Abstract
Rising salinity levels is one of the significant signs of water-quality degradation in groundwater. The alluvial Pleistocene wells in the Jericho area, Palestine show high salinity and a high susceptibility to contamination. Future exploitation and management of the water resources under these conditions will require an in-depth understanding of the sources and mechanisms of contamination. The Jericho area is located in the basin of the Jordan Valley. The basin is underlain by alluvial deposits of soil, sand and gravel of Quaternary units Q1 and Q2, and marl clay and evaporites of the upper part of unit Q2. This paper deals with the source of salinity in the wells penetrating these units, using hydrochemical tracers. The study reveals three main zones of different salinity by using different diagnostic hydrochemical fingerprinting as tracers for elucidating the sources of salinity. It was concluded that the most probable sources of salinity are (1) the geological formations of the region, which form inter-fingering layers of both the Samara and Lisan formations of Pleistocene age, where the eastern Arab Project aquifers show the highest amount of sulphate. The location and geological formation of these wells within the Lisan suggested that the source of high sulphate content is the dissociation of gypsum. (2) The NaCl water within the same area may also be upwelling from a deep brine aquifer or from a fresh-water aquifer which contains salt-bearing rocks with particles becoming finer from west to east. This noticeable high TDS to the east should be affected by the rate of pumping from the upper shallow aquifer, especially in the wells of the Arab Project which are in continuous pumping during the year. (3) The third possible source of salinity is from anthropogenic influences. This can be easily shown by the increment of nitrate, bromide and sulphate, depending on whether the location of the well is coincident with urban or agricultural areas. This reflects the addition of agricultural chemical effluents or sewer pollution from adjacent septic tanks which are mainly constructed in top gravel in the Samara layer. Further studies are required, using different geochemical and isotopic techniques, to confirm these suggested salinity sources.
Résumé
Les teneurs croissantes de salinité est l’un des signes significatifs de la dégradation des eaux souterraines. Les forages des alluvions du Pléistocène dans la zone de Jéricho, Palestine, montrent des degrés élevés en salinité et une susceptibilité aiguë aux contaminations. L’exploitation et la gestion future des ressources en eaux souterraines sous ses conditions va nécessiter une bonne connaissance des mécanismes de la contamination. La zone de Jéricho est localisée dans le bassin de la vallée du Jourdain. Le bassin est recouvert par les dépôts alluvionnaires de sols, sables, et graviers d’unités quaternaires Q1 and Q2 et de marnes, argiles et évaporites de la partie supérieure de l’unité Q2. Cet article traite de la source de salinité dans les forages pénétrants ces unités, en utilisant des traceurs hydrochimiques. La conclusion est que les sources les plus probables de salinité sont (1) les formations géologiques régionales, qui forment des inter-couches des formations de Samara et de Lisan d’âge Pléistocène, présentant des teneurs en sulfate élevées dues à la dissolution du gypse. (2) Les eaux chlorurées-sodiques dans la même zone peuvent remonter en provenance d’un aquifère profond saumâtre ou d’un aquifère d’eau douce de roches salines présentant des particules de plus en plus fines de l’ouest vers l’est. Cette TDS notable à l’est pourrait être affectée par le taux de pompage des formations superficielles, et notamment dans la zone du Projet Arabe qui est en exploitation continue toute l’année. (3) La troisième source de salinité est d’origine anthropique. Ceci est facilement démontré par l’incrémentation des nitrates, bromides, et sulfates dépendant de la localisation des forages, à proximité ou non des zones urbaines et agricoles. Ceci reflète l’utilisation des effluents agricoles et des pollutions en provenance des fosses septiques qui sont installées dans la partie supérieure des graviers de la couche de Samara. D’autres études sont requises, utilisant différentes techniques isotopiques et géochimiques, qui permettraient de confirmer ces sources suggérées de salinité.
