Abstract
Purpose
To prospectively evaluate the use of a continuous Nitinol containing memory frame patch during a TIPP-technique in the open repair of inguinal and femoral hernias.
Methods
Over a 3-year period all consecutive adult patients that needed treatment for an inguinal or femoral hernia were treated by the TIPP repair using the Rebound Shield mesh. Intra-operatively the type and size of the hernia were evaluated according to the EHS classification, as well as the size of the mesh used. Baseline characteristics for all patients were evaluated considering age, gender, BMI and American society of Anesthesiologists score. Standard X-ray was performed to evaluate mesh position. All patients were evaluated for post-operative pain using the visual analogue scale (VAS 0–10 scale).
Results
In total 289 groin hernias were operated using a nitinol containing patch in 235 patients. The mean operating time was 38 min for unilateral hernias and 59 min for bilateral hernias. The median follow-up is 21.2 months (14–33 months) during which three patients died, unrelated to the groin hernia repair. At the time of re-evaluation 12 patients (5.0 %) complained of chronic pain, with a VAS score higher than 3 after 3 months (range 3–10). Two of these patients already had severe pain pre-operatively. A total of 3 recurrences (2.9 %) were noted with strong correlation with X-ray findings.
Conclusion
A nitinol memory frame containing mesh is a valuable tool to achieve complete deployment of a large pore mesh in a TIPP repair for inguinal hernias with acceptable morbidity and a low recurrence rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior preperitoneal approaches for the repair of inguinal and femoral hernias gained attention during recent years. Possible factors related to this increased attention are the relatively high rate of chronic pain after Lichtenstein repair [1], the relatively steep learning curve for laparoscopic inguinal hernia repair [2] and the development of new mesh devices that facilitate introduction in the preperitoneal space through a minimal skin incision [3]. The virtue of the preperitoneal space for the repair of groin herniations is that it facilitates entry into the retrofascial transversalis space, thereby providing direct access to the posterior inguinal structures. The prosthesis is then pressed against the parietal peritoneum by the intra-abdominal pressure and replaces the damaged floor of the inguinal canal. The need for fixation devices, which can cause post-operative pain, can be reduced as according to Pascale’s principles the intra-abdominal pressure stabilizes the prosthesis.
The initial revival of the transinguinal preperitoneal mesh placement (TIPP), as it is called now, was initiated by reports of Edouard Pélissier who used a polyethylene memory ring patch to reinforce transversalis fascia [4]. Although this memory ring offers an easy deployment of the patch in the medial side of the preperitoneal space, previous studies by our group showed that lateral deployment of the patch is neither easy nor reliable [3, 5]. The main reason for this issue is the lack of memory on the lateral side of the prosthesis, which is meant for surgeons who want to incise the lateral side of the prosthesis to create a new internal orifice, as is done during an anterior subfascial mesh repair. As this is no longer advocated, referring to the laparoscopic techniques, there is need for a mesh device containing a continuous memory frame to facilitate efficient deployment laterally in the preperitoneal pocket. Brown investigated a continuous nitinol frame containing hernia device for its stability and effectiveness after total extraperitoneal repair (TEP), which showed acceptable results with radiographic evidence of size and shape stability after 6-month follow-up [6]. The aim of this study was to prospectively evaluate the use of a continuous Nitinol containing memory frame patch during a TIPP-technique in the open repair of inguinal and femoral hernias.
Patients and methods
Over a 3-year period all consecutive adult patients that needed treatment for an inguinal or femoral hernia were treated by the TIPP repair using the Rebound Shield mesh, Minnesota Medical Development Inc. (MMDI), Plymouth, Minnesota, USA. Also recurrent inguinal hernias after a non-mesh or non-preperitoneal mesh repair were included in the analysis as well were bilateral hernias. No specific patient selection was made and only patients in which it was impossible to explore and open the preperitoneal space were excluded from the analysis as a Lichtenstein repair was performed in these patients. Previous pelvic surgery or previous prostatectomy was no contra-indication for starting the preperitoneal procedure. Intra-operatively the type and size of the hernia were evaluated according to the EHS classification [7], as well as the size of the mesh used. Baseline characteristics for all patients were evaluated considering age, gender, BMI and American society of Anesthesiologists score. All patients were primarily evaluated for post-operative pain using the visual analogue scale (VAS 0–10 scale). No standard pre-operatively VAS measurement was performed.VAS scores were evaluated the evening after surgery, and after 3 months when the patients still complained of pain. VAS was measured as the maximum pain experienced. If the VAS score was higher than 3 on a 10-scale at that time-point, this was considered as chronic pain. An ultrasound was not routinely performed pre- or post-operatively.
