V2. Final 18O /16O and 2H/1H values were reported relative to Vienna Standard Mean Ocean Water (VSMOW); methods are documented in UC Davis (2012). Four water samples were selected for the strontium isotope ratio (87Sr/86Sr) analysis. These included a sample from A1 and A2, and two from SMC1. The samples were analysed using a triton thermal ionisation mass spectrometer at the Australian National University. The international standard NBS-987 was used as an internal check for the analyses and values of 0.710249 were obtained with a reproducibility (1σ) of ±0.000003 (n = 5). In addition, an extensive hydrochemical database from the site was also used in this investigation (Milgate 2007; Banks et al. 2009; Bestland et al. 2009).
Results and discussion
Stream salinity hydrograph
The groundwater salinity throughout the extensive groundwater monitoring network located in the SCC is relatively fresh (Table 1); most bores show lower than 1,000 mg/L TDS (total dissolved solids). Deeper bores (greater than 40 m depth) have higher salinities (up to 3,000 mg/L); however, some bores in metamorphosed sandstone units have lower salinities (down to 200 mg/L; James-Smith and Harrington 2002; Banks et al. 2009). The upslope-saline-groundwater zone represented by piezometers A1 and A2 stand out in stark contrast to the rest of the groundwater system in the area because of their high salinities. Amongst the 50 wells in total at the Scott Bottom site, the A1 and A2 piezometers are the only ones that access the salinity hotspot. Salinity levels in A1 and A2 piezometers exceed 6,000 and 15,000 mg/L TDS respectively (Banks et al. 2009).
Sam’s Creek has a large salinity increase during the waning winter wet flow (Fig. 3). There is a corresponding seasonal salinity increase in groundwater from the A1 and A2 piezometers. Surface-water salinities increase to approximately 2,500 mg/L TDS in the spring before flow ceases. The groundwater samples have the highest TDS values averaging 5,587 mg/L (A1) and 13,705 mg/L (A2). The A2 piezometer is 5 m more shallow than piezometer A1 and accesses the more saline upper part of this high-salinity groundwater zone.
There is a large salinity increase from upstream to downstream in Sam’s Creek. The farthest upstream sample site (Farm Dam) showed the lowest salinity at 105 mg/L TDS on average, which is 1.4 and 0.6% of the average salinity of the groundwater in the A1 and A2 piezometers respectively.
Early in the winter wet season, soon after commencement of flow within Sam’s Creek, water samples collected from midstream and downstream of Sam’s Creek had low salinity. Salinity levels remained comparatively low up until the middle of September. Towards the end of the period of flow, the downstream salinity levels increased significantly, while there was no significant change in the salinity of the Farm Dam water. The discovery of this large surface-water salinity increase in what is an otherwise fresh runoff area was the impetus of this study.
Chloride and elemental molar ratios
Major ion to chloride ratios are presented in Fig. 4 and provide a tool to assess the source of salinity within the groundwater and surface waters within the study area. The majority of the lowest salinity groundwater samples exhibit ratios which range from that of local rainfall to ratios of 1, potentially indicating different degrees of dissolution of vadose-zone salts during recharge; nevertheless, all groundwaters remain highly undersaturated with respect to halite. A few fresh groundwater samples show Na+/Cl− ratios as high as 1.25, indicating the addition of Na+ due to water–rock interaction, e.g. silicate weathering reactions; the latter would be consistent with the elevated Si/Cl− concentrations of these groundwater samples. In contrast, the saline groundwater portions of the aquifer (A1 and A2) show Na+/Cl− ratios below that of rainfall and suggest the preferential removal of Na+ from solution, whereby the latter is likely to reflect alterations of the groundwater chemistry due to ion exchange, i.e. the adsorption of Na+ by clays, a common process in salinized areas (Cartwright et al. 2004). Sam’s Creek water appears to lie on a mixing line between ratios indicative of rainfall and the saline, upslope groundwater of piezometers A1 and A2.
Composite diagrams of major elements (Ca2+, Na+, Si) to chloride ratios versus chloride concentration of surface water and groundwater. Samples were collected between July 2005 and October 2012 at Scott Bottom. Data sourced from Cranswick (2005), Milgate (2007), Banks et al. (2009) and this study. Rainfall ratios are from Bestland et al. (2017)
Dissolution of calcite, along with weathering of feldspars, can explain the elevated Ca2+/Cl− ratios in the fresh groundwater, while a decrease in Ca2+/Cl− ratios with increasing salinity may reflect precipitation of calcite out of the high-ionic-strength waters found up slope. This is consistent with groundwater samples in A1 and A2 being above calcite saturation.