Resumen
Los niveles ascendentes de salinidad son una de las señales significativas de la degradación de la calidad del agua en agua subterránea. Los pozos aluviales Pleistocenos en el área Jericó, Palestina, muestran alta salinidad y alta susceptibilidad por contaminación. La explotación futura y gestión de los recursos hídricos bajo estas condiciones requerirá un entendimiento profundo de las fuentes y mecanismos de contaminación. El área Jericó se localiza en la cuenca del Valle Jordano. La cuenca está compuesta de depósitos aluviales de suelo, arena, y grava de unidades Cuaternarias Q1 y Q2, y marga, arcilla y evaporitas de la parte superior de la unidad Q2. Este artículo se ocupa de la fuente de salinidad en los pozos que penetran estas unidades utilizando trazadores hidro-químicos. El estudio revela tres zonas principales de diferente salinidad mediante la utilización de diferentes indicadores diagnósticos hidro-químicos como trazadores para aclarar las fuentes de salinidad. Se concluyó que las fuentes más probables de salinidad son (1) las formaciones geológicas de la región, las cuales forman capas entrelazadas de las Formaciones Samara y Lisan de edad Pleistoceno, donde los acuíferos del proyecto Árabe oriental muestran la cantidad más alta de sulfato. La localización y formación geológica de estos pozos dentro de la Lisan sugieren que la fuente del alto contenido de sulfato es la disociación de yeso. (2) El agua que contiene NaCl dentro de la misma área puede estar ascendiendo de un acuífero profundo salado o de un acuífero con agua fresca que contiene rocas salinas que ocurren en partículas finas de oeste a este. Este contenido notablemente alto de TDS en el oriente debería estar afectado por el ritmo de bombeo que ocurre en el acuífero somero superior, especialmente en los pozos del Proyecto Árabe el cual se encuentra en bombeo continuo durante el año. (3) La tercera fuente posible de la salinidad son influencias antropogénicas. Esto puede mostrarse fácilmente por el incremento de nitrato, bromuro y sulfato dependiendo en que si la localización del pozo coincide con áreas urbanas o agrícolas. Esto refleja adición de efluentes químicos agrícolas o contaminación por aguas residuales proveniente de tanques sépticos adyacentes que se construyen en la cima de grava en la capa Samara. Se necesitan estudios más avanzados utilizando diferentes técnicas geoquímicas e isotópicas para confirmar estas fuentes de salinidad sugeridas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is one of the most valuable natural resources in the Middle East. The combination of population growth, economic and agriculture development, and an arid climate results in overexploitation of the water resources in the region. The continuous high water demand leads to a rapid degradation of the quality of fresh-water resources, as a result of the salinisation and contamination processes (Vengosh and Rosenthal 1994; Salameh 2002). The lack of sufficient water, combined with rapid water-quality deterioration, presents a serious challenge to the people in the region. In order to manage and share these water resources under conditions of accelerating degradation, it is crucial to understand the origin and mechanisms of the contamination process.
The salinity threatening these fresh-water resources is derived from different sources, both natural and anthropogenic. In general, overexploitation of fresh aquifers results in a rapid decrease of water level, which then triggers lateral as well as upwelling of deep saline waters from adjacent aquifers. Consequently, the overexploited aquifers become saline due to mixing with saline waters (Vengosh and Rosenthal 1994; Salameh 1996; Marie and Vengosh 2001). The other main factor which may have an effect on water quality is the dissolution of salts from the Lisan Samara formations. These layers are composed of soil, sand and gravel of the Quaternary units Q1and Q2, and marl clay and evaporites of the upper part of unit Q2 which infill the Jordan rift valley to a thickness of at least 2,200 m in most of the basin (EXACT 1998). In addition, human activity produces low-quality fluids which enter the aquifer and further degrade water quality. These fluids include sewage, landfill leachate and agricultural return flows. The superposition of natural and anthropogenic contaminants which affect water quality provides a scientific challenge, and requires unconventional interpretations for the origin of the salinity.
Study area
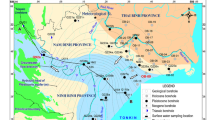
Jericho is located at the eastern boundary of the West Bank. It extends from 10 km to the north of the Dead Sea, and 7 km to the west of the Jordan River (Fig. 1). Although it has a desert climate, its location on the eastern slope of the Jerusalem and Ramallah mountains in the west, in a catchment area with abundant water resources, makes it an important agricultural district. Jericho has an area of approximately 35,330 ha. The rate of annual precipitation ranges between 120–250 mm, with an annual recharge rate of ~180 MCM (million cubic meter), while the annual consumption rate is ~140 MCM (ARIJ 1997).
