Abstract
In Canada’s western Arctic, perennial discharge from permafrost watersheds is the surface manifestation of active groundwater flow systems with features including the occurrence of year-round open water and the formation of icings, yet understanding the mechanisms of groundwater recharge and flow in periglacial environments remains enigmatic. Stable isotopes (δ18O, δD, δ13CDIC), and noble gases have proved useful to study groundwater recharge and flow of groundwater which discharges along rivers in Canada’s western Arctic. In these studies of six catchments, groundwater recharge was determined to be a mix of snowmelt and precipitation. All systems investigated show that groundwater has recharged through organic soils with elevated PCO2, which suggests that recharge occurs largely during summer when biological activity is high. Noble gas concentrations show that the recharge temperature was between 0 and 5 °C, which when considered in the context of discharge temperatures, suggests that there is no significant imbalance of energy flux into the subsurface. Groundwater circulation times were found to be up to 31 years for non-thermal waters using the 3 H-3He method.
Résumé
Dans l’Arctique de l’Ouest canadien, une décharge pérenne de bassins versants gelés est la manifestation de surface d’un système actif de flux d’eau souterraine avec des caractéristiques incluant l’entrée d’eau durant toute l’année et la formation de glace, encore que la compréhension des mécanisme de recharge de nappe et du flux dans l’environnement périglaciaire reste énigmatique. Les isotopes stables (δ18O, δD, δ13CDIC) et des gaz rares se sont avérés utiles pour étudier la recharge de nappe et le flux souterrain qui se décharge le long de rivières dans l’arctique de l’Ouest canadien. Dans ces études de six basins versants, on a établi que la recharge de nappe est un mixte de neige fondue et de précipitation. Les investigations sur tous les systèmes montrent que la nappe se recharge à travers des sols organiques à PCO2 élevée, ce qui suggère que la recharge a lieu largement durant l’été quand l’activité biologique est élevée. Les concentrations en gaz rares montrent que la température de recharge était comprise entre 0 et 5 °C, ce qui, considéré dans le contexte des températures de décharge, signifie qu’il n’y a pas de déséquilibre des flux énergétiques en sub-surface. On a trouvé des durées de circulation de l’eau de nappe jusqu’à 31 ans pour des eaux non thermales en utilisant la méthode 3H-3He.
Resumen
En el Ártico Occidental de Canadá, la descarga perenne de cuencas de permafrost es la manifestación superficial de sistemas activos de flujos de agua subterránea con características que incluyen durante el año la presencia de aguas libres y la formación de hielos, sin embargo el entendimiento de los mecanismos de la recarga de agua subterránea y el flujo en ambientes periglaciales siguen siendo enigmáticos. Los isótopos estables (δ18O, δD, δ13CDIC), y los gases nobles han demostrado ser útiles para estudiar la recarga de agua subterránea y el flujo de agua subterránea que descarga a lo largo de ríos en el Ártico occidental de Canadá. En estos estudios de seis cuencas, la recarga del agua subterránea se determinó que era una mezcla del derretimiento de la nieve y de la precipitación. Todos los sistemas investigados muestran que el agua subterránea se recarga a través de suelos orgánicos con elevada PCO2, lo cual sugiere que la recarga ocurre mayormente durante el verano cuando la actividad biológica es alta. Las concentraciones de gases nobles muestra que la temperatura de recarga fue entre 0 y 5 °C, lo cual cuando se considera en el contexto de las temperaturas de descarga, sugiere que no hay un desequilibro significativo en el flujo de energía en el subsuelo. Los tiempos de circulación de agua subterránea resultaron ser de hasta 31 años para agua no termales usando el método 3H-3He.
摘要
在加拿大西部的寒区,来自多年冻土流域的常年地下水排泄是活跃的地下水流系统在地表的表现,在地表可以看到全年开放的水域和冰的形成,然而要弄清楚冰川边缘地带地下水的补给和径流机制仍然存在很多疑惑。稳定同位素(δ18O, δD, δ13CDIC)和稀有气体被证明用来研究地下水补给和径流是很有用的,在加拿大西部寒区地下水就是沿着河流向外排泄。在本次对六个盆地研究中,地下水补给被确定为是融雪和降雨的混合。所有调查过的地下水系统显示地下水在径流过程中经过二氧化碳分压比较高的有机土壤,这表明地下水补给主要发生在生物活动比较活跃的夏季。稀有气体浓度显示地下水补给温度在0~5°C之间,在考虑到地下水排泄温度的情况下,这表明流向地下的能量流并不存在严重的不平衡。利用3H-3He方法研究发现非热水的循环时间长达31年。
Resumo
No Ártico ocidental do Canadá, a descarga perene das bacias hidrográficas com permafrost é a manifestação superficial de sistemas de escoamento de águas subterrâneas ativos com caraterísticas que incluem a ocorrência durante todo o ano de águas abertas e a formação de gelos, apesar da compreensão dos mecanismos de recarga de águas subterrâneas e do fluxo subterrâneo em ambientes periglaciais permanecer enigmática. Os isótopos estáveis (δ18O, δD, δ13CDIC) e os gases nobres têm sido úteis no estudo da recarga e do fluxo de águas subterrâneas que descarregam nos rios do Ártico ocidental do Canadá. Nos estudos de seis bacias hidrográficas, determinou-se que a recarga de águas subterrâneas era uma mistura de águas do degelo e da precipitação. Todos os sistemas investigados mostram que as águas subterrâneas recarregaram através de solos orgânicos com elevado PCO2, o que sugere que a recarga ocorre largamente durante o verão, quando a atividade biológica é alta. As concentrações de gases nobres mostram que a temperatura de recarga foi entre 0 e 5 °C, o que, quando considerado no contexto das temperaturas de descarga, sugere que não há um desequilíbrio significativo de fluxo de energia para a subsuperfície. Utilizando o método 3H-3He, os tempos de circulação de águas subterrâneas foram calculados em até 31 anos para águas não-termais.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater circulation and discharge in permafrost regimes represents an enigmatic feature of the hydrological cycle in northern catchments, yet plays an important role in river discharge and maintaining viable spawning and overwintering habitat for sea-run and freshwater fishes (Power et al. 1999; Mochnacz et al. 2010). While groundwater flow is readily documented by the formation of river icings and by reaches of open water throughout the winter, the mechanisms of groundwater recharge, subsurface flow paths and contribution to discharge remain uncertain (Woo and Marsh 2005). The flow of low-salinity perennial groundwater in permafrost terrains is considered to take place in taliks, defined as zones within permafrost regions in which temperatures remain greater than 0 °C. Saline groundwater, for which the freezing temperature is suppressed, can thereby move through permafrost terrain (permafrost being strictly a thermal condition). Accordingly, fresh groundwater can flow perennially within supra-permafrost taliks (above the permafrost table but below the depth of annual freezing), within intra-permafrost taliks (networks or pathways of unfrozen terrain within permafrost) or below the base of the permafrost (Muller 1947; Tolstikhin and Tolstikhin 1976). The distribution of taliks in permafrost basins has been well studied (French 1996; Carey and Woo 2005) and clearly linked to local climate, insolation and the conduction of heat into the soil and rock by thermal diffusion. However, the advective flow of heat into the subsurface through groundwater recharge may play a role in the formation and stability of taliks.