Groundwater samples exhibit homogenous molar Cl−/Br− ratios (ranging between 580 and 750) that are consistent with longer residence times that allow extensive groundwater mixing which homogenises the groundwater chemistry. The relative invariance of Cl−/Br− ratios with increasing Cl− concentrations provide evidence of groundwater mixing between the saline groundwater lens and the dominant groundwater system.
Overall, the major ion/Cl− ratios and Cl−/Br− ratios exhibit distinct contrasts between the compositions of Sam’s Creek water, saline groundwater at locations A1 and A2, and that of the dominant groundwater system. These differences were broadly consistent over several years of sampling. However, seasonal differences in ion/Cl− ratios for Sam’s Creek are observed (Fig. 4). Following initiation of stream flow, the creek water had ratios close to those of the Farm Dam water, which is rainfall/soil-runoff derived. Major ion ratios (Na+/Cl−, Ca2+/Cl−, Si/Cl−) in the creek attained the ratios of the saline groundwater later in the season when the creek had nearly ceased flowing. The stream salinity was still, however, only 33% of A2 in the final sample collected from Sam’s Creek. If evapotranspiration played a major role in seasonal changes in stream water chemistry, ion/Cl ratios should have remained constant, opposite to what was observed. Overall, the hydrochemical signatures indicate stream water to be mainly rainfall-runoff derived during high flows. As the water table rises during the wet season, and saline groundwater discharges into the stream, mixing and dilution with runoff occurs. During the waning stream flow, the stream consists predominantly of groundwater.
This investigation supported the findings from Poulsen et al. (2006) who determined that areas of elevated stream salinity were correlated to areas of shallow groundwater where they provide base-flow to the streams in the Eastern MLR. This study was also consistent with that of Banks et al. (2009) on Scott Creek. Scott Creek was shown to be composed of a mixture of groundwater, soil water, and surface-water runoff. The composition of Scott Creek revealed that most dissolved ions were derived from water–rock interactions in the subsurface (especially Ca2+, Mg2+, HCO3 −) as well as salts deposited in rainfall (i.e. Na+ and Cl−; Banks et al. 2009). The similarity of the concentrations of major ions (Ca2+, Mg2+, Na+, Cl− and HCO3 −) led Banks et al. (2009) to suggest that the groundwater was relatively well mixed within the fractured bedrock.
Banks et al. (2009) established that the vertical hydraulic gradient between the A1 and A2 piezometers was in a downward direction. CFC-11 (trichlorofluoromethane, CFCl3) and CFC-12 (dichlorodifluoromethane, CF2Cl2) analyses suggest shorter residence time for the groundwater upslope compared to that of the discharge zone in the valley (Banks et al. 2009). Thus, the salinity variation between upslope down-gradient groundwater and valley-bottom up-gradient groundwater is not due to processes of chemical weathering along a groundwater flow path.
Stable isotopes of water
The δ18O and δ2H composition of groundwater samples plot close to the local meteoric water line (LMWL) for Adelaide (Hughes and Crawford 2012; Kayaalp 2001), indicating that these waters are little altered from their meteoric origin and not significantly impacted by unsaturated-zone evaporation or open-water evaporation before infiltration (Figs. 5 and 6; Table 2). Shallow groundwater thereby displays the largest range of δ18O values, reflecting the variable extent of local evaporation as well as the local variability of the δ18O values of different rainfall. Sam’s Creek water samples also plot close to the LMWLs indicating only minor influence of evaporation. Surface waters are, however, enriched compared to most groundwater samples, with upstream creek waters (Sam’s Creek 2) being more enriched than downstream samples. Sam’s Creek waters cluster near the most saline groundwater samples (piezometer A2). These are enriched in δ18O by about 0.5‰ compared to both A1 and other shallow groundwater and up to about 1.5‰ compared to the main groundwater body within the SCC.
A comparison of δ18O and δ2H in relation to chloride concentrations confirms the subdued role of evaporation in altering the chemistry of surface water and groundwater in the SCC (Fig. 7). An increase in salinity is not accompanied by a trend towards heavier isotopic compositions. Instead, as salinity increases, surface-water and groundwater samples show little variation in their isotopic signature.
Age dating by Banks et al. (2009) based on CFC-12 and CFC-13 showed the apparent age of upslope groundwater (A1 and A2) to be less than that of deeper groundwater within the main groundwater body of the SCC. Similarly, Radon-222 data by Cranswick (2005) suggest that water from deeper piezometers, despite lower salinities than the upslope groundwater, have longer residence times.
