Abstract
The groundwater of major karst systems and submarine springs in the coastal limestone aquifer of Syria has been investigated using chemical and isotopic techniques. The δ18O values of groundwater range from −6.8 to −5.05‰, while those for submarine springs vary from −6.34 to +1.08‰ (eastern Mediterranean seawater samples have a mean of +1.7‰). Groundwater originates from the direct infiltration of atmospheric water. Stable isotopes show that the elevation of the recharge zones feeding the Banyas area (400–600 m a.s.l.) is higher than that feeding the Amrit area (100–300 m a.s.l.). The 18Oextracted (18O content of the seawater contribution) for the major submarine springs suggests a mean recharge area elevation of 600–700 m a.s.l., and lower than 400 m a.s.l. for the spring close to Amrit. Based on the measured velocity and the percentage of fresh water at the submarine springs outlet, the estimated discharge rate is 350 million m3/year. The tritium concentrations in groundwater (1.6–5.9 TU) are low and very close to the current rainfall values (2.9–5.6 TU). Adopting a model with exponential time distribution, the mean turnover time of groundwater in the Al-sen spring was evaluated to be 60 years. A value of about 3.7 billion m3 was obtained for the maximum groundwater reservoir size.
Résumé
L’eau souterraine des principaux systèmes karstiques et sources sous-marines de l’aquifère calcaire côtier en Syrie a été étudiée grâce à des techniques chimiques et isotopiques. Les valeurs de δ18O sont comprises entre -6.8 et -5.05‰ pour l’eau souterraine, alors que celles des sources sous-marines varient entre -6.34 et +1.08‰ (les échantillons d’eau de mer de l’Est de la Méditerranée présentent une moyenne de +1.7‰). L’eau souterraine provient de l’infiltration directe de la pluie. L’étude des isotopes stables montre que l’altitude des zones de recharge alimentant la région de Banyas (400–600m au dessus du niveau moyen de la mer, a.s.l) est plus élevée que celle de la zone alimentant la région d’Amrit (100-300m a.s.l). L’étude du 18O extracted (la teneur en 18O de la contribution de l’eau de mer) suggère, pour les principales sources sous-marines, une zone de recharge d’altitude moyenne de l’ordre de 600–700m a.s.l et inférieure à 400m pour la source proche d’Amrit. A partir de la vitesse mesurée et du pourcentage d’eau douce à la sortie des sources sous-marines, le débit est estimé à 350 million m3/an. Les concentrations en tritium dans l’eau souterraine (1.6-5.9 UT) sont basses et très proches de celles de la pluie actuelle (2.9-5.6 UT). A partir d’un modèle de distribution exponentielle dans le temps, la durée moyenne de renouvellement de l’eau souterraine pour la source de Al-sen a été évaluée à 60 ans. La taille maximum du réservoir d’eau souterraine a pu être estimée à environ 3.7 billion m3.
Resumen
Se ha investigado el agua subterránea de sistemas kársticos principales y manantiales submarinos en el acuífero costero de calizas de Siria usando técnicas químicas e isotópicas. Los valores δ18O de agua subterránea varían de -6.8 a -5.05‰, mientras que los valores en manantiales submarinos varían de -6.34 a +1.08‰ (las muestras de agua de mar del Mediterráneo tienen una media de + 1.7‰). El agua subterránea se deriva de la infiltración directa de agua atmosférica. Los isótopos estables muestran que zonas de recarga que alimentan el área Banyas (400-600 m.s.n.m.) se encuentran a mayor altura que la zona de recarga que alimenta el área Amrit (100-300 m.s.n.m.). El valor 18Oextraido (contenido 18O de la contribución de agua de mar) para los manantiales submarinos principales sugiere una elevación media para el área de recarga de 600-700 m.s.n.m. y menor que 400 m.s.n.m. para el manantial cercano a Amrit. En base a la medición de velocidad y el porcentaje de agua fresca en la desembocadura de los manantiales submarinos se estimó una tasa de recarga de 350 millones m3/año. Las concentraciones de tritio en agua subterránea (1.6-5.9 UT) son bajas y muy cercanas a los valores de lluvia actual (2.9-5.6 UT). Se evaluó el tiempo de retorno del agua subterránea en el manantial Al-sen adoptando un modelo con distribución de tiempo exponencial obteniendo un valor de 60 años. Se obtuvo un valor de aproximadamente 3.7 billones de m3 para el tamaño máximo del reservorio de aguas subterráneas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The essential groundwater in Syria is usually found in karstified limestone and dolomite aquifer systems. The recharge of these aquifer systems is related to rainfall, which is generally sporadic and irregular in space and time. During the last few decades, a dry continental climate has prevailed in the eastern Mediterranean Basin, leading to substantial decrease in average precipitation. The accelerated increase in population growth reinforces the water demand versus water resources problem in Syria. Several basins face shortage in water supply, particularly the Damascus Basin. Therefore, authorities are looking for alternative resources. The coastal area, with the important karstic reservoir, is considered to be a potential alternative resource, including the submarine springs. Submarine springs are typical phenomena in the maritime region along the eastern Mediterranean Sea. The undersea discharge of groundwater had been well known in the regions of Syria and Lebanon since the days of the Romans. Exploitation of water from these springs involved collection by a boat, using a lead funnel and leather tube, and