Groundwater flow in regions of continuous permafrost commonly occurs in karst carbonate and evaporate bedrock, with recharge occurring by diffuse flow or through dolines (Ford and Williams 2007). Karstic groundwater systems are known in numerous areas of northwestern Canada such as along the Firth River in northern Yukon (Clark and Lauriol 1997), rivers in the Norman Wells region of the Northwest Territories (NWT; Michel 1986), the Nahanni River (Brook and Ford 1980), and near Great Bear Lake, NWT (Van Everdingen 1981). In northeastern Canada, groundwater drainage occurs in permafrost on carbonate Akpatok Island (Lauriol and Gray 1990). In Alaska, there are groundwater-fed streams on the northern coast near Prudhoe Bay (Craig and McCart 1975). Many of these systems were established when the climate was warmer prior to glaciation, yet persist under the colder present conditions (Ford and Williams 2007). In the Canadian High Arctic, evidence for groundwater flow is restricted to hypersaline settings. Springs on Axel Heiberg Island (McKay et al. 2005; Omelon et al. 2006 and Pollard 2005) discharge a high total dissolved solids (TDS) mixture of subglacial water and water from a talik below a lake. These waters gain their salinity by flow through dissolution porosity in evaporite rocks. Grasby et al. (2003) describe saline perennial springs discharging supraglacially on Ellesmere Island. Groundwater may also flow through sands and gravels where conditions are appropriate, for example pro-glacial groundwater discharge in Greenland (Scholz and Baumann 1997).
The occurrence of river icings (aufeis in German, naledi in Russian or glaçage in French) is evidence for perennial groundwater discharge in permafrost regions. Icings occur when river ice freezes to the base, blocking discharge and building pressure such that the ice is breached and surface pooling occurs (Carey 1970). They were first described in Russia (Wrangel 1841; Anisimova et al. 1973), and have since been described in many polar areas such as the Brooks Range, Alaska (Yoshikawa et al. 2007); pro-glacially in Svalbard (Bennett et al. 1998); in Finland (McFadden 1990); and in Iceland (Venzke 1988)—see electronic supplementary material (Fig. 1, ESM) for map. In Canada, icings have also been observed in numerous areas. The largest icing in Canada forms annually along the Firth River, Yukon, where 12 % of basin groundwater discharge from the carbonate catchments in the British Mountains is retained annually (Clark and Lauriol 1997). In central Yukon, icings form in the North Fork Pass area (Hu and Pollard 1997). In the Canadian High Arctic, icings form at the Axel Heiberg Island hypersaline springs (Pollard 2005) and in proglacial settings such as the Akshayuk Pass of southern Baffin Island (Lacelle et al. 2006).
In smaller mountain streams (width <20 m) perennial groundwater sources maintain ice-free or below-ice discharge (Prowse et al. 2006). These areas provide spawning and overwintering habitat for northern fishes such as sea-run Dolly Varden (Salvelinus malma; Power et al. 1999; Mochnacz et al. 2010). The understanding of groundwater flow in permafrost terrain is also of importance to mining development for determining dewatering needs as well as assessing potential environmental impacts (Stotler et al. 2009).
The study of groundwater in permafrost is challenging given the limited infrastructure and very short field seasons. These conditions favour samples from baseflow discharge and perennial groundwater springs, combined with the use of geochemical and isotope tracers to elucidate recharge conditions and flow paths. Noble gas and isotopic techniques are well suited to studies of the periglacial environment given the effects of temperature on their fractionation and partitioning (e.g., Aeschback-Hertig et al. 2000). Stable isotopes 18O and D of water in periglacial watersheds can record seasonal variations as well as recharge elevation and paleo-effects (Clark et al. 2000, 2001; Michel 1986). A groundwater body with a depleted signature of 18O and D relative to surface water has been interpreted to be the result of recharge at higher elevation or as a result of recharge being dominated by snowmelt (Clark et al. 2001; Michel 1986). Alternatively, 18O has been used as a tracer of different water sources (Hayashi et al. 2004).
The concentrations and isotopic ratios of dissolved inorganic carbon (DIC) can be used to determine recharge conditions. In most cases during recharge, water dissolves CO2 from the soil where it is produced from the decomposition of organic matter. Vegetation in Yukon and NWT is primarily of the C3 variety. Vegetation of the C3 variety uses the RuBisCO enzyme to catalyse CO2 respiration, which results in vegetation with a δ13C of between −24 and −30 ‰ (Vogel 1993). When CO2 produced by the decomposition of C3 vegetation dissolves in water, the δ13CDIC is typically ca. −27 ‰ (Clark and Fritz 1997). The acidity in the water is then consumed during weathering of carbonates that have a δ13C of 0 ‰, which enriches the DIC. Open system conditions exist where there is a continual supply of CO2 so that there is equilibrium between soil CO2 and DIC, for example in shallow soils with abundant carbonate and organic material. In this case, the δ13CDIC is controlled by the pH. Under open system conditions, the final δ13C is generally between −15 and −18 ‰. Under closed system conditions water dissolves CO2 during recharge and then weathers carbonate minerals along its flow path; the end δ13CDIC will be close to −12 ‰. Greater enrichments can be generated through continued exchange between DIC and the carbonate matrix, and through incongruent dissolution of dolomite. Due to the greater partial pressure of CO2 in soil, groundwater recharge through soils dissolves more CO2 and so carries a greater weathering potential than it would under atmospheric air conditions. Based on these reactions that occur during recharge, the δ13C of DIC combined with pH and major ion geochemistry are useful tracers of groundwater recharge (Clark and Fritz1997).