Groundwater from piezometer A2 (shallowest) was more enriched than groundwater from piezometer A1 and indicates either greater evaporation of A2 groundwater or mixing with the dominant groundwater system resulting in lower salinity for A1. Stream water from the downstream site (Sam’s Creek 1) is more depleted than the upstream site (Sam’s Creek 2) suggesting that there is greater groundwater input downstream in Sam’s Creek.
A seasonal trend of isotopic enrichment in Sam’s Creek is observed (Fig. 6). As the wet season progresses, several samples taken between the start of the wet season and cessation of creek water flow show Sam’s Creek to progressively attain an isotopic composition close to that of the upslope-saline-groundwater (Fig. 6). This lends further support to the mixing model already outlined, where discharge of saline upslope groundwater and soil-water/runoff mix in Sam’s Creek. As rainfall and runoff ceases towards the end of the wet season, the proportion of groundwater input to the creek subsequently rises and creek water attains an isotopically more enriched signature.
Surface-water samples collected on 14 September 2012 had more positive water isotope values than most other surface-water samples (Fig. 6). This enrichment is probably due to a rainfall event of 25 mm on 11 September 2012. The water isotope values in the stream sites after this rainfall were more enriched and, thus, support the interpretation of an enriched rainfall event. In nearby Mackreath Creek catchment, Bestland et al. (2009) documented that lower than normal oxygen and hydrogen isotope ratios (more enriched) of a large precipitation event could be used as a natural tracer. The analysis of stable isotopes of water provides further evidence that the surface water in Sam’s Creek is a mixture of runoff water and the upslope-saline-groundwater zone.
The waters’ stable isotopic results depicting upslope-saline-groundwater input to Sam’s Creek are comparable to a study by Meredith et al. (2013) albeit on different scales. In their study, the stable isotopes of water were used to determine the influx of saline groundwater to the Darling River, New South Wales, Australia. During times of groundwater discharge, the isotopic signature was depleted and high salinities were evident. This occurred in low to zero flow conditions similar to the conditions where the salinity increase occurred in Sam’s Creek.
The results from the water isotopic signature versus chloride (Fig. 7) suggest that there is little if any evaporation that causes the salinity variation in Sam’s Creek nor the groundwater in the A1 and A2 piezometers. There were no evaporative trends significant enough to infer that the upslope-saline-groundwater system originated from evaporative enrichment. A much more likely explanation for the increased salinity could, however, be the concentration of salts in the unsaturated zone by transpiration. Transpiration influences the salinity of water; however, it does little to alter the isotopic composition of water (Zimmerman et al. 1967; Forstel 1982). Salt accumulation in the upslope-saline-groundwater zone could therefore be caused by transpiration by plants from the soil and groundwater; during recharge events, salts in the unsaturated zone can be flushed into the groundwater. This would be consistent with a study by Poulsen et al. (2006) who established transpiration to be the primary cause of elevated salinities in the Eastern MLR.
A study of the Eastern MRL by Green and Stewart (2008) revealed comparatively much greater levels of evaporative enrichment, indicating that the level in the study presented here was not substantial. Despite Sam’s Creek being constantly exposed to the atmosphere and thus subjected to evaporative processes, evaporative enrichment is insignificant.
Sam’s Creek showed little change in deuterium excess with change in δ18O‰ (Fig. 8). The average was 14.89‰ with a standard deviation of 0.41. The runoff had a slightly lower excess at 13.82‰ on average with the greatest standard deviation of 5.39‰. The deep groundwater had the highest deuterium excess at 16.65‰ on average. The A1 and A2 piezometers displayed a trend of decreasing deuterium excess with increased δ18O‰.
Deuterium excess was used to further distinguish major water components (Fig. 8). Orographic features affect the distribution of the stable isotopes of water in rain (Dansgaard 1964) including deuterium excess, and most likely contribute to the variation in isotopic ratios observed in this study. In hilly terrains such as the MLR, the fractionation of rainfall and that of the associated groundwater vary with altitude (Guan et al. 2013). Rain becomes isotopically lighter with altitude on the windward slope. This was determined to be due to progressive rain-out processes (Guan et al. 2009). At lower elevations, rainfall and corresponding groundwater is isotopically heavier due to sub-cloud evaporation and moisture exchanged with the surrounding air (Guan et al. 2009); therefore, the water from the deep piezometers could have originated from a higher altitude than the dominant groundwater system in the SCC. This also suggested that the saline water that discharged into Sam’s Creek was from a similar altitude to the A1 and A2 piezometers.
