Abstract
Ecosystems are generally linked via fluxes of nutrients and energy across their boundaries. For example, freshwater ecosystems in temperate regions may receive significant inputs of terrestrially derived carbon via autumnal leaf litter. This terrestrial particulate organic carbon (POC) is hypothesized to subsidize animal production in lakes, but direct evidence is still lacking. We divided two small eutrophic lakes each into two sections and added isotopically distinct maize litter to the treatment sections to simulate increased terrestrial POC inputs via leaf litter in autumn. We quantified the reliance of aquatic consumers on terrestrial resources (allochthony) in the year subsequent to POC additions by applying mixing models of stable isotopes. We also estimated lake-wide carbon (C) balances to calculate the C flow to the production of the major aquatic consumer groups: benthic macroinvertebrates, crustacean zooplankton, and fish. The sum of secondary production of crustaceans and benthic macroinvertebrates supported by terrestrial POC was higher in the treatment sections of both lakes. In contrast, total secondary and tertiary production (supported by both autochthonous and allochthonous C) was higher in the reference than in the treatment sections of both lakes. Average aquatic consumer allochthony per lake section was 27–40%, although terrestrial POC contributed less than about 10% to total organic C supply to the lakes. The production of aquatic consumers incorporated less than 5% of the total organic C supply in both lakes, indicating a low ecological efficiency. We suggest that the consumption of terrestrial POC by aquatic consumers facilitates a strong coupling with the terrestrial environment. However, the high autochthonous production and the large pool of autochthonous detritus in these nutrient-rich lakes make terrestrial POC quantitatively unimportant for the C flows within food webs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adjacent ecosystems are connected by reciprocal flows of resources such as energy and nutrients. A subsidy is defined as a donor-controlled resource that increases productivity of organisms in the recipient ecosystem (Polis and others 1997). The effect of subsidies on the recipient ecosystem has been well studied in streams, islands, and riparian forests (Marczak and others 2007; Masese and others 2015). A few recent studies suggest that lake ecosystems are subsidized by several forms of terrestrial organic carbon (C) (Carpenter and others 2005; Cole and others 2006), which are transferred by gravity, run-off, and wind to lakes (Polis and others 1997 and references therein). Living prey organisms originating from terrestrial ecosystems may subsidize fish in lakes (Mehner and others 2005) and may also support a substantial fraction of aquatic predator production in streams (Nakano and Murakami 2001). Terrestrial dissolved organic carbon (DOC) is another significant subsidy to lakes, but it is primarily respired by pelagic bacteria, and hence only a minor part of this allochthonous C is transferred to higher trophic levels (Kritzberg and others 2004; Berggren and others 2010; Jones and others 2012). Detrital particulate organic carbon (POC) from terrestrial ecosystems is the third form of C subsidy; its effect on lake ecosystems has rarely been studied directly (but see Bartels and others 2012), although it has been hypothesized to be an important energy source to lake animals (Carpenter and others 2005; Cole and others 2006; Babler and others 2011). Autumnal leaf litter from surrounding trees dominates the terrestrial POC source for many lakes of the temperate zone (Vander Zanden and Gratton 2011), but its effect on aquatic ecosystems has been studied experimentally only in streams (Abelho 2001). No study has yet been conducted that directly provides evidence for a stimulation of production in lake animal populations by terrestrial leaf litter POC and relates this subsidy to in-lake C supply. It is important to understand the effects of these potential subsidies, because land use change, which is a major driver of global change, can increase the exports of organic C from terrestrial ecosystems with potentially far-reaching consequences for C cycles in lakes (Larsen and others 2011).