The geology of the area is characterized by Jordan rift valley deposits mainly composed of Quaternary marl and alluvial formations. The geological formations in the Jericho district are:
-
1.
an alluvial formation (Q2, Fig. 1): this covers the eastern area adjacent to the Jordan. This formation is of Holocene–recent age, and bounded by the rift fault in the east and a fault of 12-km length to the west.
-
2.
the Lisan and Samara formations (Q1, Fig. 1): this covers the major part of the Jericho area, and is of Pleistocene–recent age. This formation includes three local faults up to 3 km long, and is bounded by the alluvial formation in the east and a fault to the west. It is mainly composed of marl chalk and conglomerates.
-
3.
a chalk and chert formation (Sc, Fig. 1): this formation occupies the western part of the Jericho district. The formation is of lower Tertiary–Senonian age. It is composed of Senonian chert and chalk deposits, structurally cut by minor faults.
-
4.
a dolomite–limestone formation (K, Fig. 1): this formation is of Cenomanian–Turonian age, and covers a very small portion of the south-western and north-western parts of the Jericho district. It is mainly composed of limestone, dolomite and marl.
The major aquifer system in Jericho forms part of the eastern basin of the West-Bank which extends along the Jordan rift valley. This system is of Quaternary Pleistocene Recent age, and mainly composed of the formations described in (1) and (2) above. The main recharge sub-basin for this system is the Jerusalem-Ramallah Mountains, in which runoff water drains through wadis flowing from the west to the Jordan rift valley in the east.
The lithology of the Pleistocene is varved marl, consisting of thin layers of gypsum and limestone, and forming dark and light alternating bands, whereas the alluvium and gravel fan formations are of Holocene age and cover the flood plains of the Jordan River. They consist of laminated marls and gravel fans.
The main sub-basin feeding the Quaternary aquifer system in the area is the Jerusalem-Ram Allah sub-basin which drains mainly through Wadi Qilt and Wadi Makkuk, the Neogene and Pleistocene Lower and Upper Cenomanian aquifers, and then flows to the east and southeast. This sub-basin also contains three major spring systems:
-
1.
the Wadi Qilt spring system: this includes Ein Qilt, Ein Fara and Ein Fawwar.
-
2.
the Wadi Makkuk (Nuaymah) spring system: this includes Ein Dyouk, Ein Nuaymah and Shosha.
-
3.
the Ein Al-Sultan spring system.
All of the above mentioned springs are used for agricultural irrigation, except Ein Al-Sultan which is also used for domestic water supply. The water quality of these springs is relatively good compared with that of other groundwater wells in the area. These wells are highly impacted by salinisation, an EC of >6,000 µS having been recorded in the eastern part of the area (PHG 1999).
The source of increasing salinity in this area is as yet not well known, although previous studies identified three major sources of salinity. These are (1) the in situ dissolution of salts within the Lisan Formation, (2) saline water from adjacent deep brine aquifers (related to over-extraction), and (3) anthropogenic effluent mainly of agricultural backflow and domestic sewer (Marie and Vengosh 2001).
In this study, further investigation of the possible sources of salinity is made, using the behaviour and character of geochemical tracers.
Data collection and method
Groundwater sampling and the selection of sample sites was aimed at providing supplementary analyses of tracers to characterize the chemical signatures of both fresh and saline groundwater, and their variation with location.
In all, 21 samples from springs and wells were collected. Two water samples correspond to springs with relatively fresh CaCO3 water, in addition to one closed well which shows the same water type; two wells are located to the north of the study area within the alluvial Samara Formation of Wadi Nuw’emeh, 10 wells are along Wadi Qilt (five west of the wadi and five east of the wadi), while the rest of the wells are located more to the east of the area.
The samples were collected in the period October–November 2003, this being the end of the dry season. Sampling localities are shown in Fig. 1.
Onsite measurements for physicochemical parameters (pH, temperature, m-value and redox potential) were carried out. The samples for anions were preserved using HgCl2 for prolonged preservation of nitrate. For accurate chloride measurement, the chloride concentration was determined in water samples for cation analyses. Most of the samples were clear, and thus no filtration was necessary. Because the samples were collected in the agricultural season, all the sampled wells had been pumping for a long period of time. Hence, no further purging was needed prior to sampling, and the samples were collected immediately. Samples for cations and anions were collected in 60-ml polyethylene bottles, and samples for tritium were collected in 500-ml bottles.
