Abstract
Introduction
With approximately 1 million ventral and inguinal hernia repairs performed in the United States each year, even small rates of complications translate into large numbers of patients. Less invasive approaches that potentially lower morbidity deserve consideration, recognizing there are many technical considerations that currently limit their use. We describe a reproducible technique and lessons learned in our laboratory that answer some existing questions with regards to the use of NOTES® for hernia repair.
Methods
A non-survival porcine model with general anesthesia was utilized in all cases. Each animal underwent transgastric peritoneal access with a percutaneous endoscopic gastrostomy (PEG) technique, and the gastrotomy was dilated with a wire-guided balloon dilatation catheter. An Esophageal Z-stent delivery device (Cook Medical, Winston-Salem, NC) was modified ex-vivo to allow us to introduce and protect a 10 × 15 cm lightweight polypropylene hernia prosthetic with pre-placed sutures. Once deployed, the sutures were pulled through the abdominal wall using a looped spinal needle technique in combination with the flexible endoscope. After the four anchoring sutures were tied, proprietary endoscopically placed tacks (Cook Medical) were placed at regular intervals between the sutures to secure the edges of the prosthetic.
Results
Hernia repairs were performed on five animals. In each case, we successfully completed prosthetic delivery and deployment into the peritoneal cavity, anchoring to the abdominal wall with full-thickness abdominal wall sutures, and endoscopically placed nitinol tacks. All prosthetics were deployed flat against the anterior abdominal wall. Operative times ranged from 65 to 120 min.
Conclusion
Transgastric abdominal wall hernia repair is feasible, consistent, and reproducible. In particular, the delivery system can successfully deliver the prosthetic across the gastric wall via a transoral route. Survival animal experiments investigating outcomes related to quality of repair, microbiology, adhesions, and visceral closure need to be done. Human studies are not recommended until these issues are formally investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are approximately 1 million hernia repairs performed in the United States annually [1]. Because of the high case volume, even low complication rates translate into large numbers of patients. As wound complications are a major part of the morbidity, eliminating abdominal wall incisions may ameliorate these complications, thus improving overall outcomes. With regards to NOTES®, many barriers exist to the successful repair of abdominal wall hernia. These include translumenal access and closure, sterile prosthetic delivery, prosthetic deployment and fixation. Naturally, clinical outcomes should be the primary metric regarding the ultimate fate of a given procedure or technique.
Methods
Five Yorkshire female pigs, 35–44 kg in weight, were used for this experiment. The animals were anesthetized and cared for according to our IACUC (Institutional Animal Care and Use Committee)-approved protocol.
Utilizing a GIF-140 diagnostic gastroscope (Olympus America, Center Valley, PA), we utilized a standard percutaneous endoscopic gastrostomy (PEG) technique to place a 0.035 inch (0.1 cm) guidewire through the abdominal wall, into the stomach, and out through the mouth. Once the wire was in place, we insufflated the peritoneal cavity to 12 mmHg with CO2 utilizing a Veress needle. We then fed extra wire into the peritoneal cavity through the abdominal wall.
A loop-anchor purse-string gastrotomy closure technique was employed prior to creation of the gastrotomy to facilitate eventual gastric closure with some animals with our previously described a technique [2]. Briefly, a single monofilament, non-absorbable suture was placed in a purse-string fashion using four full-thickness looped T-anchors (Cook Medical). A needle-knife gastrotomy was then performed in the center of the purse string, and dilated with an 18 mm, wire-guided balloon dilatation catheter (Cook Medical).
Once the gastrotomy was dilated and the endoscope was introduced into the peritoneal cavity, the 0.035 inch guidewire was then removed, and a Savary wire was placed into the peritoneal cavity through the endoscope. The animal was now considered properly prepared for prosthetic implantation.
A 10 × 15-cm lightweight polypropylene prosthetic (ProLite™ Ultra; Atrium Medical; Hudson, NH) was used. Four 2–0 silk sutures were placed at the 12, 3, 6, and 9 o’clock positions. The prosthetic was marked at one of these sutures to allow for proper orientation. The mesh was then back-loaded into a modified esophageal stent introducer (Fig. 1; Cook Medical). The stent introducer’s wire channel is separate from the lumen of the introducer that houses the prosthetic, thus keeping the prosthetic in a sterile environment during placement into the peritoneal cavity. The stent introducer was then passed over the Savary wire alongside an endoscope until the introducer tip was completely through the gastrotomy, and in the peritoneal cavity. The prosthetic was then deployed with the stent introducer under direct endoscopic (and for the first two cases, laparoscopic) vision, and the introducer was removed.