The effect strength of subsidies on a recipient ecosystem depends on several features. According to a recent review (Marczak and others 2007), the trophic group of recipient consumers and the ratio of subsidy resources to trophically equivalent autochthonous resources (those produced within the system) are significant predictors of the subsidy effect size. Among the trophic groups, subsidies affected detritivores more than omnivores, predators or insectivores (Marczak and others 2007). Furthermore, the strongest effects are found if the amount of subsidies exceeds the amount of equivalent autochthonous resources (Marczak and others 2007). Accordingly, the quantitative effect of terrestrial POC subsidies on lake food webs is difficult to predict. Leaf litter is supplied to the littoral and profundal habitats, suggesting a strong potential stimulation of benthic macroinvertebrate production (Cole and others 2006). However, leaf litter becomes part of the large lake-wide detritus pool, consisting of remains from both terrestrial and aquatic primary production that have accumulated over longer periods (Moore and others 2004; Attermeyer and others 2013). In productive lakes with high in-lake primary production, the effect size of leaf subsidies may therefore be low because detritus is dominated by autochthonous resources.
We conducted ecosystem-scale experiments in two productive lakes to test whether terrestrial POC additions increase aquatic consumer allochthony and subsidize their production. We divided both lakes into halves and added isotopically distinct maize (Zea mays L.) leaves to one section of each lake. Furthermore, we merged two independent approaches, stable isotopes, and C budget models, to quantify the flows of terrestrial C versus autochthonous C fixed by primary production within the lake to animal consumer production, as has been suggested recently (Marcarelli and others 2011). This explicitly quantitative approach extends other studies on these lakes, which have demonstrated qualitatively that even a minor incorporation of terrestrial POC (maize) into consumer tissues could be directly traced (Scharnweber and others 2014a, b). The aim of this quantitative approach is to evaluate whether the estimates of terrestrial carbon contributions to consumer tissue from mixing models correspond to the allochthonous carbon contributions to the lake-wide carbon budgets. We hypothesized a fairly close correspondence between consumer allochthony and terrestrial POC availability in the carbon budgets for benthic macroinvertebrates, but a lower allochthony relative to carbon availability in crustacean zooplankton (Marcarelli and others 2011). Our study is the first to experimentally increase terrestrial POC inputs into lakes at natural spatial and temporal scales, hence simulating an enhanced leaf litter fall to two replicate ecosystems. We further hypothesized that the enhanced supply of terrestrial, allochthonous POC (Callo) in the treatment sections of both lakes stimulates the production of benthic macroinvertebrates and crustacean zooplankton, and indirectly also enhances the production of secondary consumers, such as fish. Finally, we hypothesized that the effect size of the artificial subsidy would be low because these productive lakes are characterized by a relatively high supply of autochthonous, algal-derived organic C (Cauto); consequently, Callo should contribute only a minor proportion to total C budgets of both lakes.
Methods
General Overview
Our experiments were conducted in two small temperate shallow lakes of similar size, shape, and depth in northeastern Germany. Both lakes are hydrologically isolated and have no surface inlet. Kleiner Gollinsee (hereafter Gollinsee) is turbid and dominated by phytoplankton, whereas 22% of the area of the clearer Schulzensee is covered by submerged macrophytes (Ceratophyllum submersum L.) in summer. Both lakes are surrounded by alder trees (Alnus glutinosa (L.) Gaert.) and reed stands (Phragmites australis (Cav.) Trin. ex Steud.). Floating-leaved macrophytes (Nymphaea alba L. and Nuphar lutea (L.)) grow in both lakes and cover 3% of the area of Gollinsee and 12% of Schulzensee. Both lakes are eutrophic (ambient nutrient concentrations 35–40 µg total phosphorus L−1). In October 2010, we divided both lakes with plastic curtains sealed in the sediment, fully isolating the water volume of each section. In November 2010, we added roughly 2 t of coarsely shredded fresh maize (Zea mays L.) leaves and stems (without cobs) into one section of Gollinsee (25 g C m−2) and 3 t into one section of Schulzensee (28 g C m−2). The leaves were added into the littoral zone and prevented from floating into the pelagic zone by a rope installed at the pelagic-littoral boundary. The lake sections with experimental leaf additions are here referred to as treatment sections, as opposed to non-treated reference sections. The quantity of maize C added (per area) was about 4× higher than estimated inputs of terrestrial C by alder leaves, which occurred at the same time as the addition of maize leaves. More details on the experiments and the tracing of maize addition in food webs are published elsewhere (Attermeyer and others 2013; Scharnweber and others 2014a, 2014b).