transport to the city of Tyre in Lebanon and the island of Arados, Syria (Ghannam et al. 1998; UNESCO 2004). Several investigators have reported the occurrence of many submarine springs, some of them yielding fairly large flows, off the coasts of Syria and Lebanon (Gruvel 1930; Dubertret 1962; Kareh 1966; Moullard et al. 1967 and Hakim 1985). Syria has a coastline of 180 km, and several potential submarine spring sites. The first specific study on the coastal basin was undertaken by Selkhozpromexport (1979), which indicated that the groundwater outflows as submarine springs (from Bassieh Gulf in the north to the Lebanon border in the south) and is estimated at 1 billion m3/year approximately. Quantitative values of submarine outflow are mainly estimated according to hydrological balance considerations. Another study (Irie and Al Assad 2002) specified the location of submarine springs in Bassieh Gulf. The most important work refers to 29 spring sites stretching over 50 km coastline between the Bassieh Gulf in the north, and the Hamidiyeh village in the south (IBG/DHV 2000). The discharge rate of the springs at Bassieh Gulf and Tartous harbor were estimated using a combination of flow velocity and fresh water content. Discharge ranges from 3 m3/s at Bassieh Gulf to 3.5 m3/s at Tartous harbor. Using geochemistry (major elements) and environmental isotopes (18O, 2H and tritium), the present study concerns (1) the recharge of the karst aquifer and submarine springs, (2) the mixing rate between submarine springs water and seawater and quantifying their discharge where possible, and (3) the estimation of mean residence time of groundwater and storage capacity. The results were added to the previous studies to understand the process of water resources management.
Geological and hydrogeological setting
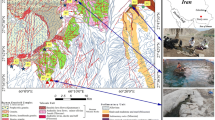
The study area (34°39′ N and 35°16′ E) is a part of the coastal basin in the west of Syria, which acts as a catchment area of about 2,100 km2 (Fig. 1). Topographic features of the coastal basin include various types of mountains, plateaus, depressions and lowland composed of Mesozoic and Quaternary complex rock formations. The coastal mountains rise to 1,560 m a.s.l. (meters above sea level). They are a monoclinal horst with a gentle anticlinal bend towards the west, where the Jurassic carbonate deposits crop out (Ponikarov 1966). The Jurassic deposits are overlain by Cretaceous, Paleogene and Neogene deposits in the west. The landmass is made up of a very thick carbonate sequence, capped by basalts on its southern side and increasingly overlain by marls on its northern side. The landmass consists of 1,200–2,000-m-thick alternating layers of dolomite, dolomitic limestone of Jurassic and Cretaceous. Upper Cretaceous (Maestrichtian) and Eocene are essentially made up of marls, with limestone intercalations; thickness of the marls averages 250–300 m. Basaltic rocks and tuff lava of the Pliocene have an average thickness of 100 m. Tectonic activity, especially during the Tertiary Period, is represented by the opening of the Ghab rift and the development of a horst structure in the coastal area. Faults of NE-SW direction displace the stratigraphic alternance with offsets up to more than 50 m. In the vadose zone, these faults act as favorable infiltration pathways for the recharge of deep lying aquifers, as well as major flow channels within the saturated zone (IBG/DHV 2000).
Location and geological map (Ponikarov 1966) and location of groundwater sampling sites in the study area: 1 alluvium (Quaternary); 2 conglomerates (Neogene); 3 basalt (Neogene); 4 chalky limestone (Paleogene); 5 dolomites, limestone and clays (Cenomanian/Turonian); 6 limestone, dolomites (U. Jurassic); 7 submarine springs; 8 well; 9 spring; 10 fault; 11 river; 12 artesian well
Three hydrological units constitute the major water reservoirs in the study area. These units are grouped under three main aquifer systems: the shallow basalt aquifer, Upper Cretaceous (Cenomanian-Tuoronian) main aquifer, and the Jurassic aquifer. Within the study region, the main water resource is confined and associated with the limestone and dolomite aquifer of the Upper Cretaceous. This aquifer is located between two aquitards of clays and marls: the Aptian-Albian at the bottom and the Maestrichtian and Eocene at the top (Fig. 2). Highly karsted, tectonically broken rocks and the step-like pattern of the slope create favorable infiltration conditions for precipitation, especially in the eastern, most elevated part of the region (Qadmous highland).
Diagrammatic representation of a cross section of the study region (Irie and Al Assad 2002)
The Jurassic aquifer has a minimum depth of 300 m and has not been exploited yet. The unconfined shallow aquifer in the basalt may discharge submarine springs close to the shoreline. Most of the submarine springs in the study area are discharged from the confined aquifer of Upper Cretaceous. Their hydraulic head is higher than the hydrostatic pressure of the salty seawater. This is confirmed by the presence of several artesian wells along the shoreline from Banyas to Hamidiyeh (IBG/DHV 2000). The spatial distribution of the submarine springs and artesian wells indicate the strong relationship with NE-SW tectonic features. Annual precipitation is 700–1,000 mm near the coast, while 1,100–2,000 mm of annual rainfall is characteristic for the highest zones near the eastern water divide. Most precipitation occurs between November and April.