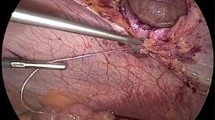
Four stab incisions were made in the abdominal wall corresponding to the locations of the sutures on the prosthetic. The prosthetic was partially unfurled and manipulated into position by using a grasping forceps via the endoscope. Once the marked suture was located, we pulled this through the abdominal wall using a looped spinal needle (Fig. 2), and repeated the process for each of the four sutures, in a technique previously described by us [3]. This was performed such that each suture tail was pulled through two separate locations of the abdominal wall via a single skin incision, as would be done for a typical laparoscopic or open ventral hernia repair. Once the sutures were tied, the mesh lay taught against the anterior abdominal wall.
Proprietary endoscopic Nitinol tacks (Fig. 3; Cook Medical), as described by Bhat et al. [4], were then passed through the working channel of the endoscope, and deployed into the abdominal wall through the periphery of the prosthetic, including the corners, at 2–3 cm intervals. The repair was completed when the mesh was tacked circumferentially (Fig. 4).
The endoscope was then withdrawn into the stomach, and the purse-string was tightened, thus closing the gastrotomy, and crimped with a friction fit collar. The excess suture was then cut with endoscopic scissors. The animal was then euthanized per our standard animal protocol.
Results
Transgastric access to the peritoneal cavity and prosthetic deployment was successful in all five cases. Additionally, in each case, the prosthetic was successfully anchored to the anterior abdominal wall with a combination of full thickness anchoring sutures and tacks solely with the transgastric flexible endoscope. The first two cases were performed with the assistance of laparoscopic visualization, primarily to obtain external video of the endoscopic maneuvering and to hasten the learning curve. The third case was performed with the endoscopist blinded to the laparoscopic view, and the final two cases were performed without the use of a laparoscope. Two of the five gastrotomy sites were not closed due to logistical constraints, and the fact that it was a non-survival model. Operative time was defined as time of endoscope insertion to gastrotomy closure or full mesh fixation, and ranged from 65 to 120 min.
Discussion
Early excitement regarding the implementation of NOTES® techniques has led to successful early human case reports [5–15], including an early human case report of a NOTES® transvaginal umbilical hernia [16]. Despite this, much scientific investigation is required prior to widespread adoption of this new surgical approach. The promise of little to no recovery coupled with a commonly performed operation has prompted us to investigate techniques to improve the current performance of hernia repair. In order to focus our efforts on a systematic pathway to developing the technique, we have broken the procedure down into the components (Table 1): abdominal access, prosthetic handling (preparation, delivery, manipulation and fixation), and visceral closure. In this non-survival porcine model, our efforts were concentrated primarily on the technical aspects of prosthetic delivery, manipulation, and fixation. Certainly, peritoneal access and secure visceral closure are critical for safety, and remain the focus of other ongoing experiments.
Other investigators have also explored NOTES® techniques for hernia repair. Hu et al. [17] made a muscular defect using a needle knife and pull-type sphincterotome; they were able to close it endoscopically using a prototype suturing device. Miedema et al. [18] simulated transgastric incisional hernia repair in a porcine model, similar to our experiment, except that they utilized only four tacking sutures, and biologic mesh (Surgisis® Gold™, Cook Medical). Sherwinter and Eckstein [19] simulated transgastric inguinal herniorrhaphy in a nonsurvival canine model employing human acellular dermal matrix (Alloderm, Lifecell, Branchburg, NJ). Interestingly, their choice of mesh fixation was with BioGlue® (CryoLife, Kennesaw, GA) delivered through the endoscope. Another study by Lomanto et al. [20] describes NOTES® transvaginal hernia repair in a survival porcine model, using Parietex™ mesh (Covidien, Mansfield, MA), covering a created defect in the abdominal wall. The mesh was secured to the abdominal wall again employing preplaced sutures, and laparoscopic tacks or fibrin glue. They reported no infections and adhesions in two of the five cases. Lastly, there is a recent case report [21] of a transrectal umbilical hernia repair in a human cadaver; obviously, troubling questions regarding infection are not assessed in this feasibility study.