Our overall intention was to quantify the effect of Callo (as terrestrial POC) on the secondary production of aquatic animals, compared to the contribution of Callo to lake-wide organic C balances. For C balances, we measured the annual autochthonous net primary production (Cauto) of phytoplankton, periphyton, submerged and emergent macrophytes (Brothers and others 2013a), and compared this to Callo inputs of dissolved organic carbon (DOC) and POC (native alder leaves and experimentally added maize leaves). All estimates were expressed as annual rates (g C m−2 y−1).
We estimated the total secondary production of crustaceans and benthic macroinvertebrates, as well as the tertiary production of fish and predatory odonates. We used stable isotope mixing models to estimate the sum of proportions of Callo sources (maize, alder) in animal tissues (consumer allochthony), and calculated the share of Callo-derived production relative to total consumer production. Consumer production supported by Callo was also divided by the lake-wide inputs of Callo, which we refer to as allochthonous ecological efficiency. Next, we divided total consumer production by the total organic C inputs to the lakes (Cauto fixation, DOCallo and POCallo inputs) to estimate the total ecological efficiency of each system. Both calculations are similar to net ecological efficiencies (Vander Zanden and others 2006) and hence reflect only the C ultimately incorporated into consumer tissues (that is, after respiratory losses). These ecological efficiencies are related to partial flow food webs (Marcarelli and others 2011) because they distinguish between Callo and Cauto contributions to the consumer-resource interaction.
Our calculations exclude secondary production by bacteria, non-crustacean zooplankton (for example, heterotrophic flagellates, ciliates, rotifers) and benthic meiofauna because we could not obtain reliable estimates of allochthony for these groups. Furthermore, we present all results as raw data, and refrain from using inferential statistics for differences between the two lakes and between sections of the lakes. Most of the annual rates we present are based on temporally or spatially replicated samplings, which are therefore not statistically independent. However, we argue that obtaining detailed ecological knowledge about entire lake ecosystems is a valid approach even in the absence of strict statistical tests (Carpenter 1989).
Sampling and Estimates of Consumer Production
Crustacean Zooplankton
Crustacean zooplankton were sampled at pelagic sites of both lakes monthly from March 2011 to February 2012 by filtering an epilimnetic mixed water sample (40 L) through a 55-µm mesh and fixing the content with 4% sugar formaldehyde (Haney and Hall 1973). Separate samples were taken in littoral and pelagic sites of both lakes. Samples were counted and identified to the genus or species level, and length was measured at the LimSa Gewässerbüro (Konstanz, Germany). Regressions were used to calculate the individual C content based on organism size (Dumont and others 1975), and calculated biomasses (mg C m−3). A carbon content of 50% dry weight was assumed (Gaedke 1992 and included references). Crustacean zooplankton secondary production was calculated based on daily production to biomass (P:B) ratios. These P:B ratios were calculated according to the individual size (w, µg dry weight) by using a linear regression model:
with α and β being taxon-specific parameters for temperatures above and below 10°C (Stockwell and Johannson 1997). Annual production was calculated by summing daily production rates over the number of days of the respective month, and summing up monthly values to the entire year (March 2011 to February 2012). Annual production per lake section was calculated as the arithmetic average of littoral and pelagic production because of comparable areas of littoral and pelagic habitats.