Sampling and analyses
Precipitation samples were collected monthly from January 2000 to March 2003 from Babjena meteoric station and Tartous station. These two stations are located on the lowland and highland respectively. Twelve springs and 21 wells tapping the coastal aquifer where sampled in September 2002 (Fig. 1). Three springs (sites Nos. 1, 2 and 12) have average discharge rates of 10.5, 3.5, and 1 m3/s respectively); six springs (sites Nos. 21–23 and 25–27) discharge at the contact zone between the Jurassic and the Upper Cretaceous. Two springs (sites Nos. 28, 29) discharge close to the coast. The wells are located along the coastal line between Banyas and Amrit. Six of them (sites Nos. 3, 4, 15, 30, 31, and 33) are artesian wells. The depths range from 150 to 285 m. A well (site No. 24) of 600 m depth is located at the contact between Jurassic and Upper Cretaceous.
The marine springs were sampled by a professional diver using appropriate equipment (Hydro-Bios, Kiel, Germany). Two sites were selected (Fig. 3a,b).
-
1.
Seven springs (BS1-BS7 in Fig. 3a) were sampled at Bassieh in October 2002. The permanent discharging springs BS1 and BS2 have the largest rate. They discharge at several points between 15 and 37 m below sea level (m b.s.l.). The remaining five springs discharge at depths between 5 to 15 m b.s.l.
-
2.
At the Tartous harbor site, five springs (TS1–TS5 in Fig. 3b) were sampled at depths from 2 to 15 m b.s.l. In addition, five seawater samples from 0.5 m depth were collected at three points at the Bassieh site and two points in the Tartous harbor.
For tritium analysis 1 L was collected; for stable isotope determinations (δ18O, δ2H), a 100-ml bottle was filled. The water for major cation and anion analyses was filtered through 0.45 μm Millipore filters, acidified (cations only) before bottling and then refrigerated.
The temperature, pH, electrical conductivity (EC) and total alkalinity (i.e. \({\text{HCO}}^{{\text{ - }}}_{{\text{3}}} \)) were measured in the field. The chemical and isotopes analyses were carried out in the laboratories of the Department of Geology in the Syrian Atomic Energy Commission (SAEC). The δ18O, δ2H analyses were performed by the mass spectrometer Delta plus. The ratios 18O/16O and 2H/1H values are reported as δ notation in per mil relative to the V-SMOW (Vienna-Standard Mean Ocean Water). The tritium, (after electrolysis) was measured by liquid scintillation counter (Quantulus 1220). Electrolytic enrichment of tritium and liquid scintillation counting are given by Florkozski (1981). Measurement accuracy for δ18O, δ2H and tritium are ±0.15, ±1.0‰ and ±0.5 TU respectively. The chemical analyses of major anions and cations were determined by an ion chromatograph (Dionex 120).
Results and discussion
Chemical composition
The results of the isotope and chemical analyses of the water samples from the coastal aquifer and submarine springs are presented in Tables 1 and 2. The groundwater from the karstified Cenomanian-Turonian aquifer is poorly mineralized as shown by its low electrical conductivity (308–825 μS/cm). Its temperature ranges from 15.1 to 20.8°C and the pH from 7.11 to 8.45. Submarine springs water is brackish (2,800–47,000 μS/cm), the temperature ranges from 22 to 27°C, and the pH is about 7.5; normally seawater has a high EC value (60,000–67,000 μS/cm).
The groundwater is characterized as calcium-magnesium and bicarbonate type, with the following ionic sequences: \({\text{HCO}}^{{\text{ - }}}_{{\text{3}}} > {\text{Cl}}^{{\text{ - }}} > {\text{SO}}^{{{\text{2 - }}}}_{{\text{4}}} \). In all cases, the concentration of Ca2+ and Mg2+ exceeds that of Na+ and K+. This water type is, in fact, a reflection of the carbonate rocks predominant in the area investigated. The Ca2+ (10–90 mg/L) and Mg2+ (15–38 mg/L) concentrations are high because of the dissolution of limestone and dolomite. The \({\text{SO}}^{{{\text{2 - }}}}_{{\text{4}}} \) content ranges from 6 to 80 mg/L, though the latter value is associated with high Ca2+ content and consequently, it comes from the gypsum dissolution present in the carbonate rocks. Concerning the calco-carbonic parameters, the PCO2 values (10−1.7–10−2.5 atm) in the groundwater samples are significantly low, and indicate that CO2 is gained along the flow path. This reduction value of PCO2 in water tends to increase the saturation of water with calcite (Bakalowicz 1979, 1994; Plagnes 2000).
All the groundwater samples are saturated with calcite, some of them slightly, but the others present saturation indices SIc >0.5. The major part of groundwater was also saturated with dolomite; in this case, the Ca/Mg ratio is low and calcite precipitation may occur.
The Ca/Mg ratio of water samples range from 1.1 to 2.7. The water samples from the contact zone between Jurassic and Upper Cretaceous had low Ca/Mg ratio values (1.1–1.5) because the water dissolves dolomite that outcrops in this sector.