Carbon, oxygen and hydrogen isotopes can indicate environments of groundwater recharge, while noble gases can inform on timing and temperature of recharge. During recharge, the gas composition of groundwater is determined by the atmospheric composition and the salinity and the temperature of the water. Once below the water table, dissolved gases do not exchange with the atmosphere and the noble gas concentration is preserved (Kipfer et al. 2002). The differing solubilities of noble gas with recharge temperature have been used in temperate regions to determine mean annual temperature in the recharge environment and to reconstruct past climates (Aeschbach-Hertig and Solomon 2012). In permafrost catchments, the noble gas record of recharge temperature can potentially provide constraints on the seasonality of recharge. In permafrost regions where the mean annual air temperature is below 0 °C, the groundwater recharge temperature can be compared with the temperature at discharge to determine if recharging groundwater advects heat into the subsurface.
Noble gas isotopes produced by radioactive decay, including 3He from tritium, 4He from alpha-decay and 40Ar from 40K provide non-reactive tracers of groundwater circulation rates. The short half-life of tritium decay (12.3 years) is useful for groundwaters actively circulating in permafrost, providing corrections for geogenic 3He production can be made (Lucas and Unterweger 2000).
Groundwater in permafrost watersheds of the northwestern Canadian Arctic
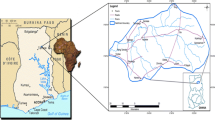
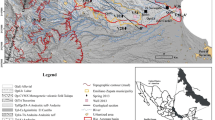
This article compiles results from two previous publications and a PhD thesis that presented data on groundwater from western Northwest Territories and Yukon Territory, Canada. Groundwater discharge has been studied in the Central Mackenzie Valley, NWT (White Sand Creek, Hodgson Creek, Smith Creek, Canyon Creek, Gibson Creek, Gayna River); and along the Fishing Branch River, Yukon and Firth River, Yukon (Fig. 1). Results from the Central Mackenzie Valley are from Utting (2012), a PhD thesis, while results from Fishing Branch River are from Utting et al. (2012) and those from Firth River are from Clark and Lauriol (1997). The study areas are located over a wide geographic area from 63° N to 68° N and 123 °W to 139 °W.
Distribution of permafrost in the Yukon and the Northwest Territories of Canada, and sampling sites (Permafrost distribution from NRCan 2003)
In the areas studied in this publication, the average annual air temperature ranges from −3.2 °C in Fort Simpson to −9.0 °C in Old Crow, allowing permafrost to persist. Total annual precipitation ranges from 265.6 mm at Old Crow to 369 mm at Fort Simpson (Environment Canada 2010). The southern part of the study area is located in extensive discontinuous permafrost which progressively becomes continuous permafrost in the northern part of the study area (NRCan 2003; Fig. 1). Permafrost thickness ranges from <10 to 100 m (Smith and Burgess 2002).
The groundwater systems included in this study are located in the Interior Platform or the Cordilleran orogen geologic provinces of Canada (NRCan 2004). Groundwater flow occurs mostly in Devonian carbonate and evaporite bedrock. Dissolution has formed karst allowing for significant groundwater flow (van Everdingen 1981; Hamilton and Ford 2002; Cinq-Mars and Lauriol 1985). Karstification processes have been occurring since at least the Cretaceous but may have slowed during the Pleistocene glaciations (van Everdingen 1981). The groundwater systems included in this study, which are located in the Northwest Territories, were glaciated during the Pleistocene, while the sites in Yukon were not glaciated (Duk-Rodkin 1999).
Field work and analytical methods
Several of the groundwater systems presented in this study were sampled during the past two decades from locations with known perennial groundwater discharge (Mutch and McCart 1974; Michel 1977, 1986). Groundwater samples were obtained from springs while additional surface water samples were collected. More recent sampling was conducted along the Firth River in June 1993 and June 1994 and along the Fishing Branch River in June 2006, July 2007 and August 2008. In August and September of 2007, some 18O and D samples were collected from streams in the Central Mackenzie Valley (Gayna River, Gibson, Hodgson, White Sand and Smith creeks). In March 2008, groundwater and surface water samples were collected in the Central Mackenzie Valley, with further surface water samples being collected in June 2008 for 18O and D. Along Smith Creek perennial groundwater springs were sampled with water temperature of up to 13.8 °C; this maintains open water all year in the creek near the springs. Similarly, along Gibson Creek warm groundwater (8.2 °C) results in open water which flows into the creek maintaining flow in winter. Along White Sand Creek, Hodgson Creek, Gayna River and Canyon Creek groundwater springs were sampled along the banks. Along Smith Creek, Gibson Creek, White Sand Creek, Hodgson Creek, Gayna River and Canyon Creek, the groundwater springs were sampled for major ions, 13CDIC and 18O and D; springs from Smith Creek, White Sand Creek and Gayna River were sampled for noble gases and tritium. Surface waters collected along these watercourses were analysed for 18O and D. Details on sample collection and analysis can be found in Clark and Lauriol (1997) for Firth River; Utting et al. (2012) for Fishing Branch River; and Utting (2012) for Central Mackenzie Valley.
As these groundwaters have temperatures above the mean annual air temperature, they could all be classified as thermal waters (White 1957). However, this type of definition is not very useful as most of these groundwaters do not have deep flow pathways where they gain heat in the subsurface. Any separation of thermal vs. non-thermal is somewhat arbitrary, but can be useful for the sake of discussion. In this manuscript, the groundwaters from Smith Creek and Gibson Creek are classified as being thermal, because of their higher temperature and higher dissolved ion concentrations.