Regarding the prosthetic choice, we chose a lightweight polypropylene prosthetic because this is the most common and well studied hernia prosthetic on the market. It represents a group of prosthetics that tolerates clinical infection relatively well, and effects a long lasting repair of abdominal wall defects compared to other types of prosthetics [22–25]. This study does not aim to compare prosthetics, or suggest which prosthetic is best for this type of repair. It is certainly possible that the prosthetic for a NOTES® hernia repair has yet to be developed. Future studies comparing prosthetic types for clinically relevant parameters such as infection rates, ease of deployment, and adhesion formation are necessary.
Delivery of the prosthetic to the posterior part of the anterior abdominal wall without injuring tissue, damaging the prosthetic or introducing infection is an obvious obstacle to performing a NOTES® hernia repair. One study examined delivery of a very small prosthetic via a transanal approach in a porcine model with both survival and non-survival techniques. Without necessarily protecting the prosthetic from the colonic flora, they found no problems associated with infection [26]. Although the small prosthetic size is not very clinically relevant, it answers some important questions about the basic principles of prosthetic delivery. On the contrary, a recent study by Buck and colleagues compared NOTES® mesh placement to laparoscopic mesh placement and found that NOTES® mesh placement had a significantly increased rate of infection, despite gastric irrigation [27]. This study also utilized a non-clinically relevant mesh size. This implies the need for a sterile delivery device, such as the one we describe. Miedema et al. [18], despite using a biologic prosthetic placed in a transgastric fashion, found microabscesses in three of five animals, indicating a likely source of contamination during prosthetic delivery.
Given that there were no infectious problems when a solid polytetraflouroethylene prosthetic was used to repair iatrogenic colonic defects in a dog model, one wonders if there is a difference between the gastric and colonic flora as it relates to prosthetic infection. There are obviously many unanswered questions about this concept [28]. One problem encountered during the transanal prosthetic placement was locating the prosthetic after delivery. The use of a unique magnetic system to assist in prosthetic location was successfully employed [26]. We have not found this necessary, as the prosthetic was easily located with the endoscope after delivery with the device via a transgastric approach, likely due to the larger prosthetic size, in addition to the predictable fashion with which the delivery system placed the prosthetic. To solve problems with contamination, we modified an esophageal stent delivery device by removing the esophageal stent, and placing the prosthetic with anchoring sutures in the chamber that housed the stent in a sterile fashion. A particularly useful property of the stent delivery device is that the wire guide is completely separate from the chamber that houses the prosthetic, further reducing the contamination risk. During deployment of the prosthetic, however, the cap is pushed off the device by a rod that also pushes the prosthetic against the cap, thus dislodging it. With the cap off, and pushed away from the delivery device, the potentially contaminated wire is briefly exposed to the prosthetic. A brief exposure to a relatively low inoculum size would theoretically reduce the chance for clinical infection, although the recent work of Buck et al. [27] seems to contradict this. One concept that we have considered is the use of an overtube, to allow a relatively smooth, less contaminated pathway for prosthetic delivery. Changing the wire once the delivery device is placed through the visceral wall may be another strategy to reduce contamination. Further modifications of the stent delivery device as such are ongoing as well. Survival studies will be needed to discover whether or not these strategies will reduce or eliminate infectious complications related to prosthetic delivery.
Manipulating the prosthetic into the correct orientation can sometimes be challenging, even during laparoscopic hernia repair. When utilizing a NOTES® technique, this challenge is magnified. In order to facilitate prosthetic manipulation, we drew from our extensive clinical experience with laparoscopic ventral hernia repair. Marking the prosthetic at the site of the first suture to pull out was difficult, so we adapted our technique to use different color sutures, determining which color would be the primary suture pulled out. The looped spinal needle technique [3] proved quite easy when performed in conjunction with a skilled flexible endoscopist.