The chemical composition of submarine springs water deviates from that of the groundwater. Obviously, the submarine spring water is a mixture of seawater and groundwater; the chemical composition depends on the mixing rate between seawater (Na–Cl) and groundwater (Ca–Mg–HCO3). All samples of the submarine springs display Na–Cl facies; only two springs had Na–Mg–Cl (at BS1) and Na–Ca–Cl (at TS2). The salinity for the two springs is 1.5 and 5 g/L respectively.
The Cl− concentration of most groundwater samples ranges from 5 to 30 mg/L, except for some samples (site Nos. 28 30 and 31), where the Cl− concentration ranged from 40 to 90 mg/L. The lowest values reflect water recharge from the highest altitude and represent the rainwater at some distance from the sea. The highest values are located close to the shoreline, and may be linked with some local lithological modification. The Cl− concentration of submarine springs water ranges from 700 to 23,000 mg/L, while the average value for the 5 samples (at 50 cm depth) of the eastern Mediterranean seawater along the Syrian coast is 27,000 mg/L. A similar Cl− concentration of 26,400 mg/L was measured in seawater along the eastern Mediterranean off the Lebanon coast (Ayoub et al. 2002). In general, the Cl− concentration of seawater is 20,000 mg/L (Drever 1997). The higher values of Cl− in the eastern Mediterranean seawater compared with that of general seawater is due to high evaporation enrichment. The average stable isotopes values of the eastern Mediterranean seawater (+1.7 and +10.8‰ for 18O and 2H, respectively) support this suggestion. The relationship between Cl− and the major cations Na+, Ca2+ and Mg2+ (Fig. 4) indicate that all the submarine spring samples at the two sites (Bassieh and Tartous) represent a simple mixing between the seawater and fresh water.
The Na/Cl ratio of groundwater samples exhibits values between 0.7 and 1.75 (Fig. 5). This range implies a further source of sodium. The enrichment of Na+ may be due to the contribution of basalt mineral solution abundant in the study area. Silica analyses should further support this conclusion. Unfortunately, data on silica content are not available for this study. Submarine springs water exhibit a Na/Cl ratio mostly between 0.7 and 0.83. The average Na/Cl ratio of the eastern Mediterranean seawater samples is only 0.68, which is less than the commonly used value of seawater (0.85; Clark and Fritz 1997).
Stable isotopes in rainwater
Precipitation is the main source of groundwater recharge in karst systems of the study area. Thus, knowledge of its isotopic composition is indispensable for this study. The rainwater samples from the stations at Tartous (5 m a.s.l.) and Babjena (1,000 m a.s.l.; Fig. 1), displayed high seasonal variation of isotopic composition (Fig. 6). The isotopic compositions at the beginning (October and November) and end of the rainy season (March and April) are characterized by relatively lighter rainfall with enriched δ18O values, and exhibit “amount effect” (a process whereby the isotopic composition of samples from light rainfall is more enriched than that of samples collected from heavy rainfall). The most isotopically depleted rainfall samples were those collected during the major rainy season (December, January and February). The δ18O values range from −6.94 to −2.83‰ with a weighted mean value of −5.2‰ at Tartous station, and from −8.94 to −3.64‰ with a weighted mean value of −7.3‰ at Babjena station. The relatively high deuterium excess “d”, (d=δ2H−8 δ18O) is a typical property of Mediterranean precipitation (Gat and Carmi 1970). The increase of “d” has been attributed to isotopic exchange with the moisture originating from the Mediterranean Sea, which is characterized by lower relative humidity conditions (Gat 1980). The values of deuterium excess are significantly higher than 10‰, and range from 12 to 25‰. Some deuterium excess values lower than 10‰ have been observed at Tartous station during the beginning and end of the rainy season. These values are most probably related to isotopic enrichment due to evaporation. The relationship between deuterium (δ D) and δ18O values monthly precipitation is given by the equation (Fig. 7).
with (R 2=0.9 and N=46)
This equation represents the coastal meteoric water line in Syria. It is similar to that of the Antalya station (Turkey) about 500 km NW of the study area (IAEA 1992), which was given by:
The slope of the coastal meteoric water line in Syria is less than the MMWL (Mediterranean meteoric water line), and suggests that much of the rainfall occurred at a mean humidity of less than 100% (Fontes and Gonfiantini 1970; Gonfiantini 1986). The δ18O precipitation value at the high elevation rain station (Babjena) is consistently lighter than the (Tartous) lower elevation station (Fig. 6), which is consistent with the well-documented elevation effect (Dansgaard 1964). The “altitude effect” is shown by a depletion of about −0.2‰ per 100 m for δ18O between the two stations. This gradient is comparable to −0.23‰ per 100 m for nine meteorological stations distributed at different altitudes (from 0 to 1,540 m a.s.l.) in the western part of Syria (Kattan 1997).
