Abstract
Floodplain and riparian ecosystems have cooler, wetter microclimatic conditions, higher water availability and greater vegetation biomass than adjacent terrestrial zones. Given these conditions, we investigated whether floodplain ecosystems allow terrestrial bird species to extend into more arid regions than they otherwise would be expected to occupy. We evaluated associations between aridity and the occurrence of 130 species using bird survey data from 2998 sites along the two major river corridors in the Murray–Darling Basin, Australia. We compared the effects of aridity on species occurrence in non-floodplain and floodplain ecosystems to test whether floodplains moderate the effect of aridity. Aridity had a negative effect on the occurrence of 58 species (45%) in non-floodplain ecosystems, especially species dependent on forest and woodland habitats. Of these 58 species, the negative effects of aridity were moderated in floodplain ecosystems for 22 (38%) species: 12 showed no association with aridity in floodplain ecosystems and the adverse effects of aridity on species occurrence were less pronounced in floodplain ecosystems compared to non-floodplain ecosystems for ten species. Greater vegetation greenness indicated that floodplain vegetation was more productive than vegetation in non-floodplain ecosystems. Floodplain ecosystems allow many terrestrial species to occur in more arid regions than they otherwise would be expected to occupy. This may be due to higher vegetation productivity, cooler microclimates or connectivity of floodplain vegetation. Although floodplain and riparian ecosystems will become increasingly important for terrestrial species persistence as climate change increases drying in many parts of the world, many are also likely to be highly affected by reduced water availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Floodplain and riparian ecosystems offer multiple values for aquatic and water-dependent biota. Floodplains are vital in providing habitat for at least some life stages of many water-dependent species (Junk and others 1989; Museth and others 2011) and riparian vegetation condition positively affects aquatic communities by maintaining water quality and natural in-stream ecosystem processes (Death and Collier 2010; Giling and others 2014). However, the importance of floodplain and riparian ecosystems for non-aquatic biota (for example, terrestrial vertebrates) is not so well known. Our limited understanding of the reliance of terrestrial biota on floodplain and riparian ecosystems is likely to have underestimated the adverse ecological effects of river regulation, the benefits of natural flooding regimes (Ballinger and Lake 2006) and the value of floodplain and riparian vegetation for terrestrial biota, particularly in drying climates (Capon and others 2013).
We define floodplain ecosystems as the interface between terrestrial and aquatic zones, consisting of terrestrial vegetation in stream channels, adjacent to water bodies and on floodplains, dominated by plant species that require periodic flooding to complete their life cycle (Naiman and others 1993). Access to water tables and flooding allow floodplain ecosystems to support more vegetation biomass than one would expect given local rainfall (Naumburg and others 2005) and to maintain more consistent production during drought (Selwood and others 2015b). Floodplain microclimates are moderated by canopy cover, topography and adjacency to water bodies (Brosofske and others 1997). The contrast in vegetation and microclimate between mesic floodplains and non-floodplain ecosystems is likely to be greater in more arid climates, where water availability is limited and temperatures are high, although the degree to which floodplain ecosystems differ from adjacent ecosystems depends on flood intermittency and groundwater depth (Stromberg and others 2006; Brand and others 2011).
Abundances of terrestrial fauna often are higher in floodplain ecosystems than in adjacent plains and upland areas and floodplain ecosystems provide seasonal habitat and drought refuges for birds (Woinarski and others 2000; Tischler and others 2013; Smith 2015). Although floodplain and riparian ecosystems sometimes have higher faunal species richness than adjacent habitats (Mac Nally and others 2000; Smith 2015), these ecosystems predominantly increase regional species richness by harbouring substantially different species assemblages (Sabo and others 2005; Pavey and Nano 2009). The contrast between faunal assemblages in floodplain and adjacent ecosystems is greater in xeric areas compared to mesic and humid locations (Sabo and others 2005). This greater contrast between floodplain and adjacent faunal assemblages in more arid regions may occur because floodplain ecosystems facilitate the occurrence of some species into more arid climates than they could otherwise occur, leading to differences between typical arid zone assemblages and more mesic assemblages in floodplain habitat.
Here, we explored whether floodplains moderate the effects of aridity on species occurrence to facilitate the occurrence of species in more arid climate zones than they otherwise would be expected to occupy. Our focal region includes the forests and woodlands in the river corridors of Australia’s largest river system, the Murray–Darling Basin. Floodplain forest and woodland occurs throughout the region, forming an extensive network of vegetation on floodplains and along rivers that is interspersed among terrestrial ecosystems ranging from humid to arid (United Nations Environment Program 1997; Bureau of Meteorology (Australia) 2015a).
Terrestrial bird assemblages in floodplain and adjacent zones are more differentiated from each other in regions with low mean annual rainfall (Tzaros 2001). Therefore, we evaluated changes in the occurrence of bird species along the two major river corridors (the Murray and Darling river bioregions) to see whether the effects of aridity on species occurrence differed between non-floodplain and floodplain ecosystems. Evidence for a reduced negative effect of aridity on species occurrence in floodplain ecosystems compared to non-floodplain ecosystems suggests a moderating effect of floodplains on the effects of aridity, allowing species to occur in more xeric regions (Figure 1). We explored whether floodplain vegetation was more productive than other vegetation. Finally, we linked species associations with aridity with species ecological traits to assess whether different guilds (foraging, nesting, diet, habitat, movement dynamics, body size) were more likely to be affected by aridity and whether species within particular guilds differed in their likelihood to have their distributions extended by floodplains.