Using both full thickness abdominal wall sutures in combination with tacks, we aimed to replicate the currently most commonly used method of prosthetic fixation for ventral hernia repair. Once the suture anchors were brought through the abdominal wall, the prosthetic was relatively flat against the anterior abdominal wall, and ready to be tacked. We utilized endoscopically delivered Nitinol anchors (Cook Medical) to tack the prosthetic between the full thickness anchoring sutures. Once all the tacks were in place, we concluded the experiment. We first evaluated this proprietary tack design and delivery system with four survival swine where the anchors were placed in the abdominal wall without a prosthetic. At necropsy 2 weeks after implantation, there were no adhesions to the tacks, which remained fixed well in the abdominal wall, and one microabscess. These animals had their gastrotomies closed using the loop-anchor purse-string system we developed previously [2]. We have since developed an alternative to the technique to place full thickness tacks through the abdominal wall and prosthetic with a needle-based delivery system directly through the abdominal wall without an incision.
Only the development of new working platforms and tools will enable more widespread use in humans that require lysis of adhesions. Adhesions not only pose a risk with abdominal wall exposure, a NOTES® approach must also consider intra-visceral adhesions for peritoneal cavity entry. The clinical benefits will also have to be studied, and may not be realized until the advent of new prosthetic and anchoring technology.
In summary, we have demonstrated the feasibility of a NOTES® ventral hernia repair by developing a reproducible technique in a porcine model that utilizes a clinically relevant prosthetic and commonly employed fixation methodology. Survival studies need to be performed to assess issues related to infection, durability of prosthetic fixation, adhesions, and visceral closure. Once fully addressed in the laboratory, human studies could be done to assess whether or not there are clinically relevant benefits.
References
Rutkow I (2003) Demographics, socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am 83(5):1045–1051
Desilets DJ, Romanelli JR, Earle DB, Surti VC, Willingham FF, Brugge WR (2009) Loop-anchor purse-string vs. endoscopic clips for gastric closure—a NOTES™ comparison study using burst pressures. Gastrointest Endosc 70(6):1225–1230 (Epub 2009, Oct 20)
Earle D (1999) A simple and inexpensive technique for closing trocar sites and grasping sutures. J Laparoendosc Adv Surg Tech A 9(1):81–85
Bhat YM, Hedge S, Knaus M, Solomon J, Kochman ML (2009) Transluminal endosurgery: novel use of endoscopic tacks for the closure of access sites in natural orifice transluminal endoscopic surgery (with videos). Gastrointest Endosc 69(6):1161–1166
Palanivelu C, Rajan PS, Rangarajan M, Parthasarathi R, Senthilnathan P, Prasad M (2008) Transvaginal endoscopic appendectomy in humans: a unique approach to NOTES-world’s first report. Surg Endosc 22(5):1343–1347 (Epub 2008 Mar 18)
Palanivelu C, Rajan PS, Rangarajan M, Parthasarathi R, Senthilnathan P, Praveenraj P (2008) Transumbilical flexible endoscopic cholecystectomy in humans: first feasibility study using a hybrid technique. Endoscopy. 40(5):428–431
Romanelli JR, Desilets DJ, Earle DB (2008) Pancreatic pseudocystgastrostomy with a peroral, flexible stapler: a human NOTES™ anastomosis in two patients. Gastrointest Endosc 68(5):981–987
Marescaux J, Dallemagne B, Perretta S, Wattiez A, Mutter D, Coumaros D (2007) Surgery without scars: report of transluminal cholecystectomy in a human being. Arch Surg 142(9):823–827
Bessler M, Stevens PD, Milone L, Parikh M, Fowler D (2007) Transvaginal laparoscopically assisted endoscopic cholecystectomy: a hybrid approach to natural orifice surgery. Gastrointest Endosc 66(1):1243–1245