Stable isotope composition of groundwater and submarine springs
The δ18O values for groundwater range from −6.8 to −5.05‰ and for δD from −31.81 to −23.82‰, with mean values of −6.00 and −28.4‰, respectively. The deuterium excess values range from 17 to 23‰ (Table 1). The samples taken from the south (Amrit area) are more enriched with δ18O, with an average value of −5.56‰, than the values found in the northern area (Banyas and Bassieh) where the average value of δ18O is −6.24‰. The spring water from the boundary between the Jurassic Mountains and the Upper Cretaceous has the lowest value of δ18O (−6.49‰). This difference is attributed to their recharge origin.
Consequently, the altitude effect of −0.23‰ per 100 m will lead to δ18O values of −8.5‰ in groundwater recharged at 1,500 m. The observed relatively enriched composition of the coastal aquifer (Upper Cretaceous) suggest a much lower mean elevation of recharge zone, situated over a large range of elevation (700±100 m or lower, see Fig. 8). According to the recharge elevations and mechanism, groundwater samples in the study area can be divided into three groups. First, groundwater from higher springs has the highest recharge elevation between, 500 and 700 m. The second group consists of the high-yield artesian wells and large springs of the Banyas and Bassieh field and produce groundwater recharged at average elevation of 400 to 600 m. Clearly, the karstic nature of this recharge region, combined with the drop in elevation caused by down-faulting, has created the high artisan pressure and yield of wells. The last group is the groundwater from Amrit area, which has the recharge elevation of 100 to 300 m. The elevation estimates of these three groups correspond more or less to the natural topographic situation, where the Upper Cretaceous aquifer outcrops in the study area. Only one sample from the Jurassic aquifer (site No. 24) has a main recharge zone elevation of more than 700 m. Isotopic data from the submarine springs at Bassieh site range from −6.34 to −31.57‰ at BS1, and from +1.08 to +9.23‰ at BS4 (Table 2). The corresponding values at the Tartous Harbor site range from −4.97 to −25.95‰ at TS2, and from −0.92 to −2.95‰ at TS1. Elevation of recharge areas to the submarine springs were calculated on the basis of 18O content. Seawater effects, calculated by chloride content, are subtracted from 18O content in accordance with the equation:
Where:
- 18Oextracted:
-
18O content (‰ SMOW) of the seawater contribution extracted
- 18Osample:
-
18O content (‰ SMOW) of the water sample
- P sea :
-
percentage of seawater contribution (calculated from chloride content)
- σ:
-
standard deviation for 18O measurement
Recharge area elevation for the water of submarine springs is between 400 and 1,000 m if the seawater is extracted. In fact, it is inferred that, as the seawater contribution rate increases, the error on recharge elevation calculation increases. The 18Oextracted values for the springs BS1, BS7 and TS2 are −6.64, −6.46, and −5.97‰ respectively, which are only 10% of the seawater rate, give a recharge area elevation between 600 and 700 m for BS1 and BS7 (Fig. 8). The last spring (TS1), close to the Amrit area, has a recharge elevation lower than 400 m. The δ18O values of Mediterranean seawater samples range from +1.49 to +1.97‰ with a mean value of +1.7‰ and the δ2H values vary from +9.9 to +11.23‰ with a mean value of +10.8‰. These values show more enrichment than those of SMOW (δ18O=0 and δ2H=0‰) defined by Craig (1961). These high values in the eastern part of the Mediterranean Sea, are due to the evaporation that causes enrichment in stable isotopes and Cl− as mentioned above. The relationship between δD and δ18O in groundwater from the coastal aquifer and submarine springs samples fits a mixing line of the following equation (Fig. 9):
with (R 2=0.99, N=48)
The slope 5.41 of the mixing line is close to the slope 6 of the local meteoric water line in the study area (LMWL). Also, Fig. 9 shows that coastal and submarine waters are correlated on the same line indicating the same hydrological origin for both. These waters come from the same aquifer related to the Cenomanian-Turonian formations. The intersection of the mixing line with the MMWL corresponds to a value of about −7 and −34‰ for δ18O and δ2H compositions, respectively. This value coincides with an intermediate isotopic composition during the major rainy season at the two stations of Tartous and Babjena, indicating that the groundwater from the Upper Cretaceous and submarine springs have originated from rainfall over lands of around and less than 700 m a.s.l.
Determination of seawater contribution by Cl content
The relationship between δ18O in the groundwater samples and the Cl− concentration is illustrated in Fig. 10. This figure shows the following results:
-
1.
Coastal fresh water reflects primarily coastal or mountain meteoric water recharge
-
2.
Different submarine springs show different amounts of mixing with seawater, and a very low mixing is observed in BS1, BS7 and TS2
-
3.
The end point in the correlation corresponds to the seawater
Taking into account Fig. 10 and resulting observations, the proportion of fresh groundwater in the submarine spring water is calculated by the following equation:
Where:
- Psea :
-
percent seawater contribution
- Clsubmarine, :
-
chloride content (mmol/L) of the submarine water sample
- Clfresh water, :
-
chloride content (mmol/L) of typical fresh groundwater
- Clseawater, :
-
chloride content (mmol/L) of a typical seawater sample
This conservative mixing equation assumes that the chemical parameter (here chloride) on which the calculation is based, is conserved during the mixing process. Since it is probably the most conservative element in groundwater chemistry, the chloride ion has been used in the seawater contribution rate calculation. In all calculations, the seawater chloride content is assumed to be 760 mmol/L which is roughly the average chloride content of seawater collected from five sites where there is no fresh water contribution. The chloride content of groundwater is assumed to be 0.56 mmol/L, which is the average chloride content of groundwater in the Upper Cretaceous aquifer. The average chloride content of rainfall at the recharge area (Babjena station) is 0.14 mmol/L, implying that about 0.4 mmol/L chloride is supplied to the groundwater as a result of evaporation and dissolution.
