Abstract
Background
Incisional hernias repaired with mesh can be expected have a lower recurrence rate than with primary repair. Biologic implants have replaced synthetic meshes in certain complex settings. We compared two porcine-dermis derived implants—cross-linked Permacol™ biologic implant and non-cross linked Strattice-firm™ tissue matrix—in a ventral hernia animal model. Our hypothesis is that cross-linked biologic implants are remodeled differently and thus behave differently than non-cross-linked biologic implants.
Methods
Eighty-nine, female Sprague-Dawley rats had a 3 × 3 cm full-thickness segment of the abdominal wall excised. A 3 × 3 cm biologic mesh, either Permacol™ or Strattice™, was secured and the skin was closed. At 1-, 3-, 6- and 12-month time intervals, rats in each group were sacrificed and the mesh was excised. The number of adhesions, surface area, mesh thickness and tensile strength were determined, and immunohistochemical analysis performed.
Results
Permacol™ biologic implant maintained thickness while Strattice™ thickness decreased significantly starting at 3 months. Adhesion area and tenacity were not significantly different between Permacol™ and Strattice™ at all time points. The tensile strength of the Permacol™ biologic implant was greater than that of Strattice™ at 3, 6 and 12 months. Migration of host cells and neo-vascularization was observed in both implant groups.
Conclusions
Cross-linked materials may prove more durable in the remodeling process as suggested by the increased thinning and weakening observed in non-cross-linked biomesh.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abdominal wall hernia repair remains a surgical challenge. Roughly 2 million patients will undergo abdominal operations each year in the United States and, of those patients, 10–23% will develop an incisional hernia [1].
However, tension-free repair with synthetic mesh has reduced the incidence of recurrence by nearly 50% when compared to primary ventral hernia repair [2]. Repair with synthetic mesh does not come without complications. The use of synthetic mesh in a known contaminated field is surgically contraindicated because of the infection risk associated with its placement at the surgical site [3]. Adhesion formation to the intestine is problematic and may lead to later complications including intestinal obstruction, difficult reoperation, and rare but devastating enteric fistulas.
Hernia repair with biologic grafts is a field that continues to grow. These grafts are described as providing an extracellular bioscaffold that becomes repopulated with new collagen deposition and neovascularized [4]. Biologic meshes may have advantages in complex repairs because of their non synthetic qualities. Those that have been shown to be most durable have been derived from pig dermis [5]. Although all xenograft biologic meshes provide a bioscaffold, not all undergo similar processing techniques and, as a result, are remodeled differently.
Permacol™ Biologic Implant (Covidien; http://www.covidien.com) is made from porcine skin that has the cellular components and genetic material removed prior to cross-linking the remaining extra-cellular matrix with non-calcifying hexamethylene diisocyanate (HMDI) to make it durable and resistant to breakdown by naturally occurring collagenases. Permacol™ is a biomaterial that has been used across a variety of surgical specialties, for urological, plastic and gynecological procedures since the 1990s [6–8].
Strattice™ Reconstructive Tissue Matrix Firm (Life Cell; http://www.lifecell.com) is also derived from porcine dermis and undergoes a proprietary processing that removes cells and significantly reduces the key components believed to play a major role in the xenogeneic rejection response. This reconstructive material demonstrated revascularization, cell repopulation and white cell migration as early as 2 weeks post implantation, and mature vascular structure at 6 months post implantation [9].
The chief proposed benefit of collagen cross-linking is to mechanically strengthen the biologic mesh and delay degradation of biologic scaffold, thus prolonging the lifespan of the tissue reinforcement [10]; of the xenograft biologic meshes available, only two have significant clinical data evaluating their performance [7]. We proposed to compare the cross-linked mesh, Permacol™, with the latest porcine-derived biologic, Strattice™, which is not cross-linked, in the areas of adhesion formation, tensile strength of mesh, surface area and thickness of mesh and “incorporation” of mesh (evaluating rat collagen type I and III and endothelial in-growth into the biologic) at various intervals over a period of 1 year.
Methods
Eighty-nine, female Sprague-Dawley rats weighing between 250 g and 275 g were randomized into two groups: Permacol™ and Strattice™. Each rat had a midline incision and 3 × 3 cm skin flaps excised. A 3 × 3 cm biologic mesh was sewn into place using running 4–0 monofilament non-absorbable suture, and the skin was closed using staples or with absorbable polyglycolic acid suture.