Major anions (NO3, SO4, Br, NH4, PO4 and Cl) were analysed using HP liquid chromatography. Concentrations of the major cations Ca, Mg, Na, Sr and K were determined by ICP-OES, and the trace elements Mn, Li, Ba, F, Si, B and Fe were analysed by ICP-MS. Due to the high chloride content, most samples needed dilution and therefore, most trace elements were under the detection limits. Alkalinity and HCO3 were measured onsite by titration. Tritium was measured by a liquid scintillation counter. The sample is mixed with a scintillation mixture of a solvent, emulsifier and solute. Tritium emits beta decay electrons, which excites the solvent. The solvent transfers its energy to the solute, which emits light photon pulses which are detected and counted.
All chemical and isotope analyses were carried out at the UFZ Environmental Research Centre Leipzig-Halle.
Results
The chemical data are shown in Table 1. The major cation and anion concentrations of the groundwater from springs and wells in the region are plotted on a Durov diagram in Fig. 2.
All the major anions and cations show an increasing trend with water path from west to east (Fig. 3). Mg, K, Na and Br show good linear correlations with the calculated TDS values, and all follow the west–east pattern, while Ca, Sr, B, SO4 and HCO3 show relatively low correlations and nitrate shows very poor correlation, only scattered nitrate values being documented (Fig. 3g).
In general, the data reflect distinctive end members of fresh water to the west and saline water further to the east, bordering five distinctive regions.
Springs and fresh-water end member
Springs in the west are characterized by Ca–Mg–CO3 water with a low degree of mineralisation (~548 mg TDS/l). Tritium units in the groundwater from the springs are ~4.5 TU, while for the adjacent Samed well the value is 1 TU. These springs, which correspond to the fresh-water end member, show low Cl contents with molar ratios of 0.0033–0.0039 for B/Cl (Fig. 4b), ~1 for Na/Cl (Fig. 4c), 0.0065–0.013 for Br/Cl (Fig. 4d), ~1.2 for Mg/Cl (Fig. 4a), ~0.18 for SO4/Cl (Fig. 4e) and 7.2–7.4*10−4 for Sr/Ca (cf. below). The groundwater shows oversaturation index values for dolomite and calcite, and undersaturation index values for gypsum and aragonite (Fig. 5a–d).
Wadi Qilt wells (western sector)
The groundwater from wells in the west of Wadi Qilt shows intermediate values between the fresh and saline end members, with a water type of Mg–Na–Ca–Cl–HCO3. However, the ion concentrations, mainly chloride, are much lower than in the eastern wells, and sometimes show values which are close to this in the fresh-water end member of the springs. Tritium units in the groundwater from these wells are between 3.3–3.9 TU. The ionic molar ratios for these wells lie between those of the two end members. They show lower saturation indices for dolomite and calcite than those of the springs, while the saturation indices for anhydrite and gypsum were higher. These wells show relatively high carbonate, boron and nitrate values.
Wadi Qilt (eastern sector)
The hydrochemistry for the springs further to the east shows trends close to those in the western sector, but with higher TDS values as well as major ion concentrations. The area is characterized by Lisan and Samara layers overlapping with the top of the Samara layer. Tritium units in the groundwater from these wells are lower than those in the wells to the west, with values between 2–3.5 TU. This sector has the same saturation indices for dolomite and calcite as the rest of the Wadi Qilt wells to the west, and much higher ones for gypsum and anhydrite (Fig. 5). Although the chloride concentrations for these wells are higher (500–900 mg/l), TDS values are >2,100 mg/l, and the molar ratios for ions are much lower; most of the molar ratios begin to take on stable trends when reaching these wells, as do the wells to the east (Fig. 3).
Wadi Nuwe’meh North
The results for the wells in this area to the north show high chloride contents (600–>700 mg/l) as well as high concentrations of major anions and cations relative to wells in the west and in Wadi Qilt West. This area is characterized by the dominance of the Samara formation with high hydraulic conductivity. The water type is Mg–Na–Cl–HCO3, the same as that for western wells, both having surplus Mg which originates from dolomite dissolution of the Samara layer. All the minerals in the groundwater from these wells are undersaturated. The molar ratios for the major ions are close to those in the eastern wells. Tritium units in the groundwater from these wells are around 1.5 TU.