Illustration of how differences in associations between species occurrence and aridity in non-floodplain ecosystems versus floodplain ecosystems can indicate the facilitation of species occurrence in more arid regions by floodplains. A moderating effect of floodplains on the association between species occurrence and aridity (= less negative in floodplain ecosystems than in non-floodplain ecosystems) indicates that floodplains may carry species into more arid regions.
Methods
Study Region
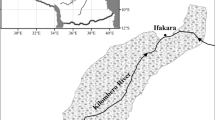
The study region consists of bioregions along the two major river corridors of the Murray–Darling Basin, which include the alluvial fans and plains of the Murray and Darling Rivers and their major tributaries (Figure 2). Vegetation in the Murray River corridor consists of Eucalyptus spp. forest and woodlands, saltbush shrublands and extensive grassland and swamp communities (Environment Australia 2000). The Darling River corridor primarily consists of woodlands and open woodlands dominated by Eucalyptus spp. (Environment Australia 2000; Breckwoldt and others 2004). The floodplain zones of these corridors are primarily dominated by river red gum (Eucalyptus camaldulensis), black box (E. largiflorens) or coolabah (E. coolabah) forests and woodlands, which are tree species that require periodic inundation to complete their life cycle (Roberts and Marston 2011; Cunningham and others 2013). Mean annual rainfall for most of the region ranges from 200 to 800 mm, increasing generally from west to east along each river (Figure 1; Bureau of Meteorology (Australia) 2015a) and the aridity of the region ranges from humid to arid (United Nations Environment Program 1997; Bureau of Meteorology (Australia) 2015a).
Location of the study region (black shaded region in inset map) and study sites (black dots on enlarged map) along the Murray and Darling River corridors. The study region consists of the Riverina and Darling River Plains bioregions as classified by Department of the Environment (2012b). Rainfall isohyet bands are shaded in grey scale with darker colours indicating higher rainfall;legend indicates maximum rainfall per band.
Bird Data
We used bird survey data from BirdLife Australia’s New Atlas of Australian Birds (Barrett and others 2003), from 01-Jan-1998 to 31-Dec-2013. All standard 2-ha/20-min surveys in native vegetation (Department of the Environment 2014) within the study region were used, which consisted of 2998 sites that were surveyed at least once (mean number of surveys = 2.3; standard deviation = 4.8), including 1547 sites within floodplain vegetation. We focused on native terrestrial birds, excluding non-native species and obligate wetland birds from the analyses. We also excluded birds of prey because they range over areas substantially greater than 2 ha. We included species that were observed in at least ten surveys (130 species) in floodplain and non-floodplain zones to allow comparisons in species occurrence between these two zones; previously, we have shown that applying a lower limit of ten produces similar results to more severe limits up to 100 occupied sites (Selwood and others 2015b).
Spatial Data
We used QGIS 2.0 (QGIS Development Team 2013) and R (R Core Team 2015) with the packages ‘raster’ (Hijmans and van Etten 2013) and ‘maptools’ (Bivand and Lewin-Koh 2013) to obtain information about each survey site using coordinates from the Atlas of Australian Birds data. We classified all survey sites as (1) floodplain ecosystems or (2) non-floodplain ecosystems (all other native vegetation). We defined floodplain ecosystems as sites located in communities dominated by river red gum, black box or coolabah, with spatial information on occurrence from (Cunningham and others 2013). Although shrublands and grasslands are present on some floodplains in the drier parts of the Murray–Darling Basin, we focus here on the more mesic woodland and forested floodplain communities. We obtained information on the vegetation community type of each site (nested within floodplain and non-floodplain ecosystem type) to control for species’ vegetation associations (Department of the Environment 2012). Information on precipitation and potential evapotranspiration at each site was obtained from spatial data modelled for 500-m grids by the Bureau of Meteorology (Bureau of Meteorology (Australia) 2015a). The aridity of each site was calculated using the inverse of the United Nations Environment Program aridity index (Ezcurra 2006), A = ET/P. Here, P is mean annual precipitation and ET is mean annual potential evapotranspiration so that higher values of A indicate higher aridity. We obtained information on native vegetation cover in the landscape surrounding sites from a digital map of native vegetation cover (Department of the Environment 2014).
Vegetation Greenness
We tested whether floodplain vegetation was likely to be more productive than non-floodplain vegetation by comparing the Normalized Difference Vegetation Index (NDVI) at surveyed sites. NDVI is a measure of vegetation greenness that provides an estimate of photosynthetic activity (Wang and others 2004). We used mean annual estimates of NDVI calculated from bands 1 and 2 of the MODIS Terra satellite 250-m imagery NASA (Atlas of Living Australia and Bureau of Rural Sciences 2015). We tested whether NDVI was higher in floodplains compared to non-floodplain ecosystems, and included aridity as a covariate in the model to account for the effects of climate on vegetation productivity. Our model was:
Here, F is binary (1= floodplain ecosystem, 0 = non-floodplain ecosystem), A i is the aridity at site i and \( \epsilon _{V} \) is the random effect of specific vegetation community type, nested within F (Department of the Environment 2012). The δs are regression parameters.