The results are shown in Table 3. According to calculation in Eq. (5), the percentage of fresh water in the submarine springs ranges from 12 to 97% at Bassieh area and from 40 to 90% at Tartous Harbor. These percentages of fresh water are plotted versus δ18O in Fig. 11, indicating a very high correlation between values (R 2=0.98). Thus, the recharge origin of submarine water seems to be an admixture of fresh water from highly karstified aquifers (Cenomanian-Turonian formation), where meteoric water infiltrates easily, and seawater.
Discharge of the submarine springs
The discharge rate of the submarine springs is estimated from the velocity measurements at BS2, TS2 and TS3 springs (IBG/DHV 2000). A robust surface-water flow meter was installed 0.5–1.0 m above the sea bottom by a diver. The flow rate represents the flow conditions at the time of the survey: 18 September 2002 for BS2 and 11 September 2002 for TS2 and TS3. It is clear that the measurements represent only the flow condition at the moment of the survey.
Based on the measured velocity and the calculated percentage of fresh water at the submarine outlet, the total discharge rate of fresh water was estimated using the following simple equation:
Where:
- Q :
-
the discharge rate in m3/s
- V :
-
is the average velocity in m/s
- A :
-
outlet surface at sea bottom in m2 and
- P(f)%:
-
proportion of fresh water
According to this equation, the discharge rate of the submarine springs is estimated to be 11 m3/s or 350 million m3/year (Table 4). The average water balance for the period 1992 to 1996 in the study area indicates that the average annual precipitation is equal to 1,050 mm/year. Runoff and evapotranspiration were estimated at 38 and 28%, respectively. Thus, a rate of 357 mm/year was estimated for the amount of infiltration recharge over the Upper Cretaceous formation. Accordingly, if the whole Cenomanian-Turonian aquifer is contributing to the recharge of the submarine springs, then an annual recharge of 750 million m3/year could be anticipated. It is certain that the whole area is not participating in the recharge of the submarine springs; consequently, the total discharge of the springs should be less than the estimated value. It is to be noted further that the previous works have suggested a total discharge of 1,000 million m3/year from the same area (Selkhozpromexport 1979). The contradictory figures shown above confirm the current uncertainty regarding the reported discharge quantities of the submarine springs. In fact, it is the author’s aim to investigate further in order to establish reliable discharge values.
Mean residence time and tritium values
Tritium is a radioactive isotope of hydrogen (t 1/2=12.32 years; Lucas and Unterweger 2000) produced naturally by cosmic rays in the upper stratosphere. However, most of tritium found in the environment was introduced during atmospheric testing of nuclear bombs in the early 1960s. These tests increased the mean tritium content in precipitation above natural levels by as much as three orders of magnitude. Since that time, the amount of tritium in precipitation has decreased almost exponentially towards natural levels (Fig. 12).
Tritium concentration in precipitation is recorded at the GNIP (Global Network of Isotopes in Precipitation) stations, Bet Dagan about 300 km to the south (data available 1960–2001), and Antalya station, approximately 500 km to the northwest of the study area (data available 1963–2002). During the overlap period of these two records, the tritium levels at both stations are nearly indistinguishable and can be taken as reference for the Syrian coast. The tritium concentrations in the precipitation that was collected at the two stations during 2000–2002 vary from 3.07 to 5.8 TU with a similar average value 4.2±0.5 TU (mean of 9 samples). During September 2002, the tritium level of coastal groundwater varied between 1.62 and 5.9 TU (Table 1), and the mean of 3H content was 4±0.5 TU. Tritium was not detected in well No. 24, with a depth of 600 m, due to the long mean residence time (MRT) in the Jurassic aquifer. Several models have been suggested as appropriate for use of tritium in the estimation of mean turnover time of groundwater (Yurtsever 1979; Zuber 1986; Maloszewski and Zuber 1996). Attempts have been made to estimate the mean turnover time (residence time) of groundwater in the Al-sen spring (No. 1). In this study, an exponential model was adopted to calculate the turnover time. Based on the available tritium records of Bet-Dagan station (completed by Tartous station for the period 2003–2005) as the tritium input function, the simulated tritium output curves were calculated using the convolution integral expressed by:
where C 0 (t) is the output tritium concentration, \(h{\left( \tau \right)} = 1 \mathord{\left/ {\vphantom {1 {\tau _{0} e^{{ - (1/\tau _{o} )t}} }}} \right. \kern-\nulldelimiterspace} {\tau _{0} e^{{ - (1/\tau _{o} )t}} }\), and \(\tau _{0} = V \mathord{\left/ {\vphantom {V Q}} \right. \kern-\nulldelimiterspace} Q\)
λ is the radioactive decay constant, and τ0 is the turnover time of the system, V is the volume of the groundwater reservoir and Q is the base flow of the spring. The tritium data were available only for the period May 1994 to June 1995 (Abou-Zakhem 2000), which concluded that the mean turnover time of groundwater in Al-sen spring is 60 years. The current study’s observed tritium content was measured from January 2005 to October 2005. The tritium output curves for different values of mean turnover time for 40, 60, 80 and 120 years are shown together with the observed monthly tritium values in Fig. 13. For the periods of observation, the tritium content in discharge from Al-sen spring showed high variations (Fig. 13). This may by due to the contribution of two components: fast flow with high tritium content (recent water) and base flow with low tritium content (older water). Comparison of the calculated output curves with the observed monthly tritium values suggests a mean turnover time between 40 and 120 years. The observed mean annual tritium during 2005 in the Al-sen springs is 3.4 TU. This value yields a mean turnover time of 60 years. During the period 1990–2004, the average discharge of base flow during the dry period (August, September and October) is 2 m3/s, which implies that the groundwater reservoir size providing the base flow would be around 3.7 billion m3. This estimation of the groundwater reservoir volume is based on a value of turnover time of 60 years.