Strontium isotopes
Strontium isotopic ratios and concentrations from this site were obtained for soil water, stream water from Sam’s Creek, stream water from Scott Creek, and groundwater from the shallow and deep part of the system (Fig. 9; Table 3). Strontium isotopes do not undergo significant mass fractionation during hydrologic processes regardless of temperature, chemical speciation or biological processes (Capo et al. 1998; Kendall and McDonnell 1998). Strontium originating from mineral weathering reactions has a 87Sr/86Sr ratio ranging between the minerals with which it is interacting and the strontium contained in precipitation. In most hydrogeologic settings, the composition of the host rock along the groundwater flow path generally dominates strontium isotopic ratios of groundwater (Kendall and McDonnell 1998; Harrington and Herczeg 2003). Atmospheric origins of strontium are dominated by marine sources especially in coastal settings such as the MLR. The 87Sr/86Sr ratio range used for precipitation in this study, of 0.7093 to 0.7107, is based on a transect of strontium isotope analyses of precipitation from the coast and then inland in the nearby state of Victoria (Raiber et al. 2009); four stations, from the coast to 100 km inland, were used to obtain the range shown in Fig. 9.
Variation of strontium isotope ratios with inverse strontium concentration. Both groundwater and surface-water samples at the Scott Bottom experimental site from 2005 to 2012 are plotted. Data sourced from Cranswick (2005); Milgate (2007); Banks (2011). Precipitation range from Raiber et al. (2009) using four stations 100 km or less from the nearby coast of the state of Victoria
The strontium isotopic ratios of the upslope-saline-groundwater zone are lower than the dominant groundwater system ranging from 0.7144 to 0.7336 compared to the dominant groundwater system which ranges from 0.7355 to 0.7503 (Fig. 9). The upslope-saline-groundwater samples, especially from piezometer A2, are distinguished by their relatively low isotopic ratios and high strontium concentrations. Soil water collected at this site and analysed by Milgate (2007) is characterised by low strontium concentrations and low strontium isotopic ratios (Fig. 9). Three relationships can be concluded from the strontium isotopic data. The mixing lines in Fig. 9 indicate: (1) mixing between the upslope-saline-groundwater zone (shallow, more saline piezometer A2) and the dominant groundwater system to produce the compositions from piezometer A1; (2) mixing between the upslope-saline-groundwater zone and the soil-water/runoff to produce the mixing line of Sam’s Creek; and (3) mixing of the dominant groundwater system and soil water to produce the mixing line of Scott Creek.
A simple mixing model was utilised whereby water from the very top of the perched saline groundwater mixed with water from the dominant groundwater system to produce the water in the lower part of the perched groundwater (Fig. 10). Strontium isotope ratios were used to quantify the relative proportions of strontium that contributed to the mixed lower part of the perched saline groundwater represented by water from piezometer A1 (Eq. 1). Strontium isotope ratios from the upper saline groundwater (piezometer A2) and the dominant groundwater system (other piezometers of moderate and deeper depth) were averaged and the three isotope ratios were used in Eq. (2) to calculate the fraction of each of the two end members.
Cross-section conceptual models of groundwater salinity at the Scott Bottom experimental site. Locations of the cross sections are illustrated in Fig. 2: a line A, b line B. The blue dashed lines in cross-section A are based on hydraulic heads in the piezometer nests. Transient flushing of accumulated salts in the thick clayey unsaturated zone, following clearing of woodland, is the preferred interpretation for the cause of the saline groundwater lens
The 87Sr/86Sr of a mixture of two end members 1 and 2 is:
where \( {M}_1^{\mathrm{Sr}} \)and \( {M}_2^{\mathrm{Sr}} \)are the masses of strontium from end members 1 and 2. End member 1 is water from piezometer A2 and end member 2 represents the average masses of strontium of samples from the dominant groundwater system. The term (87Sr/86Sr) mix is the 87Sr/86Sr ratio of the mixture, which in this case is water from piezometer A1. Rearranged, this formula gives: the fraction of end member 1; (A2) in relation to end member 2; and (dominant groundwater system) in the mixture observed in A1 (modified from Stewart et al. 1998; Green et al. 2004):
From this equation, the percentage of the dominant groundwater system represented in the mixture of groundwater in A1 was calculated at 46%. The remaining 54% is contributed by the more saline groundwater of A2.
Mixing of soil-water/runoff with upslope perched saline groundwater, as previously outlined, can be evaluated with strontium isotopes (Fig. 9). The three data points form a mixing line, with one data point indicating close to 100% soil water (high-flow sample) and one data point indicating close to 100% groundwater (low-flow sample). The seemingly wide range of strontium concentrations for soil water in Fig. 9 is misleading; strontium concentrations of these samples range from 0.024 to 0.090 ppm. Nonetheless, evaporation of soil water has produced this range of concentrations with no change in isotopic ratio.