Surgical technique
TIPP-technique was used as described by our group [3]. In brief, a 3 cm skin incision was performed under spinal or general anesthesia, depending on the patients’ preference. The incision starts half way the line between the superior anterior iliac spine towards the midline in a 30° angle to the pubic tubercle.
In all hernias, either direct, indirect or femoral, the preperitoneal space was entered bluntly through the internal ring. The epigastric vessels were then identified and retracted upwards. Gauze was introduced into the preperitoneal space to facilitate medial dissection. In that way also medial hernias were reduced easily and after that the cord was parietalized as far as possible towards the point where spermatic cord and spermatic vessels separate. By approaching the peritoneal space in this way the pubic bone is palpable and the iliac vessels are visible.
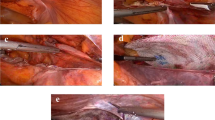
Thereafter, the lateral space is freed by digital dissection with the help of the gauze in the direction of the superior anterior iliac spine. A malleable retractor is then introduced into the free preperitoneal pocket and the Rebound Shield device is then inserted using the retractor as a slide. The choice of the surgeon to place a small (14.9 × 10.3 cm) or a large (16.1 × 11.0 cm) device depended mainly on the size of the hernia and on the constitution of the patient. Initially the device is easily introduced behind the pubic tubercle with 2–3 cm overlap to prevent medial recurrences, thereafter the device is grasped with two forceps a few cm from the lateral edge and is put into the lateral pocket. The strong memory frame of the device deploys the mesh completely in the created pocket without the need for any fixation. If possible the patient will be asked to strain or push on the nitinol frame to control its position and to check the adequate spreading of the mesh to cover the whole myopectineum of Fruchaud. In case of general anesthesia, Valsalva maneuver by increasing the pulmonary end expiratory pressure by the anesthesiologist delivers often the same result. Local long-acting anesthetic is then infiltrated around the cord intramuscularly and cutaneously. The wound is then closed with absorbable sutures in different layers.
Peri-operative data and post-operative complications were prospectively recorded. As the nitinol frame is visible on radiography, a standard X-ray in lying position was taken the same day in case of ambulatory surgery or the next day before discharge.
No limitation of daily activity post-operatively was suggested and return to work was standardized at 7–10 days if no straining had to be applied and 2–3 weeks in case of severe lifting and straining. Patients were reexamined at the outpatient clinic at 3 weeks and yearly thereafter.
In case of post-operative pain or other complications as recurrence the X-ray was used as a reference point for later evaluation of the mesh position. During analysis, the observed recurrences were retrospectively compared with the initial position of the mesh and the location of the recurrence.
Results
During the study period from September 2009 till August 2011 292 groin hernias were operated on 238 patients. In all but three patients the preperitoneal space could be adequately dissected. Due to previous open prostatectomy with lymphadenectomy three patients had a Lichtenstein procedure using a large pore polypropylene mesh. These patients are not included in this analysis.
The baseline characteristics and the distribution of the type of hernia according to the EHS classification of the 235 analyzed patients are depicted in Table 1. No obturator hernias were diagnosed in this series. In the 289 studied groin hernias a large mesh was used in 122 hernias (42.2 %) and a small mesh of 15–10 cm was used in 167 hernias (57.8 %).
The mean operating time was 38 min for unilateral hernias and 59 min for bilateral hernias. For the longer duration of surgery in some patients with chronic pain there were different explanations as the experience of the surgeon, bilateral versus unilateral hernias, associated procedure and recurrent hernias. In se there was no immediate link to the development of chronic pain.
General anesthesia was used in 184 procedures (78.3 %), while in 48 cases (20.4 %) spinal anesthesia was used. Three patients (1.3 %) underwent the procedure under local anesthesia with a mixture of long-acting ropivacaine and xylocaine, because of a contra-indication for any other type of anesthesia. Three patients (6.3 %) experienced urinary retention secondary to the spinal anesthesia, while 3 other patients experienced paralysis of the quadriceps femoris muscle for several hours due to diffusion of the local anesthetic, necessitating overnight stay in two patients.