Conclusion
From the geological and hydrogeological setting, the confined aquifers of Upper Cretaceous age feed the submarine springs as they have sufficient hydraulic head to enhance the vertical uprising of karst water. Several artesian wells along the shoreline strongly support this interpretation. In addition, it seems that the tectonic factor has an important role in the formation of marine springs near shoreline.
Stable isotopes of oxygen and hydrogen and tritium, as well as major elements, proved to be an effective monitoring tool:
-
The stable isotope composition gives evidence that the groundwater and submarine springs have the same origin, from direct infiltration of meteoric water.
-
The mean elevation of the recharge zones for high-yield artesian wells and large springs of Banyas and Bassieh field (400–600 m) is higher than that for groundwater from Amrit area (100–300 m). The 18Oextracted for the major submarine springs (BS1, BS7 and TS2) gives a mean recharge area elevation between 600 and 700 m a.s.l. for springs BS1 and BS7, and lower than 400 m a.s.l. for spring TS1, close to the Amrit area.
-
The δ18O/Cl− relationship shows that all submarine springs water is a mixture of groundwater and seawater. The percentage of fresh water in the submarine springs at the two sites (Bassieh and Tartous) ranges from 12 to 97%.
-
The independent flow velocity measurements in September 2002 were used to find the total fresh water discharge of the permanent submarine springs (BS1, BS2 and TS2, TS3), which was estimated to be 350 million m3/year. This estimation equals 45% of the calculated mass balance in the area. The contradictory figures shown above confirm the current uncertainty regarding the reported discharge quantities of the submarine springs.
-
The mean turnover time of groundwater in the Al-sen spring, found by adopting a model with exponential time distribution, is 60 years. The maximum groundwater reservoir size of Al-sen spring reaches a value of around 3.7 billion m3, on the basis of base flow of 2 m3/s and a mean residence time of 60 years.
References
Abou-Zakhem B (2000) Environmental isotopes study of the aquifer system in the coastal area, AECS-G FRSR 212, Atomic Energy Commission of Syria, Damascus, p 58
Ayoub G, Khoury R, Ghannam J, Acra A, Hamdar B (2002) Exploitation of submarine springs in Lebanon: assessment of potential. J Water Supply: Res Technol, AQUA, 51.1
Bakalowicz M (1979) Contribution de le géochimie des eaux à la connaissance de l’aquifère karstique et de la karstification. (Contribution of water geochemistry to the knowledge of karst aquifer and karst processes). PhD Thesis, P. et M. Curie Paris-6, Paris, p 269
Bakalowicz M (1994) Water geochemistry: water quality and dynamics. In: Groundwater ecology, Elsevier, Amsterdam, pp 97–127
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis, New York, p 311
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436
Drever JI (1997) The geochemistry of natural water: surface and groundwater, environments, 3rd edn. Prentice Hall, Englewood Cliffs, NJ, p 436
Dubertret L (1962) Carte géologique du Liban; Syrie et bordure des pays voisins 1/100,000, Muséum National D’Histoire naturelle, Paris, notes et mémoires sur le Moyen- Orient, T. VIII (Geological map of Lebanon; Syria and the borders of nearby countries 1/100,000, National Museum of natural history, Paris, notes and memories on the Middle-East, T VIII). International Stratigraphy lexicon, vol. III, F.10c. CNRS, Paris
Florkozski T (1981) Low-level tritium assay in water samples by electrolytic enrichment and liquid-scintillation counting in the IAEA laboratory. In: Proceedings of the International Symposium on Methods of Low-Level Counting and Spectrometry, IAEA-SM-252/63, IAEA, Vienna, p 558
Fontes JC, Gonfiantini R (1970) Composition isotopique et origine de la vapeur d”eaux atmosphérique dans la région du lac Léman. (Isotopic composition and origin of the atmospheric steam in the area of Lake Leman). Earth Planet Sci Lett 7:325–329
Gat JR (1980) The isotopes of hydrogen and oxygen in precipitation. In: Fritz P, Fontes J-ch (eds) Handbook of environmental isotope geochemistry, vol. 1, The terrestrial environmental. Elsevier, Amsterdam, pp 21–48
Gat JR, Carmi I (1970) Evolution of the isotopic composition of atmospheric water in the Mediterranean Sea Area. J Geophys Res 75:3039
Ghannam J, George M, Ayoub G, Acra A (1998) A profile of the submarine springs in Lebanon as a potential water resource. Water Int 23:278–286