Branco FAJ, Noda RW, Kondo W, Kawahara N, Rangel M, Branco AW (2007) Initial experience with hybrid transvaginal cholecystectomy. Gastrointest Endosc 66(6):1245–1248
Dolz C, Noguera JF, Martín A, Vilella A, Cuadrado A (2007) Transvaginal cholecystectomy (NOTES) combined with minilaparoscopy. Rev Esp Enferm Dig 99(12):698–702
Forgione A, Maggioni D, Sansonna F, Ferrari C, Di Lernia S, Citterio D, Magistro C, Frigerio L, Pugliese R (2008) Transvaginal endoscopic cholecystectomy in human beings: preliminary results. J Laparoendosc Adv Surg Tech A. 18(3):345–351
Zorrón R, Filguerias M, Maggioni LC, Pombo L, Lopes Carvalho G, Lacerda Oliveira A (2007) NOTES transvaginal cholecystectomy: report of the first case. Surg Innov 14(4):279–283
Zorrón R, Maggioni LC, Pombo L, Oliveira AL, Carvalho GL, Filgueiras M (2008) NOTES transvaginal cholecystectomy: preliminary clinical application. Surg Endosc 22(2):542–547
Zornig C, Mofid H, Emmermann A, Alm M, von Waldenfels HA, Felixmüller C (2008) Scarless cholecystectomy with combined transvaginal and transumbilical approach in a series of 20 patients. Surg Endosc 22(6):1427–1429
Jacobsen GR, Thompson K, Spivack A, Fischer L, Wong B, Cullen J, Bosia J, Whitcomb E, Lucas E, Talamini M, Horgan S (2010) Initial experience with transvaginal incisional hernia repair. Hernia 14(1):89–91 (Epub 2009 Apr 15)
Hu B, Kalloo AN, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Isakovich NV, Nakajima Y, Kawashima K, Kantsevoy SV (2007) Peroral transgastric endoscopic primary repair of a ventral hernia in a porcine model. Endoscopy 39:390–393
Miedema BW, Bachman SL, Sporn E, Astudillo JA, Thaler K (2009) Transgastric placement of biologic mesh to the anterior abdominal wall. Surg Endosc 23(6):1212–1218 (Epub 2009 Mar 5)
Sherwinter DA, Eckstein JG (2009) Feasibility study of natural orifice transluminal endoscopic surgery inguinal hernia repair. Gastrointest Endosc 70(1):126–130 (Epub 2009 Feb 27)
Lomanto D, Dhir U, So JBY, Cheah WK, Moe MA, Ho KY (2009) Total transvaginal endoscopic abdominal wall hernia repair: a NOTES survival study. Hernia 13:415–419
Hagen ME, Wagner OJ, Swain PC, Patel A, Inan I, Pugin F, Fasel J, Morel P (2009) Transrectal natural orifice transluminal endoscopic surgery for umbilical hernia repair in a human cadaver (with video). Gastrointest Endosc 69(6):e53–e54 (Epub 2009 Jan 18)
Bellón JM, Contreras LA, Buján J (2000) Ultrastructural alterations of polytetrafluoroethylene prostheses implanted in abdominal wall provoked by infection: clinical and experimental study. World J Surg 24(5):528–532
Gumargalieva KZ, Moiseev YV, Daurova TT, Voronkova OS (1982) Effects of infections on the degradation of polyethylene terephthalate implants. Biomaterials 3(3):177–180
Kelly ME, Behrman SW (2002) The safety and efficacy of prosthetic hernia repair in clean-contaminated and contaminated wounds. Am Surg 68(6):524–529
Blatnik J, Jin J, Rosen M (2008) Abdominal hernia repair with bridging acellular dermal matrix—an expensive hernia sac. Am J Surg 196(1):47–50 (Epub 2008 May 7)
Fong DG, Ryou M, Pai RD, Tavakkolizadeh A, Rattner DW, Thompson CC (2007) Transcolonic ventral wall hernia mesh fixation in a porcine model. Endoscopy 39(10):865–869
Buck L, Michalek J, Van Sickle K, Schwesinger W, Bingener J (2008) Can gastric irrigation prevent infection during notes mesh placement? J Gastrointest Surg 12(11):2010–2014 (Epub 2008 Aug 13)
Oh DS, Manning MM, Emmanuel J, Broyles SE, Stone HH (2002) Repair of full-thickness defects in alimentary tract wall with patches of expanded polytetrafluoroethylene. Ann Surg 235(5):708–712
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Earle, D.B., Desilets, D.J. & Romanelli, J.R. NOTES® transgastric abdominal wall hernia repair in a porcine model. Hernia 14, 517–522 (2010). https://doi.org/10.1007/s10029-010-0701-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-010-0701-0