Ambulatory treatment for this type of hernia surgery was performed in 64 patients, being 26.9 % of the population, while for the other patients the median length of hospital stay was 25 h. Reasons for overnight stay were mainly the wishes of the patient and were not related to the medical condition of the patient. Comorbidities like cardiac or pulmonary disease also were reasons for overnight stay. One patient had a bladder trauma during reduction of a large direct hernia, which needed a median laparotomy and a 3-week hospital stay because of post-operative pneumonia. Regarding other early complications, 13 patients developed a subcutaneous hematoma, in two probably due to the use of low molecular weight heparin that was indicated to replace oral anti-coagulation therapy prior to surgery. All were treated conservatively. In total six patients developed an asymptomatic seroma. Neither superficial nor deep wound infections were observed during follow-up.
The median follow-up is 21.2 months (14–33 months) during which three patients died, unrelated to the groin hernia repair. At the time of re-evaluation 12 patients (5.0 %) complained of chronic pain, with a VAS score higher than 3 after 3 months (range 3–10). Two of these patients already had severe pain pre-operatively. Five patients had a large device inserted, while seven had a small device implanted. In those patients with chronic pain, it appeared that the operating time was significantly longer than the operating time in the rest of the patients (102.4 vs. 38 min). The indication for operation in 5 out of 12 patients was a recurrent inguinal hernia. In two patients the groin was re-explored for pain and part of the nitinol frame was removed with a decrease in pain in both patients. The indication for both patients was the suspicion that the ring had something to do with the pain based on clinical examination. During exploration the position of the mesh was correct in both cases, but we decided to remove that part of the ring that was present at the location of the most severe pain. Furthermore, it appeared rather difficult to remove the complete ring as it is sewn onto the mesh textile and not in a kind of sheet, where you can pull it out easily.
A detailed report on all patients with pain after 3 months is shown in Tables 2 and 3. Our treatment of local infiltration consists of a mixture with ropivacaine 7.5 % 10 cc and 1 ml of betamethasone dipropionate 5 mg and betamethasone natrii phosphas 2 mg (Diprophos D.S.™, MSD Belgium, Brussels, Belgium). According to the location and the extent of the painful area the complete mixture or only part of it will be injected. Half of the amount will be injected 2 cm medial and cranial of the anterior superior iliac spine, and half of the amount locally, both subcutaneously and subfascially at the most painful location. If needed ultrasound can be used to identify the different layers.
We performed a sub-analysis to find a possible correlation between BMI, the use of the nitinol frame and the presence of post-operative pain. Although three patients with a BMI below 18 were operated on for an indirect hernia and in all three cases a small shield device of 15 × 10 cm was placed, none of these patients complained of chronic pain. However, the nitinol frame was consistently palpable at its upper rim in those patients. Four out of 17 patients (23.5 %) operated on with a BMI more than 30 had complaints of chronic pain. In those 4 patients all suffered from diabetes and in all four a large shield of 16 × 11 cm was inserted.
During the follow-up period a total of three recurrences were noted. There was one immediate medial recurrence on day 1 post-operatively due to a technical failure and malpositioning of the shield, visible both clinically and on X-ray (Fig. 1). Two other patients had a medial recurrence and evaluating these two patients, it was noted that the shield was not sufficiently inserted medially, hardly crossing the pubic tubercle (Fig. 2). Both patients underwent a Lichtenstein repair, leaving the original device in place.
Discussion
Adequate groin hernia repair has to tackle three different issues: it has to be an efficient repair with a low recurrence rate, it has to be safe with the incidence of post-operative chronic pain as low as possible and the post-implantation shrinkage or distortion of the mesh material used should be limited, possibly also diminishing recurrence rate and improving quality of life. Using an open transinguinal but preperitoneal mesh repair implanting a nitinol frame memory patch, we evaluated whether these issues are dealt adequately.
Biomechanically, the position of the mesh between the peritoneum and the abdominal wall muscles, the preperitoneal space, should have advantages, especially when the mesh overlaps the abdominal wall defect widely. As nicely stated by Koning et al. [8] the “upstream principle” considers this position of the mesh as the most physiological type of repair, in contrast to the onlay positioned meshes as done in a Lichtenstein repair. Several reports already showed the TIPP repair being an efficient surgical technique with low recurrence rates [5, 9–11]. Our series presented here shows a recurrence rate of exactly 1 % (3 out of 289 repairs) and is in line with these previous series.