Noble gases
Rapid exchange of noble gases with the atmosphere constrains the sites and methods of sampling. In the absence of piezometers and observation well networks in these remote settings, groundwater springs represent the best sites for noble gas sampling, using both passive diffusion gas samplers (Sanford et al. 1996; Manning et al. 2003) and water samples in annealed copper tubes. Details on the sampling and laboratory methods can be found in Utting et al. (2012) and Utting (2012). At each site sampled for noble gases, 500 ml of water was collected for tritium analysis by decay counting after electrolytic enrichment. This analysis was conducted at the University of Waterloo, Canada.
Noble gas samples were analysed at Heidelberg University, Germany, and the University of Ottawa, Canada. At Heidelberg University, samples were analysed for all noble gases using a GV 5400 mass spectrometer, following procedures based on Friedrich (2007). At the University of Ottawa, analysis was conducted on two noble gas lines. Analyses of helium isotopes and neon concentrations were conducted on a MAP 215–50 noble gas mass spectrometer using standard noble gas extraction and analysis techniques (Mohapatra and Murty 2000). Analyses for argon and krypton were conducted using an isotope dilution noble gas line with a quadrupole mass spectrometer based on the design of Poole et al. (1997).
Results and interpretation
Groundwater geochemistry
As presented in the preceding, groundwater circulation within permafrost appears to occur preferentially through secondary porosity associated with evaporite or carbonate karst terrains. First order insights to groundwater circulation can then be derived from major ion geochemistry, which provides a basis for subsequent investigations of isotopes and noble gases. Groundwaters tend to have higher dissolved solids than overland runoff derived surface water due to greater water-rock reaction time, enhanced by carbonic acid derived during transit through soils in the recharge area. The dissolved ions in groundwater may also reflect the groundwater flow path. The carbonate component of groundwater reflects the recharge conditions where CO2 is dissolved and subsequently weathers mineral material.
Water geochemistry measurements for the various study areas are presented in Table 1 and shown in Fig. 2. Geothermal groundwaters from Smith Creek and Gibson Creek catchments have higher dissolved solids and are dominated by calcium, sodium, chloride and sulphate ions. The high proportion of sodium and chloride in Smith Creek groundwater is attributed to the dissolution of halite (Michel 1986). The other groundwater systems are non-thermal and are dominated by calcium, sulphate and bicarbonate. These ions originate from the weathering of calcite and gypsum bedrock. The higher sulphate relative to bicarbonate in some of the non-thermal groundwaters is presumably due to greater interaction with gypsum rich bedrock in the subsurface.
Oxygen-18 and deuterium
Figure 3 shows δD and δ18O values measured from groundwater and surface water along the eight watercourses. Samples from the Central Mackenzie Valley were compared to the meteoric water line from Fort Simpson (δD = 7.6δ18O − 2 ‰; Hayashi et al. 2004). Samples from the Fishing Branch River and Firth River were compared to the meteoric water line from Old Crow (δD = 6.8δ18O − 21.5 ‰, Utting et al. 2012).
δ D vs. δ18O isotope results for water samples a Smith Creek, b Gibson Creek, c Canyon Creek, d White Sand Creek, e Hodgson Creek, f Gayna Creek, g Fishing Branch River, h Firth River. Plots a–f used the local meteoric water line for Fort Simpson, (δD = 7.6δ18O - 2, Hayashi et al. 2004); Plots g and h used the local meteoric water line for Old Crow (δD = 6.8δ18O – 21.6; Utting et al. 2012)
Discharging groundwater is expected to have a nearly constant δD and δ18O composition throughout the year, whereas the composition of surface water varies seasonally. In general, groundwaters along Smith Creek, Gibson Creek, Hodgson Creek, Canyon Creek, White Sand Creek and Gayna River have lower δD and δ18O values than surface water collected in these reaches (Fig. 3). Surface waters along these creeks were collected in March and June. The composition of surface water changes through the year but remains enriched compared to groundwater. Groundwater samples from the Fishing Branch River and Firth River watersheds have similar isotopic composition to the surface water. Groundwater in the Fishing Branch has a similar isotopic composition to average precipitation (Utting et al. 2012). The similar isotopic composition of surface water to groundwater is due to the very high contribution of groundwater to the river.
Groundwater recharge: DIC, calcite saturation and δ13C
Groundwater recharged through soils gains considerable weathering potential from the dissolution of CO2, which is subsequently attenuated through carbonate dissolution. Groundwater samples are near calcite saturation with indices (log IAP/Kcal) of −0.90 to 0.78. In Fig. 4, the DIC and pH of groundwater samples are shown along with the saturation curves of calcite and dolomite and the open and closed system weathering trajectories for initial soil partial pressure of CO2 (PCO2) values of 10−2.5 and 10−3.5 atm. Higher PCO2 values are more commonly associated with open system weathering reflecting the continual supply of CO2 from decomposing organic matter in the soil. The lower PCO2 of closed system conditions reflects the reduction of acidity by weathering. Given that groundwater recharge is dominantly through soils, they will, under most circumstances, have higher PCO2 values than surface waters. The δ13C of DIC in groundwater can be used as a complementary tracer. In general, a δ13CDIC near −17 ‰ suggests open system recharge through organic soil. Higher values can be associated with closed system weathering (below the water table) and/or incongruent dissolution of dolomite along the flow path, or possibly a result of methanogenesis in the subsurface (Clark and Fritz 1997).
Groundwater samples have an average PCO2 of 10−2.3 atm, which is consistent with recharge through organic soil. The δ13CDIC of groundwater ranges from –3.3 to –12.8 ‰ (see Table 1). The more depleted δ13CDIC values are consistent with closed system weathering of carbonate rocks by CO2 derived from soil. δ13CDIC values greater than –11 ‰ may be attributed to methanogenesis, as was proposed by Clark and Lauriol (1997) for the Firth River groundwater or by incongruent dissolution. It is expected that methanogenesis would cause the δ13CDOC to be more enriched than the δ13CDOC of vegetation derived carbon. The average δ13CDOC from White Sand Creek, Hodgson Creek, Smith Creek, Canyon Creek, Gibson Creek, Gayna River and the Fishing Branch River is −26.4 ‰ which does not indicate that it has been affected by methanogenesis. Incongruent dissolution of dolomite is supported by the fact that most groundwaters are supersaturated in calcite, but undersaturated in dolomite.