East Arab Project wells (saline end member)
The groundwater from wells shows the highest salinity in the Jericho area, with a chloride content of more than 1,800 mg/l for well 19-14/067 and a TDS value of 3,664 mg/l. This relatively high saline water is of the Na–Mg–Cl and Mg–Ca–Cl type. The SO4 concentration is 320 mg/l. The groundwater from these wells is highly saturated with dolomite and relatively saturated with calcite, while the saturation indices for gypsum and anhydrite are higher than the rest of the wells but still undersaturated (Fig. 5). The carbonate concentration of 440 mg/l (Fig. 2 h) is relatively high. The groundwater from these wells shows the lowest tritium units which were below the detection limit of <0.6 TU for well 19-14/067.
Discussion
The chemical data for all the springs and wells show a gradual increase for the major elements from the west of the area to the east along the water flow path. The groundwater salinity varies from fresh Ca–Mg–CO3 water in the west to relatively high-salinity Na–Mg–Cl and Mg–Ca–Cl water to the east (EC>5,000 µS), differentiating two distinctive end members. The molar ratios for most of the elements tend to be stable in the east and in Wadi Qilt East. This may be a result of a dominant source of salinity in the area. Some wells in Wadi Qilt West which show lower chloride contents also show high boron and nitrate values, while wells to the north show less anthropogenic indicators and higher chloride contents.
The linear correlation in the Durov diagram (Fig. 2) also suggests a type of dissolution or simple mixing which increases with distance from west to east and to the north. The water type varies along these paths, from Ca–Mg–HCO3 for the spring water and Mg–Na–Ca–Cl–HCO3 for the pumped wells in the west and middle of the studied area to more saline in the east where the Mg–Ca–Cl and Na–Mg–Cl water type dominates. The variability in bromide and nitrate concentrations depends on well location and agricultural/domestic activities around the borehole. The groundwater mineralisation do not really reflect the spatial trend for the major elements, this being due to variation in ion input along the water path (Fig. 5). Most of the groundwater samples show tritium units of 0.8~4.5 which reflect a water mixture between sub-modern and recent recharge (Clark and Fritz 1997). The tritium units also distinguish two differently aged end members which represent this mixing. The groundwater in the western springs and wells of the alluvial aquifer comprise mixtures of recharge from the past few years only (less than 5 years). By contrast, groundwater from wells further to the east with 3H below detection must have been recharged prior to 1952, and can be considered as sub-modern or older. The tritium level declines with increasing distance along the water path through the wadis, and emphasizes that the mechanism of recharge is not related to direct infiltration of rainfall but rather of infiltrated runoff along the wadis which drain from the Jerusalem-Ramallah Mountains (the main recharge point). In general, the variation in salinity in the Jericho district can be due to one or a combination of several sources of salinity and pollutants. These can be defined as having three main causative factors, as follows.
Saline-water source
There is good evidence of brine evolution further to the east of the study area. It is believed that the overexploitation of the wells to the east of the Jericho area has resulted in upwelling from another, deeper brine aquifer. However, its source, direction and location can not be identified. The analyses of samples from wells in the east show highly saline Ca–Cl and Na–Cl water. A Na/(Na+Cl) ratio less than 0.5 in combination with a high TDS of >500 suggest a reverse softening and a mixing of brine water in the east with fresh water in the west. Moreover, the trend of a continuous increment of salinity from the west to the middle of the area is demonstrated in the steady ratios for Na/Cl and B/Cl in the east group, with molar ratios of ~0.5 and 0.0026 (Fig. 4b, c). The low tritium concentration in the study wells emphasizes the presence of such a mixing process. The lower tritium units between <0.6–1 in the wells to the east may reflect a mixing of younger water infiltrated from wadis runoff with deep old water characterized by high salinity. This evidence could be made clearer by further studies of rare earth element concentrations and isotopic signatures.