Benthic Macroinvertebrates
Biomass (B, in g DM m−2) of benthic macroinvertebrates was estimated from eight samplings from April to November 2011 (for details see Brothers and others 2013b). Briefly, samples were collected along one transect across each lake section, which included two eulittoral (0–1 m depth), two sublittoral (1–2 m depth), and two profundal (>2 m depth) samples. For each sample, sediment from an area of 0.6 m2 was collected and stored in ethanol. Species were determined to the lowest possible taxonomic unit (mostly genus) and wet mass was measured, which was converted to dry mass using site-specific conversion factors derived from the samples. The annual production (P, g DM m−2 y−1) of macroinvertebrates was estimated separately for eulittoral, sublittoral and profundal habitats by using the allometry-based approach of Plante and Downing (1989):
where B is the mean annual biomass averaged across the eight sampling months and M max is the maximum individual mass (mg DM individual−1) for each habitat on each lake section (estimated from the April, June, and September samplings during which individuals were measured). To avoid single-individual estimates of M max, we used the average length of the largest 10% of individuals. T mean is the annual water temperature (13.6 and 14.3°C in Gollinsee and Schulzensee, respectively; measured using a stationary weather station at each lake). DM was converted to C by multiplication by 0.45 (Wetzel 2001). Lake-wide production rates were calculated as weighted averages of production rates from the three benthic habitats according to relative contributions of these habitats to the surface area of each lake section.
Fish
Abundances of the dominant fish species, roach (Rutilus rutilus L.), which represents 71 and 72% of fish abundance in Gollinsee and Schulzensee, respectively, were derived from a mark-recapture approach as described in Brothers and others (2013b). Briefly, fish were caught using an electrofishing device during five consecutive days and tagged by coded wire tags (Northwest Marine Technology, Inc., WA, USA) that were inserted into the snout region of the fish. Recaptured fish were checked for tags and population abundances were estimated by using a Schnabel multiple-census approach, adjusted by Chapman (Ricker 1975). Growth estimates for roach in 2011 were based on scale analyses. Roach were caught in September 2011 and the mean distances between the nucleus and the annuli, from three scales per fish (total of 207 individuals), were determined, which allowed back-calculating length-at-age (Fraser 1916; Lee 1920). The sum of weight increments of the different cohorts, and therefore tertiary production in 2011, was estimated using lake-specific length-mass regressions for roach. Tertiary production of the whole fish community was extrapolated according to the proportion of roach relative to the total fish biomass estimated from standardized annual fishing campaigns using four Nordic multi-mesh gillnets per lake section (set perpendicular to the shoreline, from dusk till dawn) and electrofishing (applying 15 dips for 15 seconds at each randomly chosen location) (for details see Brothers and others 2013b). These calculations underestimated young-of-the-year (YOY) fish production because we do not have reliable density estimates of YOY fish in spring and summer.
Stable Isotope Analyses and Estimation of Consumer Allochthony
In 2011, a total of 611 samples for stable isotope analysis of all primary resources (alder, maize, periphyton, seston, macrophytes, reed) and animal consumers (crustaceans, macroinvertebrates, fish) were taken from treatment and reference sections in both lakes in spring (April), summer (June), and autumn (September) (Appendix Table A1; Figure A1). Additional samples from macrophytes were collected in summer 2010. Periphyton samples were obtained by scraping the dividing curtains, reed stems, and artificial biofilm strips. Seston is usually a heterogeneous mixture of living and dead phytoplankton and other microorganisms, but may also contain large proportions of terrestrial particulate organic matter (Wilkinson and others 2013). Therefore, we corrected the seston δ13C values by a two end-member mixing model (Bade and others 2006; Taipale and others 2007) to obtain more reliable C isotope values for phytoplankton (for details see Scharnweber and others 2014a). This method resulted in seasonal mean phytoplankton δ13C values below −40‰ which, although low, are not uncommon for lakes with high respiration rates and considerable fraction of respired C in the inorganic C pool (Bade and others 2004; Karlsson and others 2008; Taipale and others 2008). By this method, we also obtained a δ13C value for the detrital POC pool within the seston. We assumed that the composition of this POC pool reflected the relative contributions of Callo (alder, maize) and Cauto (macrophytes, reed) as estimated from the lake-wide C budgets (see below).
We used stable isotope mixing models as implemented in the MixSIAR package (Stock and Semmens 2013) in R 3.01 (R Development Core Team 2012) to calculate the proportion of different food resources in the diets of consumers. Prior to mixing model analysis, we tested whether preconditions of the model, that is a valid mixing geometry and distinct resource isotope values were fulfilled. We visually inspected stable isotope biplots for resource isotopic distinctness (Appendix Figure A1). Periphyton and reeds were similar in isotope signatures; thus, we decided to pool these two resources and allocated them to Cauto.