Groundwater recharge and residence time: noble gases
Noble gas concentrations have been measured from groundwater samples collected from springs along the Fishing Branch River, Smith Creek, Gayna River and White Sand Creek (Table 2). These measurements have been used to determine groundwater recharge temperature and groundwater age.
Groundwater recharge temperature
Recharge temperatures of the groundwater springs have been estimated using the inverse modeling approach introduced by Aeschbach-Hertig et al. (1999). The approach constrains temperature and excess air to match modeled dissolved noble gas contents with measured concentrations. Different models exist to describe dissolved noble gases in groundwater as a result of equilibrium dissolution and excess air incorporation (Aeschbach-Hertig and Solomon 2012). The MATLAB program Noble 90 (Peeters et al. 2003) was used to model the noble gas concentrations and to vary the model parameters including temperature, excess air, and fractionation to find the best fit for the observed concentrations. The program minimises χ 2, the error-weighted sum of the model data deviations of the individual noble gases, which at the same time provides a measure of the goodness of the achieved fit (Aeschbach-Hertig et al. 1999). Due to changes in pressure, recharge elevation is a necessary input to constrain the noble gas model and is estimated for each watershed (Table 3). The calculations based on the closed-system equilibration model for dissolved noble gases (Aeschbach-Hertig et al. 2000) return groundwater recharge temperatures between 0 ± 3 and 5 ± 1 °C (Table 3). Groundwater samples from Gayna River and White Sand Creek return recharge temperatures between 0 ± 3 and 1 ± 1 °C, and along the Fishing Branch River between 0 ± 3 and 5 ± 1 °C, which is realistic for these systems. The very high helium concentrations of the Smith Creek sample resulted in problems with measuring the neon concentration, meaning the recharge could not be modelled accurately.
3H-3He: groundwater residence time
Helium concentrations and isotope ratios are dependent on the groundwater flow path and groundwater age (Kipfer et al. 2002). Samples from Smith Creek thermal springs had high helium concentrations reflecting a deep groundwater flow path and generally longer residence time. Groundwater from cool springs along Fishing Branch River, White Sand Creek, and Gayna River had lower helium concentrations reflecting a shallower groundwater flow path. Smith Creek groundwater had much higher geogenic helium concentrations due to the deeper flow path and longer residence time of this thermal groundwater. Figure 5 shows 3He/4He vs. Ne/He of the noble gas samples, where Ne is strictly of atmospheric origin while 3He and 4He may have a geogenic component from U and Th decay and 3He from 3H decay. The high proportion of crustal helium from Smith Creek groundwater is evident by the very low Ne/He. Smith Creek water had tritium concentrations below the detection limit (<0.8 TU) and a 3He/4He of 3.01 ± 0.14 × 10−8 which is close to the average crustal value (~2.0 × 10−8; Kipfer et al. 2002). The 3He/4He of non-thermal groundwater samples ranged from 1.0 × 10−6 to 1.49 × 10−6 and tritium concentrations ranged from 9.2 to 16.3 TU. The concentrations of helium isotopes, neon and tritium were used to determine the groundwater residence time of samples with measureable tritium.
The age of groundwaters which have recharged since the atmospheric testing of nuclear weapons can be determined using 3H-3He dating. The amount of tritogenic 3He from 3H decay is determined by accounting for other sources of 3He such as excess air and crustal helium. This is calculated in Eq. 1 (Kipfer et al. 2002):
where 4Hem is the measured concentration of 4He, R m is the measured 3He/4He, R eq is the 3He/4He for atmospheric equilibrium (1.36 × 10−6), R ter is the 3He/4He from crustal sources, 4Heeq is the helium concentration in air equilibrated water, L ex is the He/Ne of the excess air component (2.88 × 10−1), Nem is the measured neon, Neeq is the equilibrium concentration of neon at recharge and R ex is the 3He/4He for excess air (1.384 × 10−6), (Kipfer et al. 2002). For the groundwaters from White Sand Creek and Gayna River, the 3He/4He value from Smith Creek (3.01 × 10−8) was used as the crustal value for R ter for groundwater in this region as this groundwater system contained no measurable tritium and is considered close to a purely crustal signal. R ter for groundwater in the Fishing Branch River watershed was determined by regression between air equilibrated water through the data point which obtains the lowest value for R ter on a plot of 3He/4He vs. Ne/He. The value determined for the Fishing Branch River was 6.25 × 10−7. The value of 3HeTri obtained from Eq. 1 is used in Eq. 2 (Tolstikhin and Kamenskiy 1969) to determine groundwater residence time:
where 12.3 years is the half-life of tritium, 3HeTri is from tritium decay, and 3H is tritium in water. Results of Eq. (2) are listed in Table 3.
Groundwater circulation rates are highly variable for the different flow systems. Smith Creek groundwater has no measurable tritium (< 0.8 TU) and a crustal helium signature. This water recharged prior to the 1950s and is classified as submodern. The non-thermal groundwaters contain less crustal helium and have been dated successfully with the 3H-3He method. Groundwater along the Fishing Branch River ranges in age from 0 ± 2 to 18 ± 1 years. Groundwater sampled along White Sand Creek was determined to have a groundwater age of 30 ± 3 years. Groundwater sampled along Gayna River was dated at 24 ± 2 years (duplicate sample result 21 ± 2 years).
Discussion
Within the watersheds studied, the measurements of δD, δ18O, δ13CDIC, Ne, Ar, Kr and Xe were used to provide information on groundwater recharge. As δD and δ18O are dependent on temperature, they provide insights to recharge elevation and potentially the seasonality of recharge. Seven of the groundwater systems studied indicate some degree of depletion of δ18O and δD. Although depleted isotopic values could be the result of recharge dominated by snowmelt, these values have been interpreted to be the result of recharge at higher elevation with a mix of precipitation through the year. This interpretation is based on the DIC and δ13CDIC results which support recharge through organic rich soil. Recharging water is a blend of snowmelt and summer rains.