Evaporites and in situ rock weathering
Generally, all the wells and springs show HCO3/SiO2 ratios of >10 and TDS>500, which suggests carbonate weathering along the water path. The Mg/(Ca+Mg) ratios, which are over 0.5 in most cases (Fig. 4c), indicate dolomite dissolution, which overlaps with the Lisan layer in most of the area. In addition to calcite precipitation, especially for the wells in the east where the Ca/(Ca+SO4) ratios are 0.75–0.95, there is surplus calcium from the suggested Ca–Cl brine, beside gypsum or carbonate dissolution of the Lisan layer. The results show two main evaporitic effects. These are mainly the effect of the Samara layer and its dolomite to the west, and the effect of the Lisan layer and its gypsum and calcite to the east and north. Figure 5 shows that the groundwater in the west is oversaturated with respect to dolomite and calcite, and becomes less saturated further to the east of Wadi Qilt and Nuw’emeh North. This trend changes for the wells to the east where another input of Mg-rich salts raises the saturation indices for dolomite and calcite in a trend which increases until it reaches the saline end member at the eastern Arab Project wells. The Ca and HCO3 concentrations are lowered by the precipitation of other minerals such as gypsum and aragonites. Relatively high SO4 concentrations in the east, in parallel with a low saturation index of gypsum, indicate gypsum dissolution. Moreover, the increase in ion content, mainly Mg from a suggested brine source to the east, favours dolomitization of the calcite and aragonite dominating the entire sequence. Sr/Ca molar ratios of 0.0025–0.0155 emphasize this dolomitization process (Sass and Starinsky 1997).
Except for the springs which show relatively fresh water, the ratios of Na/Cl (0.4 east–0.7 west), Mg/Cl (0.18 east–0.5 west) and SO4/Cl (0.015 east–0.15 west) show values which are even smaller than the Lisan leaching ratios which have values of 0.54, 0.23 and 0.05 respectively (Salameh 2002), and much lower than seawater ratios. The Br/Cl ratios (0.006 east–0.014 west) are higher than that of seawater (~0.0016). Although the groundwater from many of the studied wells show different Br/Cl molar ratios which may also have been affected by dissolution of salts, the variation between this value and those from the wells is mainly due to more Br input, suggesting two sources of origin: (1) halite dissolution from the evaporitic Lisan layer with Br/Cl molar ratios different to those of brine water or seawater, and (2) an anthropogenic source of bromide which is used widely in the form of methyl bromide in this agricultural area.
Anthropogenic influences
The greatest anthropogenic effect on the groundwater quality in the Jericho area is due to agricultural activities, which have three main indicators: (1) bromide, which is injected as a fumigant into the land in the form of methyl bromide gas; (2) NO3 from nitrogen fertilizers, pesticides and manure; and (3) potassium, magnesium and calcium, which may appear in surplus amounts due to irrigation backflow and infiltration with soil into the groundwater.
The wells in the middle and west of the Jericho area are susceptible to agricultural influence. This is more clearly present in the east, although the high chloride content and the influence of natural evaporites make this indicator more ambiguous. Figure 6 makes this picture clearer. The iron factory site, as a non-agricultural area, was taken as an end member for the sewage input, and the Arab Project well, where there is extensive agriculture with less sewage input, was taken as the other end member. There are two different trend lines for the two end members and the mixing points of the two influences in between. Nitrate values vary with activities and location. NO3 values increase significantly in the wells in the agricultural and animal farm areas or near septic tanks, for example, Sabiru Rantizi well 19-14/06. This well is found near an animal farm, in an area which has widespread agricultural activities and where pesticides are used extensively. The same values are shown by the iron factory well which taps the Lisan layer in the east, south of Jericho, but which has essentially no agricultural activities. In this case, the main reason for the high nitrate level is the common use of septic tanks. Locating the septic tanks in this area may not be easy, especially as the area is formed mainly by limestone and gypsum. The nature of the soft rock reflects a highly fractured texture through which pollutants can easily pass. The continuous input of potassium in the groundwater from wells to the east may reflect an agricultural source; this is a cumulative value incorporating the potassium coming from sylvite in this extensive agricultural area within the Arab Project Area to the east of Jericho.
Conclusions
The high salinity in the shallow Pleistocene aquifer in the Jericho plain area is a function of three main contributing sources. Figure 7 shows the relation between the molar ratios of Sr/Ca versus Cl, and the distribution of the groundwater samples between the three main end members with respect to typical brine, freshwater and Lisan leachate Sr/Ca molar ratios.