A critical review of the literature shows a tendency of studies to focus more on pain, especially chronic pain as a primary outcome instead of recurrence rate [1, 12–15]. This shift in focus is probably not only due to the incidence of chronic pain (19–63 %) exceeding the risk of recurrences (2.0–5.5 %) but also because of the important impact on quality of life. Detailed questionnaires used to evaluate this chronic pain in several clinical studies suggest that a significant proportion is of neuropathic origin [16]. Neuropathic pain together with central disturbances and numbness might be caused by damage to well defined sensory nerves from different types of surgical trauma or secondary nerve damage from an inflammatory response leading to nerve compression. Willaert et al. [17] showed in a recent Cochrane review on open preperitoneal techniques for inguinal hernia, that there is evidence, although not strong, that a preperitoneal repair causes less or at least comparable chronic pain compared to Lichtenstein procedure. The only randomized controlled trial in this respect is recently published by Koning et al. [9] showing indeed significant lower incidence of chronic pain at 1 year after surgery in TIPP treated patients versus Lichtenstein treated patients. In contrast, Lundstrom et al. [18] stated that an open preperitoneal technique is an independent risk factor for chronic pain. We believe that an anterior approach with no subfascial mesh placement and no fixation in that region already diminishes the chances for developing chronic pain. This is confirmed as well by other recent reports on nerve handling and self-fixating meshes [19, 20]. The technique used in our series only has to handle the ilioinguinal nerve before entering the preperitoneal space. No further anterior subfascial dissection is performed so that nerve damage to both the iliohypogastric and the genitofemoral nerve should be equal as in other posterior approaches by open or laparoscopic techniques. The cases of chronic pain in our series were analyzed thoroughly and the main issues causing the pain were neuropathy improving with infiltration or treatment by pain specialists and chronic irritation by the mesh/nitinol ring. This can of course not be neglected, but is in line with other reports on posterior approaches [9].
Regarding mesh behavior and handling properties, performing TIPP using a macroporous flat mesh is technically demanding. The considerable increase in flexibility of this type of mesh makes it difficult for surgeons to handle or manipulate at the time of insertion and positioning. Some companies incorporated absorbable filaments into their weave to add stiffness and facilitate implantation, but by doing so this may contribute to the increased surface area and potentially to a higher infection risk. Previous reports already described the use of memory containing devices to facilitate introduction of a mesh in the preperitoneal space without further need for fixation as the memory ring, together with the intra-abdominal force (Laplace), keeps the mesh in place [4, 11]. So far, the memory ring reports on TIPP mainly involve a non-continuous polyethylene ring inside a polypropylene mesh [3–5, 9–11, 21]. The main disadvantage of this device, at least in our experience, is the interruption at the lateral edge of the mesh, as this regularly interferes with an adequate deployment of the patch laterally. Therefore, we investigated the continuous nitinol memory frame to solve this issue in TIPP repair. The stabilization of the mesh with the stiffness provided by the addition of the nitinol memory frame made it remarkably easy to handle. The elasticity of the frame allows the device to easily adjust to human anatomy offering a way to diminish the need for fixation and its possible complications.
An additional benefit of the continuos nitinol frame might be the reduction or even prevention of mesh shrinkage, as shown earlier by Maillart and colleagues [11] for the interrupted memory device by ultrasound verification and by Torres-Villalobos et al. [22] and Brown [6] for this nitinol frame device using classical X-ray control, both in experimental and in clinical settings. This advantage may be attributed in part to the mesh characteristics, as it is not the mesh itself that shrinks but rather the surface area reduction that is caused by connective tissue contraction during the consolidation of scar tissue. However, another important factor might be the nitinol frame as it has a high tensile strength that allows it to keep the mesh in a constant shape, possibly preventing shrinkage.
Nitinol is a so-called ‘smart metal alloy’ of nickel and titanium, with two equal proportions of each. The term “smart” refers to the capability of nitinol to remember its original shape. Nitinol is a very well known component in various areas of (bio) medicine as cardio-vascular stents, dental braces and spectacle frames, but not that much is known about the behavior of the memory frame during ingrowth in human tissue.
Using a continuous nitinol memory frame is a new application of this alloy and it has been shown in vascular applications that both bending and compression forces may contribute to wire fracture [23], resulting in in-stent restenosis [24].