Conceptual model
The conceptual model relies on the specific hydrogeology of this site (Fig. 10). The regolith-saprolite zone of the study site consists of heavy clay that has been weathered from the metamorphosed shale bedrock. This zone forms a thick clay layer over much of the Scott Bottom site (Banks et al. 2009; Bestland et al. 2016). Such clayey zones have been shown to restrict infiltration and cause salinity issues due to poor drainage. Cartwright et al. (2013) demonstrated that shallow saline groundwater can occur where the geology and porosity of the soil restricts drainage. These zones can have very low permeability and slow infiltration rates that hinder the flushing of salts (Bennetts et al. 2007; Rengasamy 2006). Shallow groundwater such as this can be prone to direct evaporation through capillary action (Turner et al. 1987; Salama et al. 1999; Lamontagne et al. 2005). This causes salts to accumulate in the unsaturated zone, especially near the evaporation front, which can cause significant increases in salinity (Barnes and Allison 1988). At the Scott Bottom site, the clayey soil and saprolite along with the thickness of this zone are thought to be a critical factor in the formation of the upslope-saline-groundwater zone.
Based on the hydrochemical data presented here and the hydrogeologic context of this site, the following conceptual model is presented. Salts have accumulated in the thick, clayey unsaturated zone due to low rates of infiltration and recharge. These salts (dissolved solids) are sourced primarily from precipitation as indicated by strontium isotopes, but with a component of soil-saprolite weathering input. The salts accumulated over some unknown time span in the unsaturated zone. Evapotranspiration from woodland vegetation combined with the thick low-permeability unsaturated zone would have led to very low rates of recharge. During heavy rainfall events, some recharge would have flushed a portion of the accumulated salts to the water table. Following clearing of much of the woodland vegetation and its replacement with short-rooted pasture vegetation, an increase in infiltration and recharge is envisioned. The flushing of accumulated salts would then have formed the upslope-saline-groundwater zone. The presence and/or extent of an upslope-saline-groundwater zone prior to land clearing are unknown. It is thought that this flushing of accumulated salts is a transient process. Further, as recharge increased and the water table rose, saline groundwater would have discharged into Sam’s Creek. The upslope-saline-groundwater zone and its discharge into Sam’s Creek are thought to be transient features because (1) the strong density inversion of the saline groundwater zone above the dominant groundwater system would not be stable over time, and (2) there is a lack of salt scalds in the valley bottom. Thus, this process has not reached equilibrium with the dominate water system nor has it caused salt to accumulate in downslope areas where the water table is shallow.
Most Australian native vegetation is efficient in utilising infiltrating rainfall (Allison and Hughes 1983; Schofield 1992). Following land clearing of native woodland over 100 years ago and the expansion of short-rooted pasture grasses, increased recharge would be expected and could result in raising the water table and flushing accumulated salts. With increased recharge, steady-state salt movement through soil-regolith should result in a decrease in groundwater salinity. A pronounced shallow-groundwater-salinity decrease, widespread in the Clare Valley area of South Australia, has been interpreted as being caused by land clearing of woodland and increased recharge resulting in freshening of the shallow groundwater (Love et al. 2002; Stewart 2005; Bestland et al. 2017).
The origin of the accumulated salts in the unsaturated zone is analogous to the common Australian feature of dryland salinity. Prior to European settlement, groundwater salinity concentrations in Australia were in long-term equilibrium. In agricultural regions, settlers cleared most of the native vegetation and replaced it with annual crop and pasture species. This vegetation allowed a larger proportion of rainfall to recharge to the groundwater (George et al. 1997; Walker et al. 1999). As a result, water tables have risen, often to the point where evaporation from shallow groundwater has led to salt accumulation and salt scalds as well as input of salty water into surface waters (Earl 1988; Evans 1994).
At the Scott Bottom site, analysis of the stable isotopes of water indicates that transpiration was the dominant process for the accumulated salts. It is thought that the woodlands that previously dominated the area caused this transpiration. The subsequent removal of this natural transpiration pump due to deforestation could have caused the water table to rise. This conceptual model is supported by a study in the Clare Valley, South Australia (Love et al. 2002), which investigated vertical groundwater salinity variations. In that study, it was shown that much of the chloride present within the groundwater represented residual chloride which predated clearing of the native vegetation. Removal of native vegetation within the last 100 years was postulated to have caused recharge rates to have increased. The leaching of chloride was proposed as being regulated by the rate of diffusion of salts into fractures. If the fractures were widely spaced it would take a considerably long time for the salts to leach. The areal extent of the saline groundwater in the wider area is currently unknown and to answer this question additional bores, covering a more comprehensive section of the landscape need to be drilled.