At 1, 3, 6 and 12 months, six rats in each group were sacrificed. The anterior abdominal wall skin was elevated and a subsequent trap door incision was created. The parietal surface of the mesh was evaluated for adhesion formation utilizing a previously validated adhesion grading system [11], and the adhesions were photographed. The entire abdominal wall, including the implant, was excised and rephotographed to determine surface area utilizing ImageJ (NIH; http://www.rsbweb.nih.gov/ij/). The imaging program was validated by comparing its results to the surface area as measured by tracing on transparency film. The thickness of the mesh was also measured at numerous areas using a digital caliper. The biologic mesh was then harvested in order to perform tensile strength measurements. A 0.5 × 2 cm central portion of the mesh was excised along with a 0.5 cm wide portion of mesh/muscle interface, from a lateral area, perpendicular to the interface (Fig. 1a,b). Both of these excised portions were tested to find the breaking strength of the mesh itself, and that of the mesh/muscle interface, using an Instron-Sacks biaxial testing system in uniaxial mode. A 2 cm gauge length and a strain rate of 0.825 mm/min were used for the mesh; the strain rate was increased to 1.625 mm/min for the mesh/muscle interface. A portion from the peripheral segment of the mesh, abutting the muscle, was sent to pathology for anti-rat collagen type I and III, anti-rat endothelial cell CD31 antibody, hematoxilyn and eosin (H&E), Trichrome, and Sirius red staining.
Statistical analysis
Adhesion tenacity and tensile strength were compared using two-tailed t test comparing means, with P < 0.05 being considered statistically significant.
Results
Of the initial 89 animals, 17 out of 42 implanted with Strattice™ and 24 out of 47 implanted with Permacol™ had to be sacrificed due to complications that consisted of skin separation and early herniation (Table 1). This complication rate is to be expected secondary to not utilizing drains to prevent seromas and utilizing the mesh in a bridging position.
At 1, 3, 6 and 12 months, neither adhesion tenacity nor adhesion area were significantly different between Permacol™ and Strattice™ mesh implants (Fig. 2a,b).
The surface area of the implanted mesh did not differ in either Permacol™ or Strattice™ animals at 1, 3, or 6 months at the time of ex-plantation. However, the surface area of the implanted Strattice™ was significantly greater when compared with animals implanted with Permacol™ at the ex-plantation time of 12 months (Fig. 3a). The thickness of the control Permacol™ and Strattice™ was fairly uniform, at 1.1–1.3 mm and 1.1–1.5 mm, respectively. Both Permacol™ and Strattice™ lost 0.2–0.3 mm of thickness over the first 30 days but that loss was not statistically different from either control measurements or between the two groups. Mesh thickness in animals implanted with Permacol™ were statistically higher at 3, 6 and 12 months post-implantation when compared to animals having Strattice™ implanted (Fig. 3b).
Pre-implantation tensile strengths of Permacol™ and Strattice™ biologics were not statistically different from one another nor were they at 1 month post-implantation. At 3, 6 and 12 months post-implantation, Permacol™ biologic implant demonstrated statistically higher tensile strengths when compared to their Strattice™ cohorts (Fig. 4).
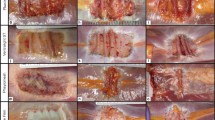
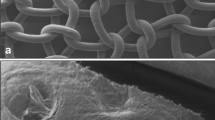
Initial histological evaluation did not demonstrate any significant differences in H&E staining for the 1-month time point. Both had endovascular in-growth as well as host collagen infiltration. By the 3-month time point there was a significant increase in inflammatory cells within the Strattice™ mesh; however, there was no significant difference in collagen or vessel density between either mesh. The 6-month histology did not demonstrate a significant difference in vessel density but showed a continued increase in inflammatory cells and a disorganization and decrease in collagen content in the Strattice™ mesh. The 6-month vessel density was 74.66 vessels/cm2 and 64.68 vessels/cm2, for Permacol™ and Strattice™, respectively. The vessel density at 12 months for Permacol™ was 34 vessels/cm2 (Fig. 5a). We were unable to perform vascular density analysis with any confidence secondary to the significant thinning of the Strattice™ mesh at 12 months (Fig. 5b). There was a significant influx of inflammatory cells and disorganization in much of the mesh that was harvested.
Discussion
Multiple methods for abdominal wall reconstruction have been used with different rates of success. Most are in general agreement that midline fascial approximation with a mesh underlay, plus or minus component separation, provides better outcomes. However, we chose to utilize a bridging technique rather than an underlay technique for a multitude of reasons. Our main purpose was to evaluate the remodeling process of the respective biologic implant. Using an underlay would have added numerous variables to our immunohistochemistry analysis of the mesh as well as tensile strength testing. Our bridging technique provided us an opportunity to evaluate the mesh itself without uncontrolled variables.
Biomeshes have been introduced to the hernia repair field with potential benefits of superior biocompatibility, reduced adhesion formation, degradability, and decreased risk of post-op infection in contaminated operation fields [12]. With these goals in mind, we set out to determine whether there was an observable difference in using non-cross-linked and cross-linked biological meshes in the repair of ventral hernias over the period of 1 year.