Species Occurrence
We evaluated the occurrence of individual species in the Murray River and Darling River bioregions to test whether individual species occurrence decreased with increasing aridity (A) in non-floodplain ecosystems, and whether this negative association was less, or absent, in floodplain ecosystems (Figure 1). We estimated the probability p i of observing the species during a single survey at site i, and modelled the number of observations as a Binomial variable: y i ~ Binomial(n i , p i ), where n i was the number of surveys conducted at a site.
For each species, the model was
Here, the estimated effect of the aridity for site i nested within river corridor c (Murray or Darling), γ c[i], depends on whether the site is on a floodplain (F i = 1) or not (F i = 0), with \( \beta_{1} \)being a measure of the effect of aridity in non-floodplain ecosystems and \( \beta_{2} \) being the difference of that effect in floodplain ecosystems. We included F as a covariate in the model to account for species associations with floodplains, thereby allowing us to identify a moderating effect of floodplains on aridity (β 2) that is independent of species apparent preference or disfavour of floodplain habitat. Where the effect of aridity is negative (β 1 < 0), β 2 > 0 indicates a moderating influence of floodplains, resulting in a less negative or positive association with aridity in floodplains. We included specific vegetation type (V, nested within F) and the percentage cover of native vegetation (C) in the surrounding landscape (500 m radius) to account for species–habitat associations and for the effects of landscape context (Thomson and others 2009). The river corridor-specific aridity effects (\( \beta_{1,c} , \beta_{2,c} \)) and covariate effects (\( \beta_{3,c} , \beta_{4,c} , \beta_{5,c} \)) comprise overall mean (fixed) effects, B n , and random variation with variance σ 2 n . The spatial random error components (\( \epsilon_{i} \)) are for sub-region (Interim Biogeographic Regionalisation for Australia, Department of the Environment 2012b) (ɛ 1) and site (ɛ 2), which were modelled as independent and identically distributed. We used linear combinations to estimate the marginal posterior distributions for the mean association between species occurrence and aridity in the floodplain ecosystem (B fr = B 1 + B 2).
Species Traits
We grouped species into guilds within the following ecological traits: body mass, nesting substrate, foraging substrate, diet, habitat preference and movement dynamics (Table 1). We tested whether (1) the effects of aridity on species occurrence (B 1) and (2) moderating effect of floodplains on aridity effects (B 2 for species where B 1 > 0) were related to species ecological traits. We used a random effects model to partition the variation in B 1 and B 2 among species traits. We included as a random effect the phylogeny of our species constructed from Jetz and others (2012) to control for species relatedness. Models were weighted by the inverse variance of the species-specific posterior distributions of the response variables B 1 and B 2. The proportion of variation explained by each trait and phylogeny was calculated from the posterior means of the random effects variance parameters (Gelman, 2005). We used uninformative (flat) inverse-Wishart priors. Posterior distributions were sampled with chains of 100,000 iterations after 15,000 iteration burn-in periods and a thinning rate of 100. Three independent chains were run for each model and convergence was checked using the Gelman–Rubin diagnostic test in the package ‘coda’ in R (Plummer and others 2006).
Model Fitting
Species occurrence and vegetation greenness models were fitted using integrated nested Laplace approximations (INLA) using the INLA package (Rue and others 2009). INLA uses deterministic approximations to posterior marginal distributions for Bayesian inference, which makes it an accurate and computationally efficient solution for large datasets (Rue and others 2009). We used the MCMCglmm package (Hadfield 2010) in R to fit the species trait models because this package allows for the incorporation of a phylogenetic tree and weighting of the response variable based on uncertainty. We considered a posterior probability of Pr (parameter > 0) > 0.9 as strong evidence that the parameter had a positive association to the response variable, and a posterior probability of Pr (parameter < 0) > 0.9 as strong evidence for a negative association (Kass and Raftery 1995).
Results
Vegetation Greenness
Floodplain vegetation had greater NDVI compared to non-floodplain vegetation (δ 1 = 2.25, SD = 0.39, Pr(\( \delta_{1} \) > 0) > 0.9) after accounting for the effects of aridity, which had a negative association with NDVI (δ 2 = −10.8, SD = 0.27, Pr(\( \delta_{2 } \) > 0) > 0.9).
Species Occurrence
For all but one species (yellow-throated miner Manorina flavigula), the associations between species occurrence and aridity were in the same direction for both river corridors, so we report on the mean trend across river corridors. Of the 130 species that we analysed, 58 (45%) showed strong evidence of lower occurrence with higher aridity in non-floodplain ecosystems (Pr (B 1 < 0) > 0.9, Figure 3, see Table S1 in Supporting Information), but the effect of aridity was moderated in floodplain ecosystems for 22 of these species (38%) (Pr (B 2 > 0) > 0.9, Figure 3A; Table 2): 12 species showed no association with aridity in floodplain ecosystems (Pr (B fr < 0) and Pr(B fr > 0) < 0.9) and, while still negative, the adverse effects of aridity on species occurrence were less in floodplain ecosystems compared to non-floodplain ecosystems for ten species (Figure 3A). Only nine species showed a more negative association with aridity in floodplain ecosystems than in non-floodplain ecosystems (Pr (B 2 < 0) > 0.9, Figure 3C), whereas the other 27 species showed no substantial difference in the negative association with aridity in floodplain ecosystems compared to non-floodplain ecosystems (Figure 3B). Twenty-nine species (22%) showed a positive association between occurrence and aridity (Pr (B 1 < 0) > 0.9) in non-floodplain ecosystems; the associations differed for just seven of these species in floodplain ecosystems: two had a more positive association with aridity in floodplain ecosystems and five species had a less positive association with aridity in floodplain ecosystems (see Table S1 in Supporting information). The occurrence of 43 species in non-floodplain ecosystems was not strongly related to aridity (see Table S1 in Supporting information).