Conclusions
Many of the concepts outlined in this paper are scarcely documented in scientific literature. Intermittent streams are not commonly sampled due to their transitory flow. Upslope-saline-groundwater zones in hilly landscapes could be widespread in areas such as the MLR. The study here has documented the connection between an intermittent creek and an upslope-saline-groundwater zone. The probable mechanism for the late season salinity increase in Sam’s Creek was determined to be the water-table rise of the underlying saline groundwater during wet season recharge. This study was undertaken to better understand the interactions between stream flow, rainfall, groundwater and land-use changes, with a view to better understanding the sustainability of these water resources.
The results presented here document saline groundwater input into the intermittent Sam’s Creek. The saline groundwater, accessed by two piezometers (A1 and A2), is thought to be caused by flushing of accumulated salts following clearing of native woodland and subsequent increased recharge. The origin of the upslope-saline-groundwater zone is thought to be caused by a combination of the thick clayey soil-saprolite zone and the clearing of woodland. The low permeability of the clay soil and saprolite causes low infiltration and recharge and the accumulation of salts from evapotranspiration, as demonstrated by water isotope analysis. This situation, including the upslope-saline-groundwater zone, is thought to be transient. Because the thick clayey soil and saprolite zone extends over large areas of the MLR, these upslope-saline-groundwater zones could occur on a much broader scale.
References
ACME Labs (2012) ACME analytical laboratories: pricing brochure. acmelab.com/pdfs/Acme_Price_Brochure.pdf. Accessed 12 July 2012
Allison G, Hughes M (1983) The use of natural tracers as indicators of soil-water movement in a temperate semi-arid region. J Hydrol 60(1–4):157–173
Allison G, Cook P, Barnett S, Walker G, Jolly I, Hughes M (1990) Land clearance and river salinisation in the western Murray Basin, Australia. J Hydrol 119(1–4):1–20
Anderson TA (2013) Origin of high salinity water in an ephemeral stream, Scott Creek, Mount Lofty Ranges. Honours Thesis, Flinders University, Adelaide, South Australia
Banks EW (2011) Hydrogeological and hydroclimatic controls on surface water-groundwater interactions. PhD Thesis, Flinders University, Adelaide, South Australia
Banks EW, Wilson T, Green G, Love A (2007) Groundwater recharge investigations in the Eastern Mount Lofty Ranges, South Australia. DWLBC Rep 20, Dept. of Water, Land and Biodiversity Conservation, Adelaide, South Australia, 105 pp
Banks EW, Simmons C, Cranswick R, Love A, Werner A, Bestland E, Wood M, Wilson T (2009) Fractured bedrock and saprolite hydrogeologic controls on groundwater/surface-water interaction: a conceptual model (Australia). J Hydrogeol 17(8):1969–1989
Barnes C, Allison G (1988) Tracing of water movement in the unsaturated zone using stable isotopes of hydrogen and oxygen. J Hydrol 100(1):143–176
Bennetts DA, Webb JA, McCaskill M, Zollinger R (2007) Dryland salinity processes within the discharge zone of a local groundwater system, southeastern Australia. Hydrogeol J 15(6):1197–1210
Bestland EA, Stainer G (2013) Down-slope change in soil hydrogeochemistry due to seasonal water table rise: implications for groundwater weathering. Catena 111:122–131
Bestland EA, Milgate S, Chittleborough D, Vanleeuwen J, Pichler M, Soloninka L (2009) The significance and lag-time of deep through flow: an example from a small, ephemeral catchment with contrasting soil types in the Adelaide Hills, South Australia. Hydrol Earth Syst Sci 13:1–14
Bestland EA, Liccioli C, Soloninka L, Chittleborough DJ, Fink D (2016) Catchment-scale denudation and chemical erosion rates determined from 10 be and mass balance geochemistry (Mt. Lofty Ranges of South Australia). Geomorphology 270:40–54
Bestland E, George A, Green G, Olifent V, Mackay D, Whalen M (2017) Groundwater dependent pools in seasonal and permanent streams in the Clare Valley of South Australia. J Hydrol Reg Stud 9:216–235
BOM (2007) Climate statistics for Australian sites Bureau of Meteorology. http://www.bom.gov.au/climate/averages/tables/ca_vic_nos.shtml. Accessed 22 September 2016