Measurements of δ13CDIC were used to determine if recharge occurs in organic soils and if it is under open or closed system conditions. The average PCO2 of groundwaters is 10−2.3 atm with the CO2 derived from organic soils. The δ13CDIC of groundwater ranges from −3.3 to −12.8 ‰. This range of δ13CDIC is interpreted to be derived from the dissolution of soil CO2 and the subsequent weathering of marine carbonates under closed system conditions likely with incongruent dissolution of dolomite affecting some samples. The incongruent dissolution of dolomite along the flow path is supported by the supersaturation with calcite, but undersaturation in dolomite. These processes occur in a similar way in non-permafrost watersheds (Clark and Fritz 1997).
Figure 6 shows the groundwater temperature vs. noble gas recharge temperature. In general, the noble gas recharge temperature is significantly lower than the groundwater temperature at discharge (P <0.01). Groundwaters along White Sand Creek and Gayna Creek, like groundwater from the Fishing Branch River (Utting et al. 2012), have noble gas recharge temperatures that are some 2–3 °C lower than the measured discharge temperatures, signifying no significant advective flux of heat into the subsurface. By contrast, the higher discharge temperatures suggest groundwaters gain heat from elevated ground temperatures in the low-elevation discharge area. Thermal groundwater systems like Smith Creek must gain significant heat in the deeper subsurface to reach >10 °C.
Based on the noble gas results and the δD, δ18O and δ13CDIC of groundwater, it is proposed that groundwater recharge occurs through soil, often in the upper elevations of these watersheds. Six of seven watersheds in the Mackenzie Valley possess groundwater with some isotopic depletion, which suggests recharge at higher elevations. Recharge at these elevations may, to some degree, reflect permafrost distribution as affected by thermal inversions. In the Central Mackenzie Valley, thermal inversions are common in winter (Eley 1974). Under normal conditions it is coldest at higher elevations; however, during inversions this is reversed, potentially resulting in thinner permafrost just below the tree line, to become thicker again at higher elevations, as noted in the Franklin Mountains on the east side of the Mackenzie River (~550 m a.s.l.; Taylor et al. 1998). If zones of thinner permafrost exist just below the tree line, they may act as recharge zones. Recharge water appears to represent precipitation from the entire year. However, recharge may be from variable elevations. Michel (1977, 1986) proposed that recharge may occur from draining of ephemeral lakes underlain by karst in the Franklin Mountains; however, this is not consistent with the δ13CDIC values presented here. There is abundant karst in the Franklin Mountains and there is likely recharge in karst; however, 13CDIC values indicate recharge through organic soils. Recharge must happen during the summer when soils are producing CO2, and when groundwater temperatures are >0 °C; this water may subsequently flow into karst. The same observations hold true for the Firth River and Fishing Branch River watersheds; however, there was no observable elevation effect.
All groundwater samples had tritium concentrations indicating modern recharge except for Smith Creek groundwater (<0.8 TU). Previous research also found Smith Creek groundwater to have low tritium (7 ± 8, 16 ± 8 TU, Michel 1977). The very low tritium concentrations of Smith Creek groundwater indicate groundwater recharge greater than 45 years ago. A small amount of tritium may have been present in early samples due to minor contamination with meteoric water, which in the 1970s had a much higher concentration. Non-thermal groundwaters had groundwater ages ranging from 0 ± 2 to 31 ± 3 years. The ages of non-thermal groundwaters (generally greater than 1 year) suggest that groundwater discharge is probably relatively constant from year to year.
Summary and conclusion
Figure 7 presents a conceptual diagram of the flow system derived from the above results. Despite permafrost conditions, perennial groundwater flow occurs primarily through karst carbonate and evaporite bedrock in Canada’s western Arctic. The discharge of this perennial groundwater is manifested in the form of icings and open water year round. Groundwater has δ18O and δD levels that are either close to the value of mean annual precipitation or are in some cases more depleted. These results suggest that recharge is a mix of annual precipitation and where values are depleted recharge likely occurs at higher elevations. In some areas, permafrost at higher elevations may be thinner than expected due to temperature inversions in winter; this thinner permafrost may allow for enhance groundwater recharge. PCO2 (10−2.3 atm) and δ13C (−3.3 to −12.8 ‰) indicate a biogenic supply of CO2 which is consumed during closed system weathering of marine carbonate material. As such recharge likely occurs through organic-rich soils which overlay fractured bedrock, the weathering of carbonate bedrock results in groundwaters which are often supersaturated in calcite. Noble gas results indicate groundwater recharge temperatures are close to 0 °C, and give no evidence for significant advection of heat into the subsurface during recharge. Groundwater ages are related to groundwater flow path. Non-thermal groundwaters dated by 3He-ingrowth from tritium were found to have circulation times largely on the order of two to three decades, and so an indication that these karst groundwater systems have substantial flow paths and significant storage. Thermal groundwater along Smith Creek is classified as sub-modern based on its lack of measureable tritium.
Isotopic methods suggest that recharge processes in permafrost terrain are not unlike those in non-permafrost terrain. Permafrost reduces the amount of recharge, but in the watersheds studied does not preclude it. The more extensive the permafrost, the more groundwater flow is concentrated in macro-porosity. In other watersheds, even with non-carbonate bedrock, groundwater may flow perennially if there are the appropriate conduits to concentrate flow and heat to prevent freezing.