Species associations with aridity in non-floodplain ecosystems (light grey) and floodplain ecosystems (dark grey) for species that had a negative association with aridity in non-floodplain ecosystems (Pr (B 1 < 0) > 0.9). Bars represent 80% credible intervals for posterior distributions, central line indicates mean. Species are arranged according to those with (A) strong evidence for a less negative association with aridity in floodplain ecosystems (Pr (B 2 > 0) > 0.9), these species are listed in Table 2 (B) no evidence for a difference in the association with aridity between ecosystems and (C) strong evidence for a more negative association with aridity in floodplain ecosystems (Pr (B 2 < 0) > 0.9). All species and mean effect sizes are listed in Table S1.
Species Traits
Habitat guild explained most of the variation in species response to aridity (53%), while other traits and phylogeny explained very little variation (<4% each; Figure 4A). Species that occupy forest and woodland habitats on average were more likely to have a negative association with aridity in non-floodplain ecosystems (Pr (effect > 0) > 0.9) (Figure 4B). Species traits explained little variation in whether floodplain ecosystems mediated the negative effect of aridity on species occurrence (Figure 4C); 6% of the variation in B 2 was attributed to nesting substrate (Figure 4C, D), whereas other traits and phylogeny each explained less than 2% of variation (Figure 4C).
Mean (central line) and 95% credible intervals for (A) variance components by species trait for species response to aridity index, with habitat guild explaining the most variation in species associations with aridity; (B) estimated effects of aridity index on each habitat guild (effects < 0 = negative association with aridity; (C) variance components by species trait for the extension of species distributions by floodplain ecosystems, with nesting guild explaining the highest amount of variance among the species traits; and (D) estimated effects of floodplains on species aridity association by nesting guild (positive effect indicates floodplain ecosystems facilitate species occurrence in more arid regions). Asterisks indicate effects with Pr > 0.9.
Discussion
The occurrence of 45% of terrestrial bird species in non-floodplain ecosystems along the Murray and Darling river corridors was negatively associated with aridity. Aridity can influence the occurrence of faunal species through two mechanisms: (1) abiotic conditions that impose direct physiological limitations on species occurrence, and (2) limitations on occurrence based on the availability of habitat resources provided by vegetation, which itself is influenced by aridity. Temperatures in arid landscapes fluctuate greatly, maximum temperatures are high, rainfall is sporadic and free water is limited (Davis and others 2013). As aridity increases, energy needs and evaporative water loss increase, which can result in selective pressure on biota related to basal metabolic rates (Tieleman and others 2003). Arid ecosystems are resource limited compared to more mesic ecosystems because low water availability limits primary production (Knapp and Smith 2001). Tree cover generally decreases in more arid regions (Schulze and others 1996; Scholes and others 2002). Among habitat guilds, the occurrences of species that prefer forest and woodland habitat were most negatively affected by aridity, suggesting that declines in tree cover in arid regions may play an important role in limiting species occurrence.
Floodplains moderated the negative effects of aridity on the occurrence of species in the Murray and Darling river corridors, with 38% of species that were negatively affected by aridity in non-floodplain ecosystems showing weaker or no decrease in occurrence with higher aridity in floodplain ecosystems. Conversely, there was little difference in the association between aridity and species occurrence in floodplain ecosystems for species whose occurrence was positively associated with aridity in non-floodplain ecosystems. Along the aridity gradient, floodplain sites had higher NDVI than other vegetation, indicating that floodplain forest and woodland is more productive than other vegetation (Wang and others 2004), probably due to higher water availability and greater soil fertility (Naumburg and others 2005; Ballinger and Lake 2006). These outcomes suggest that floodplain ecosystems act as a ‘green tongue’, allowing species to occupy drier regions than they would otherwise (Figure 1). Floodplain ecosystems moderated the effect of aridity across habitat, foraging, nesting and dietary guilds, which suggests that there may be greater amounts of a broad range of resources in floodplain forests and woodlands compared to other habitats in arid regions. Floodplain vegetation of the Murray–Darling Basin is dominated by species of Eucalyptus, which provide a higher abundance of food (for example, nectar and carbohydrate rich exudates) and nesting resources (for example, hollows) for birds compared to tree species that dominate non-floodplain in arid zones, such as Acacia spp. (Pavey and Nano 2009; Tischler and others 2013). Resource availability in floodplain ecosystems is likely to be more reliable during periods of low rainfall because floodplain vegetation has access to water through streams, floods or groundwater (Selwood and others 2015b).