Capo RC, Stewart BW, Chadwick OA (1998) Strontium isotopes as tracers of ecosystem processes: theory and methods. Geoderma 82(1):197–225
Cartwright I, Weaver TR, Fulton S, Nichol C, Reid M, Cheng X (2004) Hydrogeochemical and isotopic constraints on the origins of dryland salinity, Murray Basin, Victoria, Australia. Appl Geochem 19(8):233–1254
Cartwright I, Gilfedder B, Hofmann H (2013) Chloride imbalance in a catchment undergoing hydrological change: Upper Barwon River, southeast Australia. Appl Geochem 31:187–198
Chittleborough D, Smettem K, Cotsaris E, Leaney F (1992) Seasonal changes in pathways of dissolved organic carbon through a hillslope soil (Xeralf) with contrasting texture. Soil Res 30(4):465–476
Cranswick R (2005) Hillslope scale geological controls on surface water–groundwater interaction: evidence of active recharge to a fractured rock aquifer. Honours Thesis, Flinders University, Adelaide, Australia
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16(4):436–468
Drexel JF, Preiss WV, Parker A (1993) The geology of South Australia: the Precambrian. Mines and Energy, South Australia, Geological Survey of South Australia, Adelaide, Australia, 54 pp
Earl G (1988) Stream salinities and salt loads in the Goulburn and Broken River catchments. Rural Water Commission of Victoria, Maffra, Australia
Evans W (1994) Regional salt balances and implications for dryland salinity management. Water Down Under 94: Groundwater papers; Preprints of papers, Engineers Australia. Barton, Australia, pp 349–354
Forstel H (1982) 18O/16O ratio of water in plants and in their environment. In: Schmidt H, Forstel H, Heizinger K (eds) Stable isotopes. Elsevier, Amsterdam, pp 503–509
George R, McFarlane D, Nulsen B (1997) Salinity threatens the viability of agriculture and ecosystems in Western Australia. Hydrogeol J 5(1):6–21
Greeff G (1994) Ground-water contribution to stream salinity in a shale catchment, RSA. Ground Water 32(1):63–70
Green G, Bestland EA, Walker GS (2004) Distinguishing sources of base cations in irrigated and natural soils: evidence from strontium isotopes. Biogeochemistry 68:199-225
Green G, Stewart S (2008) Interactions between groundwater and surface water systems in the Eastern Mount Lofty Ranges. Dept of Water, Land and Biodiversity Conservation, Adelaide, South Australia
Guan H, Simmons CT, Love AJ (2009) Orographic controls on rain water isotope distribution in the Mount Lofty Ranges of South Australia. J Hydrol 374(3):255–264
Guan H, Zhang X, Skrzypek G, Sun Z, Xu X (2013) Deuterium excess variations of rainfall events in a coastal area of South Australia and its relationship with synoptic weather systems and atmospheric moisture sources. J Geophys Res Atmos 118(2):1123–1138
Harrington G (2004a) Hydrogeological Investigation of the Mount Lofty Ranges, Progress Report 3: borehole water and formation characteristics at the Scott Bottom research site, Scott Creek Catchment. Report DWLBC 2004/03, Dept. of Water, Land and Biodiversity Conservation, Adelaide, South Australia
Harrington G (2004b) Hydrogeological Investigation of the Mount Lofty Ranges, Progress Report 4: groundwater–surface water interactions in the Scott Creek, Marne River and Tookayerta Creek catchments. Report DWLBC 2004/03, Dept. of Water, Land and Biodiversity Conservation, Adelaide, South Australia
Harrington GA, Herczeg AL (2003) The importance of silicate weathering of a sedimentary aquifer in arid central Australia indicated by very high 87Sr/86Sr ratios. Chem Geol 199:281–292
Hughes CE, Crawford J (2012) A new precipitation weighted method for determining the meteoric water line for hydrological applications demonstrated using Australian and global GNIP data. J Hydrol 464:344–351
James-Smith J, Harrington G (2002) Hydrogeological Investigation of the Mount Lofty Ranges, Progress Report 1: hydrogeology and drilling phase 1 for Scott Creek Catchment, Dept. for Water, Land and Biodiversity Conservation, Adelaide, South Australia
Jolly I, Williamson D, Gilfedder M, Walker G, Morton R, Robinson G, Jones H, Zhang L, Dowling T, Dyce P (2001) Historical stream salinity trends and catchment salt balances in the Murray-Darling Basin, Australia. Mar Freshw Res 52(1):53–63
Kayaalp A (2001) Application of rainfall chemistry and isotope data to hydro-meteorological modelling. PhD Thesis, Flinders University, Adelaide, South Australia
Kendall C, McDonnell J (1998) Isotope tracers in catchment hydrology. Elsevier, Amsterdam