References
Aeschbach-Hertig W, Solomon DK (2012) Noble gas thermometry in groundwater hydrology. In: Burnard P (ed) The noble gases as geochemical tracers. Advances in Isotope Geochemistry. Springer, Heidelberg, Germany
Aeschbach-Hertig W, Peeters F, Beyerle U, Kipfer R (1999) Interpretation of dissolved atmospheric noble gases in natural waters. Water Resour Res 35:2779–2792
Aeschbach-Hertig W, Peeters F, Beyerle U, Kipfer R (2000) Palaeotemperature reconstruction from noble gases in ground water taking into account equilibration with entrapped air. Nature 405:1040–1044
Anisimova N, Nikitina N, Piguzova V, Shepelyev V (1973) Water sources in central Yakutia. In: Proc. Second International Conference on Permafrost, Yakutsk, USSR, July 1973
Bennett M, Huddart D, Hambrey M, Chienne J (1998) Modification of braided outwash surfaces by aufeis: an example from Pedersenbreen, Svalbard. Z Geomorphol 42:1–20
Brook G, Ford D (1980) Hydrology of the Nahanni Karst, northern Canada and the importance of extreme summer storms. J Hydrol 46:103–121
Carey KL (1970) Icing occurrence, control and prevention, an annotated bibliography. Cold Region Research and Engineering Laboratory special report, US Army Corps of Engineers, Washington, DC, 151 pp
Carey SK, Woo M (2005) Freezing of subarctic hillslopes, Wolf Creek Basin, Yukon, Canada. Arctic Antarct Alpine Res 37(1):1–10
Cinq-Mars J, Lauriol B (1985) Le karst de Tsi-it-toh-Choh: notes préliminaires sur quelques phénomènes kastiques de Yukon septentrional [The karst Tsi-it-toh-Choh: perliminary notes on some karst phenomena of northern Yukon]. Ann Soc Geol Belg 107:185–195
Clark I, Fritz P (1997) Environmental Isotopes in hydrogeology. Lewis, New York
Clark I, Lauriol B (1997) Aufeis of the Firth River Basin, northern Yukon, Canada: insights into permafrost hydrology and karst. Arct Alp Res 29:240–252
Clark ID, Douglas M, Raven K, Bottomley DJ (2000) Recharge and preservation of glacial meltwater in the Canadian Shield. Ground Water 38:735–742
Clark ID, Lauriol B, Harwood L, Marschner M (2001) Groundwater contributions to discharge in a permafrost setting: Big Fish River, NWT. Arct Antarct Alp Res 33:62–69
Craig P, McCart P (1975) Classification of stream types in Beaufort Sea drainages between Prudhoe Bay, Alaska, and the Mackenzie delta, N.W.T., Canada. Arct Alp Res 7:183–198
Duk-Rodkin A (1999) Glacial limits map of Yukon Territory. Open file report 3694. Geological Survey of Canada, Ottawa
Eley F (1974) Mesoscale climatic study of Norman Wells, NWT Canada, Environmental-Social Committee, northern pipeline, Department of Indian and Northern Affairs, Ottawa, pp 56
Environment Canada (2010) National Climate Data and Information Archive. Environment Canada, Ottawa
Ford D, William P (2007) Karst hydrogeology and geomorphology. Wiley, West Sussex, UK
French H (1996) The periglacial environment. Addison Wesley Longman, Vancouver, Canada
Friedrich R (2007) Grundwassercharakterisierung mit Umwelttracern: Erkundung des Grundwassers der Odenwald-Region sowie Implementierung eines neuen Edelgas-Massenspektrometersystems [Groundwater characterization by environmental tracers: exploration of groundwater in the Odenwald region, as well as implementation of a new noble gas mass spectrometer system]. PhD Thesis, University of Heidelberg, Germany
Grasby SE, Allen CC, Longazo TG, Lisle JT, Griffin DW, Beauchamp B (2003) Supraglacial sulfur springs and associated biological activity in the Canadian High Arctic: signs of life beneath the ice. Astrobiology 3:583–596
Hamilton J, Ford D (2002) Karst geomorphology and hydrogeology of the Bear Rock Formation: a remarkable dolostone and gypsum megabreccia in the continuous permafrost zone of Northwest Territories, Canada. Carbonates Evaporites 17:114–115
Hayashi M, Quinton WL, Pietroniro A, Gibson JJ (2004) Hydrologic functions of wetlands in a discontinuous permafrost basin indicated by isotopic and chemical signatures. J Hydrol 296:81–97
Hu X, Pollard W (1997) The hydrologic analysis and modelling of river icing growth, North Fork Pass, Yukon Territory, Canada. Permafr Periglac Process 8:279–294
Kipfer R, Aeschback-Hertig W, Peeters F, Stute M (2002) Noble gases in lakes and groundwaters. In: Porcelli D, Ballentine CJ, Wieler R (eds) Noble gases in geochemistry and cosmochemistry. Mineralogical Society of America, Washington, DC, 642 pp
Lacelle D, Lauriol B, Clark I (2006) Effect of chemical composition of water on the oxygen-18 and carbon-13 signature preserved in cryogenic carbonates, Arctic Canada: implications in paleoclimatic studies. Chem Geol 234:1–16
Lauriol B, Gray JT (1990) Drainage karstique en milieu de pergélisol: le cas de l’île d’Apakok, T.N.O, Canada [Karst drainage in permafrost: the case of Apakok Island, NWT, Canada]. Permafr Periglac Process 1:129–144
Lucas LL, Unterweger MP (2000) Comprehensive review and critical evaluation of the half-life of Tritium. J Res Nat Inst Stand Technol 105(4):541–549
Manning AH, Solomon DK, Sheldon AL (2003) Applications of a total dissolved gas pressure probe in ground water studies. Ground Water 41:440–448
McFadden T (1990) The Kilpisjärvi Project. J Cold Regions Eng 4(2)
McKay C, Anderson D, Pollard WH, Heldman JL, Doran PT, Fritsen C, Priscu J (2005) Polar lakes, streams and springs as analogs for the hydrological cycle on Mars. In: Water on mars and life [Advances in astrobiology and biogeophysics]. Springer, Berlin, pp 219–233
Michel FA (1977) Hydrogeologic studies of springs in the Central Mackenzie Valley, Northwest Territories, Canada. MSc Thesis, University of Waterloo, Canada
Michel FA (1986) Hydrogeology of the Central Mackenzie Valley. J Hydrol 85:379–405
Mochnacz NJ, Schroeder BS, Sawatzky CD, Reist JD (2010) Assessment of northern Dolly Varden, Salvelinus malma malma (Walbaum, 1792), habitat in Canada. Canadian Manuscript Report of Fisheries and Aquatic Sciences 2926, Fisheries and Oceans Canada, Ottawa, vi + 48 pp