Following the implantation of biomaterials, the body initiates a reparative process that either aids in the integration of the biomaterial or induces a disproportionate inflammatory response that could end in excessive fibrosis with resultant scarring and encapsulation or rapid mesh degradation, leading to graft weakening and hernia repair failure [10]. The use of Permacol™ and Strattice™ biologic meshes had an observed wound complication rate of 19–26% in our model of ventral hernia repair. These complications consisted of skin separation and early herniation secondary to separation of the implant from the host muscle as determined upon autopsy. Bridging defects in clinical studies also reveals similar early hernia recurrence rates. The rate of seroma formation in both groups accounted for the vast majority of wound complications. Seroma formation after ventral hernia repair is one of the most common post-operative complications [13]. Drains were not used as would be our practice in human patients to control seroma formation.
The pre-implantation mesh thicknesses did not vary statistically between Permacol™ and Strattice™ biologics. However, there was a higher degradation rate observed in the Strattice™ biologic starting at 3 months and continuing until the end of the experiment at 12 months. This higher degradation rate was also noted in the Strattice™ mesh surface area at 12 months post-implantation. This is suggestive that cross-linking has a benefit in lessening the degradation rate of the implant.
In addition to adding initial strength, one of the primary functions of biologic meshes is to act as a natural scaffold through which the body’s reparative process ultimately replaces the mesh by gradually degrading and/or reabsorbing, remodeling and replacing the mesh with native tissue. Proper mesh integration depends on cellular and fibrovasular in-growth, followed by tissue remodeling [10]. A bridging method utilizing both cross-linked (Permacol™) and non-cross linked (Strattice™) biologic meshes was used to observe the migration of host cells from the periphery as well as to observe the role that peritoneal fluid played in re-vascularization and re-collagenation in-growth into the biologic implant in the rat, and to assess neocollagen deposition. Cross-linked materials may prove more useful in the remodeling process as suggested by the increased thinning and increased weakness beginning at the 3-month time point in the non-cross-linked biomesh.
More studies evaluating the remodeling process and durability of emerging biologic mesh products are needed. Animal models have demonstrated the lack of durability and eventual failure of human dermis-derived biologic meshes when used in abdominal wall repair [5]. Thus, such studies could provide valuable data regarding efficacious and appropriate use of costly products in clinical settings.
References
den Hartog D et al (2010) Open surgical procedures for incisinal hernias. The Cochrane Database of Systematic Reviews: Issue 5
Jin J, Voskerician G, Hunter S, McGee MF, Cavazzola LT, Schomisch S, Harth K, Rosen MJ (2009) Human peritoneal membrane controls adhesion formation and host tissue response following intra-abdominal placement in a porcine model. J Surg Res 156:297–304
Saettele TM, Bachman SL, Costello CR, Grant SA, Cleveland DS, Loy TS, Kolder DG, Ramshaw BJ (2007) Use of porcine dermal collagen as a prosthetic mesh in a contaminated field for ventral hernia repair: a case report. Hernia 11:279–285
Harth KC, Rosen MJ (2009) Major complication associated with xenograft biologic mesh implantation in abdominal wall reconstruction. Surg Innov 16(4):324–329
Gaertner WB, Bonsack ME, Delaney JP (2007) Experimental evaluation of four biologic prostheses for ventral hernia repair. J Gastrointest Surg 11(10):1275–1285
Wotton FT, Akoh JA (2009) Rejection of Permacol® mesh used in abdominal wall repair: a case report. World J Gastroenterol 15(34):4331–4333
Hiles M, Ritchie RDR, Altzer AM (2009) Are biologic grafts effective for hernia repair? A systematic review of the literature. Surg Innov 16:26–37
Harper C (2001) Permacol: clinical experience with a new biomaterial. Hosp Med 62(2):90–95
LifeCell Corporation (2009) Strattice™ Reconstructive Tissue Matrix. http://www.lifecell.com/strattice-reconstructive-tissue matrix/. Accessed 16 July 2010
Orenstein SB, Qiao Y, Klueh U, Kreutzer DL, Novitsky YW (2010) Activation of human mononuclear cells by porcine biologic meshes in vitro. Hernia 14(4):401–407
Duran B, Ak D, Cetin A, Cetin A, Guvenal T, Cetin M, Imir AG (2003) Reduction of postoperative adhesions by N, O-carboxymethylchitosan and spermine neonoate in rats. Exp Anim 52(4):267–272
Petter-Puchner AH, Fortelny RH, Walder N, Mittermayr R, Ohlinger W, van Griesnsven M, Redl H (2008) Adverse effects associated with the use of porcine cross-linked collagen implants in an experimental model of incisional hernia repair. J Surg Res 145:105–110
Jin J, Schomisch S, Rosen MJ (2009) In vitro evaluation of the permeability of prosthetic meshes as the possible cause of postoperative seroma formation. Surg Innov 16(2):129–133
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulier, K.E., Nguyen, A.H., Delaney, J.P. et al. Comparison of Permacol™ and Strattice™ for the repair of abdominal wall defects. Hernia 15, 315–319 (2011). https://doi.org/10.1007/s10029-010-0777-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-010-0777-6