Cooler and wetter microclimatic conditions in floodplain ecosystems may facilitate the occurrence of species in more arid regions than they could otherwise occur based on annual rainfall or temperature. We did not collect data on site microclimate conditions, and available climate data are at a more coarse resolution than our sampling units (Bureau of Meteorology (Australia) 2015c, 2015b). However, extensive canopy cover and proximity to water may moderate microclimate in floodplain ecosystems, resulting in lower daytime temperatures and higher relative humidity than in adjacent habitats (Brosofske and others 1997; Danehy and Kirpes 2000), especially in arid zones where non-floodplain habitat has relatively low canopy cover. Arid zones experience lower night-time temperatures compared with more mesic regions with higher canopy cover, which may place relatively greater energetic demands on birds during the night (Körtner and others 2001). Higher vegetation cover in floodplain ecosystems is likely to provide greater availability of micro-refuges for animals to shelter from hot daytime conditions and also warmer nighttime conditions. Relief from the pressure of climate conditions in floodplain ecosystems may allow some species to expand farther into arid zones. Access to water in arid zones can assist birds to ameliorate the effects of evaporative water loss (Fisher and others 1972), so that proximity to water in floodplain ecosystems may facilitate species occurrence in arid regions (McCluney and Sabo 2009).
Riparian and floodplain ecosystems provide an interconnected network of vegetation throughout landscapes and over regions (Fremier and others 2015). Despite extensive clearing for agriculture, the floodplain forests and woodlands of the Murray–Darling Basin remain some of the largest and more contiguous vegetation types in south-eastern Australia (McGinness and others 2010). The higher connectivity of floodplain vegetation compared to other vegetation types, particularly in much modified landscapes, may contribute to the broader distribution of species ranges in floodplain ecosystems along these river corridors. Changes in species composition with increasing loss of landscape cover are less apparent in riparian vegetation than in other vegetation types (Bennett and others 2014). It is possible that floodplain ecosystems facilitate the extension of species distributions into arid zones through the provision of more continuous corridors of vegetation for movement of individuals and therefore, meta-population persistence, which may otherwise be hindered in more fragmented non-floodplain vegetation. There is genetic evidence that this is the case for a woodland-dependent marsupial, the yellow-footed antechinus Antechinus flavipes (Lada and others 2008).
Our finding that floodplains facilitate the occurrence of species in more arid regions is consistent with greater distinctness of floodplain and riparian faunal assemblages from adjacent assemblages in arid regions than in wetter regions (Woinarski and others 2000; Sabo and others 2005). Previous work in our study region found that terrestrial bird assemblages are more differentiated between floodplain and adjacent habitats along the Murray River as mean annual rainfall decreases (Tzaros 2001). The extension of species distributions into arid regions along tongues of floodplain vegetation probably contributes to greater differences in local species composition between floodplain and other vegetation, and so, increases local and regional species richness (Sabo and others 2005).
Areas that provide relief from high temperatures, have greater water availability, and support more vegetation biomass will become increasingly important for species persistence as climate change increases drying in many parts of the world (Hartmann and others 2013; Reside and others 2014). Floodplain ecosystems provide drought refuges for fauna from which individuals can disperse when conditions moderate (Seabrook and others 2011; Selwood and others 2015b) and more stable species assemblages during drought than other vegetation types (Haslem and others 2015; Nimmo and others 2016). Here, we show that floodplain ecosystems moderate the effect of aridity on species occurrence, which further highlights the important role that these zones play in enabling species persistence in drying climates. Riparian zones enabled the persistence of mesic plant communities in past geological periods of climate drying (Meave and others 1991) and floodplain ecosystems have the potential to be important areas for species persistence and adaptation under human-induced climate change (Capon and others 2013).
The degree to which floodplain ecosystems differ from surrounding uplands depends on flood frequency and duration, flow intermittency and groundwater depth (Brand and others 2011). Human land-use and drying climate conditions may reduce the contrast in vegetation productivity between floodplain and non-floodplain ecosystems by affecting water availability. Multiple anthropogenic pressures have affected the floodplain ecosystems of the Murray–Darling river system (Mac Nally and others 2011). Reduced flood frequency and changed flooding regimes have led to extensive dieback of floodplain forests and woodlands, and the drying climate is exacerbating these effects (Horner and others 2009). Changes to groundwater depth and salinity have contributed to forest dieback (Cunningham and others 2011). Therefore, the ameliorative effect of floodplains on aridity is likely to be less than it was prior to regulation of the Murray–Darling river system. The ongoing pressures of human land-use and a drying climate may further dampen the capacity for floodplain ecosystems to carry bird species into more arid climate zones.
Floodplains are not immune to the effects of climate drying and terrestrial fauna in these ecosystems may have limited resistance to drought and recovery in wetter periods (Selwood and others 2015a). Many floodplain ecosystems have been much disturbed by human land use, which may further limit their resistance to climate change (Whitford and others 1999). Floodplain ecosystems around the world have experienced reduced stream-flow and inundation due to water extraction for human land use, high levels of urban and agricultural development (Tockner and Stanford 2002), disruptive land uses such as grazing, timber extraction and secondary impacts such as salinity and alien plant invasions (Richardson and others 2007; Mac Nally and others 2011). The maintenance of intact floodplain vegetation and ecological restoration of modified floodplain ecosystems will maximize the value of these areas for terrestrial fauna in arid zones, particularly as climate change intensifies (Seavy and others 2009). We have shown that floodplain ecosystems are important for contributing to regional terrestrial bird assemblages. Management of floodplain ecosystems, including environmental water allocations, should include the setting and monitoring of goals for terrestrial biota alongside aquatic and water-dependent biota, which are usually the focus for management in such ecosystems.