The allochthony of crustaceans was estimated using a mixing model with only phytoplankton and the mix of detrital POC from seston as the two potential resources. We used phytoplankton, periphyton+reed, macrophytes, alder, and maize as potential resources in mixing models of benthic macroinvertebrates. For omnivorous fish (roach, rudd Scardinius erythrophthalmus L., tench Tinca tinca L., perch Perca fluviatilis L.), we used benthic macroinvertebrates and crustaceans, and included Isopoda (Asellus aquaticus L.) as a separate resource due to their distinct isotope signature. For secondary consumers (omnivorous fish and Odonata), we estimated the indirect contribution of primary resources via the consumption of benthic prey and crustaceans using ratio calculations (Brauns and others 2011). Mixing modeling was conducted using fractionation factors of 0.4 ± 1.3 for δ13C and 3.4 ± 1.0 for δ15N (Post 2002), concentration dependence (Phillips and Koch 2002), and the MixSIAR model option without a residual error term (Parnell and others 2013). Convergence of each mixing model was verified using the plots and diagnostic test provided by the software. We calculated a global standard deviation (SD) for each estimated proportion, as based on the sum of variances of the estimates for single resources.
Lake-Wide Organic C Budgets
All organic Callo inputs were calculated for a period spanning March 2010 until February 2011, incorporating experimental maize additions, annual contributions of leaf fall from surrounding alder trees, and the net flux of groundwater DOC. Because of logistic constraints, we could not repeat these estimates in 2011, but assume that there was little difference in alder leaf litter fall and t-DOC input between 2010 and 1011. The supply of organic Callo was calculated following methods outlined by Brothers and others (2013b). Briefly, the C input from alder leaves was measured directly using floating leaf traps installed at multiple locations around the periphery of each lake. The net DOC input to each lake was calculated as the difference between input and output of groundwater DOC to each lake using measurements made every two to four weeks from sampling wells constructed in the immediate vicinity of each lake (Rudnick and others 2015).
All Cauto fixation rates were calculated for a period spanning April 2011 to March 2012. Following Scharnweber and others (2014a), organic Cauto was considered to be the sum of organic C production within the lake area, which included littoral reeds growing at or immediately beyond the lakeshore, along with phytoplankton and periphyton, and aquatic macrophytes. Gross primary production (GPP) was calculated separately for the pelagic and littoral zones for each lake section, following methods outlined by Brothers and others (2013a, b).
Briefly, phytoplankton and periphyton GPP were calculated using photosynthesis-irradiance (PI) curves. The GPP of C. submersum was calculated from maximum biomass and C content measurements made in July 2010 and the GPP of Aphanothece stagnina from in situ glass bottle experiments and core exposures. To estimate net primary production (NPP) which is truly available to secondary producers, producer-specific GPP was multiplied by correction factors (phytoplankton: 0.6, epipelon: 0.23, epiphyton: 0.55, Aphanothece: 0.6, Ceratophyllum: 0.4) (see Brothers and others 2013a). The annual organic C load of floating-leaved and emergent macrophytes into the lakes was considered to be equivalent to the maximum biomass measured in July 2010.
Results
Annual autochthonous net primary production was slightly higher in the treatment sections of Schulzensee (312 g C m−2 y−1) and Gollinsee (348 g C m−2 y−1) than in the respective reference sections of both lakes (Schulzensee 263 g C m−2 y−1, Gollinsee 337 g C m−2 y−1). Based on lake-wide C balances, the input of Callo from maize and alder POC plus DOC via groundwater to total in-lake C supply summed up to 8.8% (treatment) and 2.4% (reference) in Gollinsee, and 11.3% (treatment) and 4.3% (reference) in Schulzensee (Figure 1). In other words, in-lake C fixation by algae and vascular plants accounted for at least about 90% of total organic C inputs to consumers, even in sections receiving maize inputs.