Mohapatra RK, Murty SVS (2000) Search for the mantle nitrogen in the ultramafic xenoliths from San Carlos, Arizona. Chem Geol 164:305–320
Muller S (1947) Permafrost or permanently frozen ground and related engineering problems. Military Intelligence Division, Ann Arbor, MI
Mutch RA, McCart P (1974) Springs within the northern Yukon drainage system (Beaufort Sea Drainage). In: McCart P (ed) Fisheries research associated with proposed gas pipeline routes in Alaska, Yukon and Northwest Territories.. Canadian Arctic Gas Study, pp 34
Omelon C, Pollard W, Andersen D (2006) A geochemical evaluation of perennial spring activity and associated mineral precipitates at Expedition Fjord, Axel Heiberg Island, Canadian High Arctic. Appl Geochem 21:1–15
Peeters F, Beyerle U, Aeschbach-Hertig W, Holocher J, Brennwald MS, Kipfer R (2003) Improving noble gas based paleoclimate reconstruction and groundwater dating using 20Ne/22Ne ratios. Geochim Cosmochim Acta 67:587–600
Pollard WH (2005) Icing processes associated with high Arctic perennial springs, Axel Heiberg Island, Nunavut, Canada. Permafr Periglac Process 16:51–68
Poole JC, McNeill GW, Langman SR, Dennis F (1997) Analysis of noble gases in water using a quadrapole mass spectrometer in static mode. Appl Geochem 12:707–714
Power G, Brown RS, Imhof JG (1999) Groundwater and fish: insights from northern North America. Hydrol Process 13:401–422
Prowse TD, Wrona FJ, Reist JD, Gibson JJ, Hobbie JE, Le’vesque LMJ, Vincent WF (2006) Climate change effects on hydroecology of arctic freshwater ecosystems. Ambio 35(7):347–358
NRCan (Natural Resources Canada) (2003) The atlas of Canada: permafrost. Government of Canada, Ottawa
NRCan (Natural Resources Canada) (2004) The atlas of Canada: geological provinces. Government of Canada, Ottawa
Sanford WE, Shropshire RG, Solomon DK (1996) Dissolved gas tracers in groundwater: simplified injection, sampling and analysis. Water Resour Res 32:1635–1642
Scholz H, Baumann M (1997) An ‘open system pingo’ near Kangerlussuaq (Søndre Strømfjord), West Greenland. Geol Greenl Surv Bull 176:104–108
Smith S, Burgess M (2002) A digital database of permafrost thickness in Canada. Open file 4173, Geological Survey of Canada, Ottawa
Stotler RL, Frape SK, Ruskeeniemi T, Ahonen L, Onstott TC, Hobbs MY (2009) Hydrogeochemistry of groundwaters in and below the base of thick permafrost at Lupin, Nunavut, Canada. J Hydrol 373:80–95
Taylor A, Nixon M, Eley J, Burgess M, Egginton P (1998) Effects of atmospheric inversions on ground surface temperatures and discontinuous permafrost, Norman Wells, Mackenzie valley, Canada. In: Lewkowicz A, Allard M (eds) Proceedings of the 7th International Conference on Permafrost, Yellowknife, NT, June 1998, pp 1043–1047
Tolstikhin IN, Kamenskiy IL (1969) Determination of ground-water ages by the T-3He Method. Geochem Int 6:810–811
Tolstikhin N, Tolstikhin O (1976) Groundwater and surface water in the permafrost region (translation). Inland Waters Directorate, Government of Canada, Ottawa, 22 pp
Utting N (2012) Geochemistry and noble gases of permafrost groundwater and ground ice in Yukon and the Northwest Territories, Canada. PhD Thesis, University of Ottawa, Canada
Utting N, Clark ID, Lauriol B, Wieser M, Aeschbach-Hertig W (2012) Origin and flow dynamics of perennial groundwater in continuous permafrost terrain using isotopes and noble gases: case study of the Fishing River, Northern Yukon, Canada. Permafr Perigl Process 23(2):91–106. doi:10.1002/ppp.1732
van Everdingen RO (1981) Morphology, hydrology and hydrochemistry of karst in permafrost terrain near Great Bear Lake, Northwest Territories. Government of Canada, Ottawa, 53 pp
Venzke J (1988) Observation on icings phenomena in the Icelandic subarctic-oceanic environment. Geookodynamik 9:207–220
Vogel JC (1993) Variablity of carbon isotope fractionation during photosynthesis. In: Ehleringer JR, Hall AE, Farquhar GD (eds) Stable isotopes and plant carbon-water relations. Academic, San Diego, CA, pp 29–38
White DE (1957) Thermal waters of volcanic origin. Geol Soc Am Bull 68:1637–1658
Woo M, Marsh P (2005) Snow, frozen soils and permafrost hydrology in Canada, 1999–2002. Hydrol Process 19:215–229
Wrangel F (1841) A journey to the northern shores of Siberia and along the Arctic Ocean made in 1820–1924. Harper, New York
Yoshikawa K, Hinzman L, Kane D (2007) Spring and aufeis (icing) hydrology in Brooks Range, Alaska. J Geophys Res 112:1–14
Acknowledgements
Thanks to Brewster Conant Jr. for help in the field and ideas during this research. Also thanks to Paul Middlestead, Wendy Abdi, Patricia Wickham, Ping Zhang, Ratan Mohapatra and Monika Wilk who assisted with isotopic and geochemical analyses. Thanks to all those who helped with field work including André Pellerin, Billy Nukon, Geoff Cramond, Angelina Buchar, Lisa Tellier and Marielle Fortin-McCuaig. Funding for student travel to N.W.T. and Yukon and was provided by the Northern Scientific Training Program. Funding for helicopter transport was provided by the Yukon Geological Survey, the Polar Continental Shelf Project and Fisheries and Oceans Canada. This work was funded through NSERC Discovery and Northern Supplement grants to I.D. Clark.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the theme issue “Hydrogeology of Cold Regions”
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 368 kb)
Rights and permissions
About this article
Cite this article
Utting, N., Lauriol, B., Mochnacz, N. et al. Noble gas and isotope geochemistry in western Canadian Arctic watersheds: tracing groundwater recharge in permafrost terrain. Hydrogeol J 21, 79–91 (2013). https://doi.org/10.1007/s10040-012-0913-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-012-0913-8