Another possible side-effect is pitting corrosion and possible release of nickel ions, of which the effects might be toxic [25]. For the use in groin hernia repair, the groin being indeed an area of frequent bending and compression, fracture of the individual wires probably will not entail serious complications, but the consequences of long-term contact between the nitinol wires and the iliac vessels and nerves are unknown. The toxicity issue is of course important to mention as most of the hernia patients might live with their device implanted for many years. As the follow-up of this study is rather short in that regard, this study cannot turn down possible chronic consequences. Therefore, it seems important to inform not only surgeons implanting these devices but also to provide the hernia patients with objective information on these issues.
An advantage of nitinol is the possibility to identify the frame with a simple X-ray. Although it may not be advocated to perform a X-ray after each inguinal hernia, in case of complications this might be an additional tool to identify the correct etiology of the problem. Related to adequate surgical technique, an X-ray helps visualizing the position of the mesh. Using the two-dimensional X-ray it is not yet clear what the optimal positioning should be. Without three-dimensional view, there is a difference in patients having an X-ray in standing versus laying down position and the correct flat deployment of the mesh is impossible to evaluate; however, the correlation for medial and lateral overlap is clear. Also in our series there was a strong correlation between our recurrences and the initial position of the frame. However, other patients that had a suboptimal X-ray positioning did not at all develop post-operative pain or a recurrence of the hernia up till now. Further investigation and follow-up is needed to clarify this.
In conclusion, although this is only a prospective registration of our experience with this nitinol frame containing mesh, it seems a safe and reliable mesh to be used during inguinal hernia repair and facilitates mesh positioning and deployment during TIPP repair.
References
Bay-Nielsen M, Perkins FM, Kehlet H (2001) Pain and functional impairment 1 year after inguinal herniorrhaphy: a nationwide questionnaire study. Ann Surg 233(1):1–7 (Epub 2001/01/05)
Hynes DM, Stroupe KT, Luo P, Giobbie-Hurder A, Reda D, Kraft M et al (2006) Cost effectiveness of laparoscopic versus open mesh hernia operation: results of a Department of Veterans Affairs randomized clinical trial. J Am College Surg 203(4):447–457 (Epub 2006/09/27)
Berrevoet F, Sommeling C, de Gendt S, Breusegem C, de Hemptinne B (2009) The preperitoneal memory-ring patch for inguinal hernia: a prospective multicentric feasibility study. Hernia J Hernias Abdom Wall Surg 13(3):243–249 (Epub 2009/02/10)
Pelissier EP (2006) Inguinal hernia: preperitoneal placement of a memory-ring patch by anterior approach. Preliminary experience. Hernia: J Hernias Abdom Wall Surg 10(3):248–252 (Epub 2006/06/08)
Berrevoet F, Maes L, Reyntjens K, Rogiers X, Troisi R, de Hemptinne B (2010) Transinguinal preperitoneal memory ring patch versus Lichtenstein repair for unilateral inguinal hernias. Langenbeck’s archives of surgery/Deutsche Gesellschaft fur Chirurgie 395(5):557–562 (Epub 2009/08/01)
Brown RB (2011) NiTiNol hernia device stability in inguinal hernioplasty without fixation. JSLS J Soc Laparoendosc Surg/Soc Laparoendosc Surg 15(2):160–164 (Epub 2011/09/10)
Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J et al (2009) European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia : J Hernias Abdom Wall Surg 13(4):343–403 (Epub 2009/07/29)
Koning GG, Andeweg CS, Keus F, van Tilburg MW, van Laarhoven CJ, Akkersdijk WL (2012) The transrectus sheath preperitoneal mesh repair for inguinal hernia: technique, rationale, and results of the first 50 cases. Hernia: J Hernias Abdom Wall Surg 16(3):295–299 (Epub 2011/12/02)