Gonfiantini R (1986) Environmental isotopes in lake studies. In: Fritz P, Fontes J-ch (eds) Handbook of environmental isotope geochemistry, vol. 1, The terrestrial environmental. Elsevier, Amsterdam, pp 113–168
Gruvel A (1930) Quelques sources sous-marines observées sur les cotes Libano Syriennes (Some submarine springs observed on the Libano-Syrian coast), vol 191. Comptes-rendus de l’ Academic des Sciences, Paris
Hakim B (1985) Recherches hydrologiques et hydrochimiques sur quelques karsts Méditerranéens Liban, Syrie et Maroc (Hydrological and hydrochemical research on some Mediterranean karsts of Lebanon, Syria and Marocco). University of Lebanon, Beirut, p 701
IBG/DHV (2000) Project of supplying part of the water demand of Damascus city and its countryside from the Syrian coastal area water surplus, Hydrogeological investigation, NR.1, Phase 1-first stage, Damascus water supply and sewerage authority, Damascus, Syrian Arab Republic
International Atomic Energy Agency (1992) Statistical treatment of data on environmental isotopes in precipitation; Tech. Rep. Series No. 331, IAEA, Vienna
Irie M, Al Assad A (2002) Basic study of submarine springs in Bassiya Gulf, Ministry of Irrigation, Water Research Center. Japan Overseas Cooperation Volunteers, Damascus, p 82
Kareh RP (1966) The submarine springs of Chekka: exploitation of a confined karstic aquifer discharging into the sea. MSc. Thesis, American University of Beirut, Lebanon
Kattan Z (1997) Chemical and environmental isotope study of precipitation in Syria. J Arid Environ 35:601–615
Lucas LL, Unterweger MP (2000) Comprehensive review and critical evolution of the half-life of tritium. J Res Natl Stand Techno 105(4):541–549
Maloszewski P, Zuber A (1996) Lumped parameter models for the interpretation of environmental tracer data. In: Manual on mathematical models in isotope hydrology. IAEA, Vienna, pp 9–58
Moullard L, Mijatovic B, Kareh RP, Massad B (1967) Exploitation d’ une nappe Karstique captive a exutoires sous-marins, problèmes poses et solution adoptée, cote libanaise (Exploitation of confined karst aquifer with discharge underwater: problems installations and adopted solution) vol 73. In: Hydrology of fractured rocks (I-Proc. Symposium Dubrovnic), Assoc. Int. Hydrol. Sci., Gentbrugge, pp 273–250
Plagnes V (2000) Structure et fonctionnement des aquifères karstiques: caractérisation par la géochimie des eaux. (Structure and function of the karstic aquifers: characterization by the geochemistry of water). Documents du BRGM 294, BRGM, Orléans, pp 376
Ponikarov VO (1966) The geology of Syria, Explanatory notes on the map of Syria, Scale 1:500 000, Part II. Mineral deposits and underground water resource, Technoexport, Moscow, pp 120
Selkhozpromexport (1979) Hydrogeological and hydrological surveys and investigation in four areas of Syrian Arab Republic, coastal area, vol. II, Hydrology. Georgian State Institute for Design of Water Resources Development Projects., Tbilisi, USSR, pp 173
UNESCO (2004) Submarine groundwater discharge. Management implications, measurements and effects. IHP-VI, Series on Groundwater No. 5, ICO Manuals and Guides No. 44, UNESCO, Paris
Yurtsever Y (1979) Environmental isotopes as a tool in hydrogeological investigations of southern karst regions of Turkey. Int. Seminar on Karst Hydrogeology, Antalya, October 1979, pp 269–293
Zuber A (1986) Mathematical models for the interpretation of environmental radioisotopes in groundwater systems, chap 1. In: Fritz P, Fontes J Ch (eds) Handbook of environmental isotope geochemistry, vol. 2. Elsevier, Amsterdam, pp 1–59
Acknowledgements
The author would like to thank I. Othman, Director General of AECS for use of their facilities during this study. I am also grateful to M. Geyh, M. Bakalowicz, W.R. Agha and S. Rammah for their helpful comments and useful discussion. The author thanks R. Naser, Head of the Geology Department at the AECS, and the staff of the laboratories for their cooperation in performing the isotopic and chemical analyses. The work was financially supported in part by the Supreme Council of Sciences (Res-Pro, No. 9. /2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Charideh, A., Rahman, A. Environmental isotopic and hydrochemical study of water in the karst aquifer and submarine springs of the Syrian coast. Hydrogeol J 15, 351–364 (2007). https://doi.org/10.1007/s10040-006-0072-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-006-0072-x