Kretchmer P (2007) Determining the contribution of groundwater to stream flow in an upland catchment using a combined salinity mixing model and modified curve number approach. Honours Thesis, Flinders University, Adelaide, South Australia
Lamontagne S, Leaney FW, Herczeg AL (2005) Groundwater–surface water interactions in a large semi-arid floodplain: implications for salinity management. Hydrol Process 19(16):3063–3080
Love A, Cook P, Harrington G, Simmons C (2002) Groundwater flow in the Clare Valley. Report DWR02 3, Dept. for Water Resources, South Australia, 43 pp
Meredith K, Hollins S, Hughes C, Cendón D, Stone D (2013) The influence of groundwater/surface water exchange on stable water isotopic signatures along the Darling River, NSW, Australia. In: Ribeiro L, Stigter TY, Chambel A, Conesso de Melo M, Medeiros A (eds) Groundwater and ecosystems, vol 18. CRC, Boca Raton, FL, pp 57–68
Milgate SA (2007) Hydrochemical investigation of flow pathways through quartz-sand and duplex soils during a storm event: Mackreath Creek, Mount Lofty Ranges. Honours Thesis, Flinders University, Adelaide, South Australia, Adelaide, South Australia
Pichler M (2009) Characterization of spatial and seasonal changes of dissolved organic carbon in the soils of a South Australian Catchment, PhD Thesis, Flinders University, Adelaide, South Australia
Poulsen DL, Simmons CT, Le Galle La Salle C, Cox JW (2006) Assessing catchment-scale spatial and temporal patterns of groundwater and stream salinity. Hydrogeol J 14(7):1339–1359. doi:10.1007/s10040-006-0065-9
Preiss WV (1987) The Adelaide Geosyncline: Late Proterozoic stratigraphy, sedimentation, palaeontology and tectonics. Dept. of Mines and Energy, Adelaide, South Australia
Raiber M, Webb JA, Bennetts DA (2009) Strontium isotopes as tracers to delineate aquifer interactions and the influence of rainfall in the basalt plains of southeastern Australia. J Hydrol 367:188–199
Ranville JF, Chittleborough DJ, Beckett R (2005) Particle-size and element distributions of soil colloids: implications for colloid transport. Soil Sci Soc Am J 69:1173–1184
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57(5):1017–1023
Salama RB, Otto CJ, Fitzpatrick RW (1999) Contributions of groundwater conditions to soil and water salinization. Hydrogeol J 7(1):46–64
Schofield N (1992) Tree planting for dryland salinity control in Australia. Agrofor Syst 20(1–2):1–23
Stevens DP, Cox JW, Chittleborough DJ (1999) Pathways of phosphorous, nitrogen, and carbon movement over and through texturally differentiated soils, South Australia. Aust J Soil Res 37:679–693
Stewart BW, Capo RC, Chadwick OA (1998) Quantitative strontium isotope models for weathering, pedogenesis and biogeochemical cycling. Geoderma 82(1):173–195
Stewart S (2005) Clare prescribed water resources area groundwater monitoring status report 2005. DWLBC 2005/18, Government of South Australia, Adelaide, 43p
Taylor JK, Thompson B, Shepherd R (1974) The soils and geology of the Adelaide area. Geological Survey of South Australia, Adelaide, South Australia
Turner J, Arad A, Johnston C (1987) Environmental isotope hydrology of salinized experimental catchments. J Hydrol 94(1):89–107
UC Davis (2012) Stable Isotopes Facility homepage. University of California, Davis, CA. http://stableisotopefacility.ucdavis.edu/index.html. Accessed 12 Februaury 2012
Walker GR, Gilfedder M, Williams J (1999) Effectiveness of current farming systems in the control of dryland salinity. CSIRO Land and Water, Clayton, South Australia
Zimmerman U, Ehhalt D, Munnich K (1967) Soil water movement and evapotranspiration: changes in the isotopic composition of water. From ‘Isotopes in Hydrology’ conference, IAEA, Vienna
Acknowledgements
This project was funded by a Flinders University Program Grant (2005). Access to South Australian Water Corporation property is gratefully acknowledged. Field assistance by Olanrewaju Abiodun and Robbie Andrews was helpful. Editorial assistance by Kate Osborne is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, T.A., Bestland, E.A., Soloninka, L. et al. A groundwater salinity hotspot and its connection to an intermittent stream identified by environmental tracers (Mt Lofty Ranges, South Australia). Hydrogeol J 25, 2435–2451 (2017). https://doi.org/10.1007/s10040-017-1637-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-017-1637-6