Lake wide organic C supply (g C m−2 y−1) including net primary production by phytoplankton, periphyton (Epiphyton, Epipelon, Aphanothece), Ceratophyllum macrophytes and reed (all autochthonous, in gray) and allochthonous C sources (in white) from alder leaves and maize leaves (treatment sections only) as well as net (groundwater inflow minus outflow) t-DOC balances in Gollinsee and Schulzensee.
Average consumer allochthony as calculated by mixing models of the eight consumer groups varied between 1 and 60% across all groups and the four lake sections (Figure 2). However, standard deviations of allochthony estimates were broad and included zero for crustaceans and odonates (Figure 2). The unweighted grand mean of consumer allochthony across all eight groups was slightly higher in the treatment section of Gollinsee (40%) than in the reference section of this lake (35%), in particular caused by a higher allochthony of chironomids and gastropods. In contrast, average allochthony was identical in both sections of Schulzensee (27%). On average across all consumer groups, Callo incorporation into consumer tissues was dominated by alder (22–35%), whereas the average contribution from maize in the treatment sections was low (6% in Gollinsee, 5% in Schulzensee) (Figure 2). Accordingly, there was a disproportionately high use of Callo by consumers, compared to the low percentage of Callo of total C available in all four lake sections (Figure 3A). However, this imbalance was caused only by the disproportionally high use of alder by consumers relative to the alder supply in the C balances; in contrast the proportion of C from maize in consumers was similar to the relative proportion of maize in the C balances (Figure 3B).
Annual average percentages (samples from spring, summer and autumn) of allochthony of consumers (mean contribution of allochthonous C from alder (gray) and maize (black) to animal tissue ± SD), as estimated from mixing models of stable isotopes of carbon and nitrogen, in treatment sections (A, C) and reference sections (B, D) of Gollinsee and Schulzensee, respectively. The grand mean allochthony across the eight consumer groups per lake section is indicated by the dotted line. Crust crustacean zooplankton; Chiron Chironomidae; Ephem Ephemeroptera; Trichopt Trichoptera; Isop Isopoda; Gastrop Gastropoda; Odon Odonata; Fish omnivorous fish.
Percentage of allochthonous C of total C supply plotted against consumer allochthony (%), as averaged (±SD) from the eight main consumer groups from crustaceans, benthic macroinvertebrates and fish in treatment and reference sections of Gollinsee and Schulzensee (A). Allochthonous C sources include alder leaves and maize leaves (treatment sections only). The same as (A), but allochthony split into the two major POC sources, alder and maize leaves (B). The 1:1 line shows consumer allochthony in proportion to allochthonous C supply.
Total secondary and tertiary production (supported by both Callo and Cauto) was generally higher in the reference than in the treatment sections of both lakes (Figure 4). The overall taxonomic composition of zooplankton biomass was similar in the treatment and reference sections of both lakes (Figure A2a), but there were substantially higher biomasses of gastropods and odonates in the reference than in the treatment sections of both lakes (Figure A2b). Total secondary production (excluding bacteria, non-crustacean zooplankton and benthic meiofauna) was dominated by the production of crustacean zooplankton in treatment and reference sections of both lakes (3.5–12.8 g C m−2 y−1), whereas production by benthic macroinvertebrates was substantially lower (0.97–1.94 g C m−2 y−1) (Figure 4A, B). Tertiary production rates ranged between 0.24 and 0.49 g C m−2 y−1 (Figure 4C) and were dominated by production of odonates (range 0.19 to 0.46 g C m−2 y−1), whereas production of fish (>1 year old) was a magnitude lower (range 0.023 to 0.047 g C m−2 y−1). Secondary production as based on Callo was higher in the treatment than in the reference sections of both lakes for crustaceans (Figure 4A) and higher in the treatment than in the reference sections of Gollinsee for macroinvertebrates (Figure 4B). In contrast, allochthonous secondary production of benthic macroinvertebrates in Schulzensee (Figure 4B) and tertiary production of odonates and fish in both lakes (Figure 4C) were similar or higher in the reference than in the treatment sections. However, the macroinvertebrate group with highest biomass and production (except gastropods) was chironomids in both lakes (Figure A2b), and Callo-based secondary production of chironomids was higher in the treatment than in the reference sections of Gollinsee (treatment: 0.18 g C m−2 y−1, reference: 0.10 g C m−2 y−1) and Schulzensee (treatment: 0.10 g C m−2 y−1, reference: 0.09 g C m−2 y−1). In total, the sum of allochthonous secondary production of crustaceans and benthic macroinvertebrates was almost twice as high in the treatment as in the reference sections of Gollinsee (treatment: 0.71 g C m−2 y−1, reference: 0.37 g C m−2 y−1) and Schulzensee (treatment: 1.74 g C m−2 y−1, reference: 0.97 g C m−2 y−1), primarily driven by the higher Callo-based production of crustaceans in the treatment sections.