Relationship between Sr/Ca molar ratios and Cl concentrations. The data suggest three inputs to the groundwater. The brine constitutes the most significant input, with a ratio of ~0.008 for the Arab Project wells in the east. The iron factory well shows a ratio close to that of the Lisan leachate. Most of the wells undergo mixing between the three end members
In general, the hydrochemical and isotopic data clearly indicate three contributions.
-
1.
The evolution of calcium chloride in the wells to the east which are relatively rich in strontium and Fe in parallel with low molar ratios for most of the elements compared to seawater reflect a reverse softening and a mixing of brine water.
-
2.
The continuous increment of the major anions and cations, with chloride molar ratios which bear Lisan molar ratios, suggest the in situ dissolution of rocks as an additional source of salinity. There is a decrease in water saturation with dolomite and calcite for most of the wells in the west, in parallel with an increase of Mg, Ca and HCO3. These elements demineralise under the effect of the other brine effluent when the water reaches the eastern wells. The high Mg input favours calcite dolomization in which the calcium present in surplus amounts forms Ca–Cl brine in the eastern wells.
-
3.
The anthropogenic effects from sewage and agriculture are clearly visible in the wells which have low chloride content, but by no means low brine effects, this lower chloride with higher molar ratios, and high Br and nitrate suggesting the input of different water which contains pollutants. This water may result from agricultural backflow or sewage effluent.
The lithological formation of the area has a two-edge effect on the salinity increment in the area, the one being the in situ weathering of the rock. The other is the structure of the formation, which consists of dolomite and marl from the highly transmissive Samara layer with coarse gravel and high hydraulic conductivity, and the Lisan layer with fine silts and low hydraulic conductivity but highly fractured structure, where both serve as a good medium for pollutant transmission and storage.
Many aspects are still not clear and need further research using other technical investigations. More sampling for isotopes (deuterium, nitrogen15 and sulphur34) and pesticide indications were taken during the course of this study. When these analyses are completed, the mode, the tracks and the source of salinity and pollutants in the groundwater within the Jericho area will be clearer.
References
ARIJ (1995) Environmental profile for the West Bank, vol 2: Jericho District. Applied Research Institute Jerusalem
ARIJ (1997) Water resources, Chap 8. In: The status of the environment in the West Bank. Applied Research Institute Jerusalem, pp 95–107
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis, New York, USA, 328 pp
EXACT (1998) Overview of Middle East water resources—water resources of Palestinian, Jordanian and Israeli interest. Compiled by US Geological Survey for Executative Action Team, Washington, 44 p
Marie A, Vengosh A (2001) Sources of salinity in groundwater from Jericho area, Jordan valley. J Ground Water 39:240–248
PHG (1999) Water quality and hydrogeology of the eastern aquifers bordering the Jordan Valley—Jericho District. Palestinian Hydrology Group Jerusalem Tech Rep
Salameh E (1996) Water quality degradation in Jordan: impacts on environment, economy and future generating resource base. Friedrich Ebert Stiftung, Royal Society for Conservation of Nature, Amman, Jordan, 179 pp
Salameh E (2002) Sources of water salinities in the Jordan Valley area/Jordan. Acta Hydrochim Hydrobiol 29:329–362
Sass E, Starinsky A (1997) Behaviour of strontium in subsurface calcium chloride brines: southern Israel and Dead Sea rift valley. Geochim Cosmochim Acta 43(6):885–895
Vengosh A, Rosenthal E (1994) Saline groundwater in Israel: its bearing on the water crisis in the country. J Hydrol 156:389–430
Acknowledgements
We express our deep gratitude to the German Federal Ministry of Science and Research (BMBF) (Dr. S. Kieffer and Dr. H.J. Metzger) for funding and support this Project, and for the Country of Germany for their continuous support of the Palestinian people. Further thanks goes to all Colleages in Palestine Hydrology Group-PHG (Dr. Tamimi and Dr. Ghanem) and all other Partners in this Project for their joint work activities and Cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khayat, S., Hötzl, H., Geyer, S. et al. Hydrochemical investigation of water from the Pleistocene wells and springs, Jericho area, Palestine. Hydrogeol J 14, 192–202 (2006). https://doi.org/10.1007/s10040-004-0399-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-004-0399-0