REFERENCES
Atlas of Living Australia and Bureau of Rural Sciences. 2015. Normalised difference vegetation index (NDVI*100). Australia: Atlas of Living Australia.
Ballinger A, Lake PS. 2006. Energy and nutrient fluxes from rivers and streams into terrestrial food webs. Mar Freshw Res 57:15–28.
Barrett G, Silcocks A, Barry S, Cunningham R, Poulter R. 2003. The new atlas of Australian birds. Hawthorn East, (VCT): Birds Australia (Royal Australasian Ornithologists Union).
Bennett AF, Nimmo DG, Radford JQ. 2014. Riparian vegetation has disproportionate benefits for landscape-scale conservation of woodland birds in highly modified environments. J Appl Ecol 51:514–23.
Bivand R, Lewin-Koh N. 2013. maptools: Tools for reading and handling spatial objects. R package version 0.8-23.
Brand LA, Stromberg JC, Goodrich DC, Dixon MD, Lansey K, Kang D, Brookshire DS, Cerasale DJ. 2011. Projecting avian response to linked changes in groundwater and riparian floodplain vegetation along a dryland river: a scenario analysis. Ecohydrology 4:130–42.
Breckwoldt R, Boden R, Andrew J. 2004. The Darling. Canberra: Murray–Darling Basin Commission.
Brosofske KD, Chen J, Naiman RJ, Franklin JF. 1997. Harvesting effects on microclimatic gradients from small streams to uplands in western Washington. Ecol Appl 7:1188–200.
Bureau of Meteorology (Australia). 2015a. Climate Data Online. Australia: Bureau of Meteorology.
Bureau of Meteorology (Australia). 2015b. Gridded daily rainfall metadata. Australian Government.
Bureau of Meteorology (Australia). 2015c. Gridded daily temperature metadata. Australian Government.
Capon SJ, Chambers LE, Mac Nally R, Naiman RJ, Davies P, Marshall N, Pittock J, Reid M, Capon T, Douglas M, Catford J, Baldwin DS, Stewardson M, Roberts J, Parsons M, Williams S. 2013. Riparian ecosystems in the 21st Century: hotspots for climate change adaptation? Ecosystems 16:359–81.
Christidis L, Boles W. 2008. Systematics and taxonomy of Australian birds. Canberra: CSIRO Publishing.
Cunningham SC, Thomson JR, Mac Nally R, Read J, Baker PJ. 2011. Groundwater change forecasts widespread forest dieback across an extensive floodplain system. Freshw Biol 56:1494–508.
Cunningham SC, White M, Griffioen P, Newell G, MacNally R. 2013. Mapping floodplain vegetation types across the Murray–Darling Basin using remote sensing. Canberra: Murray–Darling Basin Authority.
Danehy RJ, Kirpes BJ. 2000. Relative humidity gradients across riparian areas in eastern Oregon and Washington forests. Northwest Sci 74:224–33.
Davis J, Pavlova A, Thompson R, Sunnucks P. 2013. Evolutionary refugia and ecological refuges: key concepts for conserving Australian arid zone freshwater biodiversity under climate change. Glob Change Biol 19:1970–84.
Death RG, Collier KJ. 2010. Measuring stream macro invertebrate responses to gradients of vegetation cover: when is enough enough? Freshw Biol 55:1447–64.
Department of the Environment. 2012. Australia—Present Major Vegetation Groups—NVIS Version 4.1 (Albers 100m analysis product). Canberra: Australian Government
Department of the Environment. 2014. Natural areas of Australia—100 metre. Canberra: Australian Government.
Dunning JBJ. 2007. CRC Handbook of Avian Body Masses, 2nd edn. Florida: CRC Press.
Environment Australia. 2000. Revision of the interim biogeographic regionalisation for Australia (IBRA) and development of version 5.1. Canberra: Department of Environment and Heritage.
Ezcurra E. 2006. Global Deserts outlook. Nairobi, Kenya: United Nations Environment Programme.
Fisher CD, Lindgren E, Dawson WR. 1972. Drinking patterns and behavior of Australian desert birds in relation to their ecology and abundance. The Condor 74:111–36.
Fremier AK, Kiparsky M, Gmur S, Aycrigg J, Craig RK, Svancara LK, Goble DD, Cosens B, Davis FW, Scott JM. 2015. A riparian conservation network for ecological resilience. Biol Conserv 191:29–37.
Gelman A. 2005. Analysis of variance—why it is more important than ever. Ann Stat 33:1–53.
Giling DP, Grace MR, Thomson JR, Mac Nally R, Thompson RM. 2014. Effect of native vegetation loss on stream ecosystem processes: dissolved organic matter composition and export in agricultural landscapes. Ecosystems 17:82–95.
Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22.
Hartmann DL, Tank AMGK, Rusticucci M, Alexander LV, Brönnimann S, Charabi Y, Dentener FJ, Dlugokencky EJ, Easterling , Kaplan A, Soden BJ, Thorne PW, Wild M, Zhai PM, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM. 2013. Observations: atmosphere and surface. In: Stocker TF, Qin D, Eds. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. New York (NY): Cambridge University Press.