Koning GG, Keus F, Koeslag L, Cheung CL, Avci M, van Laarhoven CJ et al (2012) Randomized clinical trial of chronic pain after the transinguinal preperitoneal technique compared with Lichtenstein’s method for inguinal hernia repair. Br J Surg 99(10):1365–1373 (Epub 2012/09/11)
Koning GG, Vriens PW (2012) Anterior preperitoneal repair of extremely large inguinal hernias: An alternative technique. Int J Surg Case Rep 3(2):45–48 (Epub 2012/01/31)
Maillart JF, Vantournhoudt P, Piret-Gerard G, Farghadani H, Mauel E (2011) Transinguinal preperitoneal groin hernia repair using a preperitoneal mesh preformed with a permanent memory ring: a good alternative to Lichtenstein’s technique. Hernia: J Hernias Abdom Wall Surg 15(3):289–295 (Epub 2011/02/01)
Callesen T, Bech K, Kehlet H (1999) Prospective study of chronic pain after groin hernia repair. Br J Surg 86(12):1528–1531 (Epub 1999/12/14)
Nienhuijs SW, Boelens OB, Strobbe LJ (2005) Pain after anterior mesh hernia repair. J Am College Surg 200(6):885–889 (Epub 2005/06/01)
Poobalan AS, Bruce J, King PM, Chambers WA, Krukowski ZH, Smith WC (2001) Chronic pain and quality of life following open inguinal hernia repair. Br J Surg 88(8):1122–1126 (Epub 2001/08/08)
Poobalan AS, Bruce J, Smith WC, King PM, Krukowski ZH, Chambers WA (2003) A review of chronic pain after inguinal herniorrhaphy. Clin J Pain 19(1):48–54 (Epub 2003/01/07)
Aasvang EK, Brandsborg B, Christensen B, Jensen TS, Kehlet H (2008) Neurophysiological characterization of postherniotomy pain. Pain 137(1):173–181 (Epub 2007/11/03)
Willaert W, De Bacquer D, Rogiers X, Troisi R, Berrevoet F (2012) Open Preperitoneal Techniques versus Lichtenstein Repair for elective Inguinal Hernias. Cochrane Database Syst Rev 7:CD008034 (Epub 2012/07/13)
Lundstrom KJ, Sandblom G, Smedberg S, Nordin P (2012) Risk factors for complications in groin hernia surgery: a national register study. Ann Surg 255(4):784–788 (Epub 2012/03/16)
Hsu W, Chen CS, Lee HC, Liang HH, Kuo LJ, Wei PL et al (2012) Preservation versus division of ilioinguinal nerve on open mesh repair of inguinal hernia: a meta-analysis of randomized controlled trials. World J Surg 36(10):2311–2319 (Epub 2012/05/31)
Kingsnorth A, Gingell-Littlejohn M, Nienhuijs S, Schule S, Appel P, Ziprin P et al (2012) Randomized controlled multicenter international clinical trial of self-gripping Parietex ProGrip polyester mesh versus lightweight polypropylene mesh in open inguinal hernia repair: interim results at 3 months. Hernia: J Hernias Abdom Wall Surg 16(3):287–294 (Epub 2012/03/29)
Pelissier EP (2009) Preperitoneal memory-ring patch for inguinal hernia. Re: Preperitoneal memory-ring patch for inguinal hernia: a prospective multicentric feasibility study, Berrevoet et al. (2009) Hernia (in press) doi:10.1007/s10029-009-0475-4. Hernia: J Hernias Abdom Wall Surg 13(4):451–452 Epub 2009/03/21
Torres-Villalobos G, Sorcic L, Ruth GR, Andrade R, Martin-del-Campo LA, Anderson JK (2010) Evaluation of the rebound hernia repair device for laparoscopic hernia repair. JSLS: J Soc Laparoendosc Surg Soc Laparoendosc Surg 14(1):95–102 (Epub 2010/06/10)
Shabalovskaya SA (2002) Surface, corrosion and biocompatibility aspects of Nitinol as an implant material. Bio-med Mater Eng 12(1):69–109 (Epub 2002/02/16)
Early M, Kelly D (2011) The consequences of the mechanical environment of peripheral arteries for nitinol stenting. Med Biol Eng Comput. 49:1279–1288
Pound BG (2010) Corrosion behaviour of nitinol in blood serum and PBS containing amino acids. J Biomed Mater Res Part B: Appl Biomater 94B:287–295
Conflict of interest
FB declares no conflict of interest that directly relates to this study; AV declares no conflict of interest; JB declares no conflict of interest; RT declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berrevoet, F., Vanlander, A., Bontinck, J. et al. Open preperitoneal mesh repair of inguinal hernias using a mesh with nitinol memory frame. Hernia 17, 365–371 (2013). https://doi.org/10.1007/s10029-013-1110-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-013-1110-y