Haslem A, Nimmo DG, Radford JQ, Bennett AF. 2015. Landscape properties mediate the homogenization of bird assemblages during climatic extremes. Ecology 96:3165–74.
Higgins PJ. 1999. Parrots to dollarbird. In: Handbook of Australian, New Zealand and Antarctic birds (Vol. 4). Melbourne: Oxford University Press.
Higgins PJ, Davies SJJF. 1996. Snipe to pigeons. In: Handbook of Australian, New Zealand and Antarctic birds (Vol. 3). Melbourne: Oxford University Press.
Higgins PJ, Peter JM. 2002. Pardalotes to shrike-thrushes. In: Handbook of Australian, New Zealand and Antarctic birds (Vol. 6). Melbourne: Oxford University Press.
Higgins PJ, Peter JM, Cowling SJ. 2006. Boatbill to starlings. In: Handbook of Australian, New Zealand and Antarctic birds (Vol. 7). Melbourne: Oxford University Press.
Higgins PJ, Peter JM, Steele WK. 2001. Tyrant-flycatchers to chats. In: Handbook of Australian, New Zealand and Antarctic birds (Vol. 5). Melbourne: Oxford University Press.
Hijmans RJ, van Etten J. 2013. raster: Geographic data analysis and modeling. R package version 2.1-16.
Horner GJ, Baker PJ, Mac Nally R, Cunningham SC, Thomson JR, Hamilton F. 2009. Mortality of developing floodplain forests subjected to a drying climate and water extraction. Glob Change Biol 15:2176–86.
Junk WJ, Bayley PB, Sparks RE. 1989. The flood pulse concept in river-floodplain systems. Can Spec Publ Fish Aquat Sci 106:110–27.
Kass RE, Raftery AE. 1995. Bayes factors. J Am Stat Assoc 90:773–95.
Knapp AK, Smith MD. 2001. Variation among biomes in temporal dynamics of aboveground primary production. Science 291:481–4.
Körtner G, Brigham RM, Geiser F. 2001. Torpor in free-ranging tawny frogmouths (Podargus strigoides). Physiol Biochem Zool 74:789–97.
Lada H, Mac Nally R, Taylor AC. 2008. Distinguishing past from present gene flow along and across a river: the case of the carnivorous marsupial (Antechinus flavipes) on southern Australian floodplains. Conserv Genet 9:569–80.
Lislevand T, Figuerola J, Székely T. 2007. Avian body sizes in relation to fecundity, mating system, display behavior, and resource sharing. Ecology 88(6):1605.
MacNally R, Cunningham SC, Baker PJ, Horner GJ, Thomson JR. 2011. Dynamics of Murray–Darling floodplain forests under multiple stressors: The past, present, and future of an Australian icon. Water Resour Res. doi:10.1029/2011WR010383.
Mac Nally R, Soderquist TR, Tzaros C. 2000. The conservation value of mesic gullies in dry forest landscapes: avian assemblages in the box-ironbark ecosystem of southern Australia. Biol Conserv 92:293–302.
Marchant S, Higgins PJ. 1990. Ratites to ducks. In: Handbook of Australian, New Zealand and Antarctic birds (Vol. 1). Melbourne: Oxford University Press.
McCluney KE, Sabo JL. 2009. Water availability directly determines per capita consumption at two trophic levels. Ecology 90:1463–9.
McGinness HM, Arthur AD, Reid JRW. 2010. Woodland bird declines in the Murray–Darling Basin: are there links with floodplain change? Rangel J 32:315–27.
Meave J, Kellman M, MacDougall A, Rosales J. 1991. Riparian habitats as tropical forest refugia. Glob Ecol Biogeogr Lett 1:69–76.
Museth J, Johnsen SI, Walseng B, Hanssen O, Erikstad L. 2011. Managing biodiversity of floodplains in relation to climate change. Int J Clim Change Strateg Manag 3:402–15.
Naiman RJ, Decamps H, Pollock M. 1993. The role of riparian corridors in maintaining regional biodiversity. Ecol Appl 3:209–12.
Naumburg E, Mata-Gonzalez R, Hunter RG, Mclendon T, Martin DW. 2005. Phreatophytic vegetation and groundwater fluctuations: a review of current research and application of ecosystem response modeling with an emphasis on Great Basin vegetation. Environ Manag 35:726–40.
Nimmo DG, Haslem A, Radford JQ, Hall M, Bennett AF. 2016. Riparian tree cover enhances the resistance and stability of woodland bird communities during an extreme climatic event. J Appl Ecol 53:1365–2664.
Pavey CR, Nano CEM. 2009. Bird assemblages of arid Australia: Vegetation patterns have a greater effect than disturbance and resource pulses. J Arid Environ 73:634–42.
Plummer M, Best N, Cowles K, Vines K. 2006. CODA: Convergence diagnosis and output analysis for MCMC. R news 6:7–11.
QGIS Development Team. 2013. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Project OSGF editor.
R Core Team. 2015. R: a language and environment for statistical computing. Computing RFfS editor. Vienna, Austria: http://www.R-project.org/.
Reside AE, Welbergen JA, Phillips BL, Wardell-Johnson GW, Keppel G, Ferrier S, Williams SE, Vanderwal J. 2014. Characteristics of climate change refugia for Australian biodiversity. Austral Ecol 39:887–97.
Richardson DM, Holmes PM, Esler KJ, Galatowitsch SM, Stromberg JC, Kirkman SP, Pyšek P, Hobbs RJ. 2007. Riparian vegetation: degradation, alien plant invasions, and restoration prospects. Divers Distrib 13:126–39.
Roberts J, Marston F. 2011. Water regime for wetland and floodplain plants: a source book for the Murray–Darling Basin. Canberra: National Water Commission.
Rue H, Martino S, Chopin N. 2009. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc 71:319–92.
Sabo JL, Sponseller R, Dixon M, Gade K, Harms T, Heffernan J, Jani A, Katz G, Soykan C, Watts J, Welter J. 2005. Riparian zones increase regional species richness by harboring different, not more, species. Ecology 86:56–62.
Scholes RJ, Dowty PR, Caylor K, Parsons DAB, Frost PGH, Shugart HH. 2002. Trends in savanna structure and composition along an aridity gradient in the Kalahari. J Veg Sci 13:419–28.
Schulze E-D, Mooney H, Sala O, Jobbagy E, Buchmann N, Bauer G, Canadell J, Jackson R, Loreti J, Oesterheld M. 1996. Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia 108:503–11.
Seabrook L, McAlpine C, Baxter G, Rhodes J, Bradley A, Lunney D. 2011. Drought-driven change in wildlife distribution and numbers: a case study of koalas in south west Queensland. Wildl Res 38:509.
Seavy NE, Gardali T, Golet GH, Griggs FT, Howell CA, Kelsey R, Small SL, Viers JH, Weigand JF. 2009. Why climate change makes riparian restoration more important than ever: recommendations for practice and research. Ecol Restor 27:330–8.
Selwood KE, Clarke RH, Cunningham SC, Lada H, McGeoch MA, Mac Nally R. 2015a. A bust but no boom: Responses of floodplain bird assemblages during and after prolonged drought. J Anim Ecol 84:1700–10.
Selwood KE, Thomson JR, Clarke RH, McGeoch MA, Mac Nally R. 2015b. Resistance and resilience of terrestrial birds in drying climates: do floodplains provide drought refugia? Glob Ecol Biogeogr 24:838–48.
Smith JE. 2015. Effects of environmental variation on the composition and dynamics of an arid-adapted Australian bird community. Pac Conser Biol 21:74–86.
Stromberg JC, Lite SJ, Rychener TJ, Levick LR, Dixon MD, Watts JM. 2006. Status of the riparian ecosystem in the upper San Pedro River, Arizona: application of an assessment model. Environ Monit Assess 115:145–73.
Tieleman BI, WIlliams JB, Bloomer P. 2003. Adaptation of metabolism and evaporative water loss along an aridity gradient. Proc R Soc Lond Series B 270:207–14.
Tischler M, Dickman CR, Wardle GM. 2013. Avian functional group responses to rainfall across four vegetation types in the Simpson Desert, central Australia. Austral Ecol 38:809–19.
Tockner K, Stanford JA. 2002. Riverine flood plains: present state and future trends. Environ Conserv 29:308–30.
Tzaros CL. 2001. Importance of riparian vegetation to terrestrial avifauna along the Murray River, south-eastern Australia (MSc Thesis). School of Ecology and Environment. Victoria: Deakin University.
United Nations Environment Program. 1997. World atlas of desertification. London: UNEP.
Wang J, Rich PM, Price KP, Kettle WD. 2004. Relations between NDVI and tree productivity in the central Great Plains. Int J Remote Sens 25:3127–38.
Whitford W, Rapport D, deSoyza A. 1999. Using resistance and resilience measurements for ‘fitness’ tests in ecosystem health. J Environ Manag 57:21–9.
Woinarski JCZ, Brock C, Armstrong M, Hempel C, Cheal D, Brennan K. 2000. Bird distribution in riparian vegetation in the extensive natural landscape of Australia’s tropical savanna: a broad-scale survey and analysis of a distributional data base. J Biogeogr 27:843–68.
ACKNOWLEDGEMENTS
We thank the late Shaun Cunningham for many useful discussions and for providing the floodplain vegetation spatial data. Hania Lada and the Arthur Rylah Institute compiled the species trait information. We thank Jian D. L. Yen and James R. Thomson for statistical advice. H.A. Ford, J.D.L. Yen, the Clarke laboratory and two anonymous reviewers provided valuable feedback. K.E.S acknowledges the support of the Holsworth Trust Wildlife Research Endowment and BirdLife Australia’s Stuart Leslie Bird Research Award. R.M. acknowledges the support of the Australian Research Council (grant LP120200217). We thank the many BirdLife Australia Atlasers whose contributions made this work possible.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
KS and RM conceived the study, KS analysed the data, KS wrote the paper with contributions from RM, RHC and MM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10021_2016_59_MOESM1_ESM.docx
Supplementary material 1 (DOCX 118 kb) Appendix S1 Parameter estimates for the effect of aridity on species occurrence in non-floodplain and floodplain vegetation
Rights and permissions
About this article
Cite this article
Selwood, K.E., Clarke, R.H., McGeoch, M.A. et al. Green Tongues into the Arid Zone: River Floodplains Extend the Distribution of Terrestrial Bird Species. Ecosystems 20, 745–756 (2017). https://doi.org/10.1007/s10021-016-0059-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-016-0059-y