Annual secondary production (g C m−2 y−1) of crustaceans (A), benthic macroinvertebrates (B) and tertiary production of fish and odonates (C) in treatment and reference sections of Gollinsee and Schulzensee, split according to contributions from allochthonous and autochthonous C sources, as estimated by mixing models of stable isotopes of C and nitrogen. Note the different scalings of y-axes.
Allochthonous ecological efficiency (allochthonous production of crustaceans plus benthic macroinvertebrates divided by Callo inputs) was 2.1% (treatment) and 4.4% (reference) in Gollinsee, and 4.4% (treatment) and 8.2% (reference) in Schulzensee (Figure 5A). Total ecological efficiencies (total production of crustaceans plus benthic macroinvertebrates divided by Callo plus Cauto) in Gollinsee and Schulzensee, respectively, were 1.2 and 1.5% (treatment) or 3.0 and 5.4% (reference) (Figure 5B).
Ecological efficiencies (percentage of C incorporation into secondary production on lake-wide C supply) in treatment and reference sections of Gollinsee and Schulzensee. A Allochthonous ecological efficiency, the percentage of allochthonous C sources (alder, maize) within secondary production of crustaceans and benthic macroinvertebrates relative to the total allochthonous C supply from alder and maize leaves and net (groundwater inflow minus outflow) DOC influx, and B total ecological efficiency, the percentage of allochthonous-based (alder, maize) and autochthonous-based (phytoplankton, periphyton, macrophytes) secondary production of crustaceans and benthic macroinvertebrates relative to the total C supply from autochthonous C fixation and allochthonous C input. The percentages add to 100% when considering the C not incorporated into the production rates.
Discussion
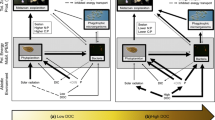
Our results demonstrate that the experimental addition of terrestrial POC did not subsidize total secondary and tertiary production of crustaceans, benthic macroinvertebrates and fish in the treatment sections of either eutrophic lake. In contrast to our expectations, total consumer production was actually higher in the reference than in the treatment sections of both lakes. However, allochthony of crustaceans and some benthic macroinvertebrate groups increased in response to addition of maize C, and hence secondary production as based on Callo was about twice as high in the treatment as in the reference sections of both lakes. The higher Callo-based secondary production suggests that the consumers in part replaced Cauto by the added Callo from maize. Furthermore, allochthony of almost all consumers was substantially higher than would be expected given the minor share of Callo input relative to the total C inputs, which were dominated by Cauto fixation in both lakes. These two results demonstrate the disproportional importance of terrestrial leaf litter as C source in particular for benthic animal consumers. However, the ecological efficiencies of animal consumers were very low, suggesting that crustaceans and benthic macroinvertebrates were not limited by C supply. Therefore, the absolute quantity of terrestrial POC entering a lake in the form of leaves seems to be of minor importance, due to the high autochthonous production and because the large pool of autochthonous detritus buffers the effects of allochthonous C inputs.
Experimental Test of the Subsidy Concept
In a meta-analysis of the ecological effects of resource subsidies from 115 datasets, no single study on lakes was included (Marczak and others 2007). Furthermore, we are not aware of any other more recent study that estimated animal production rates in lakes in response to experimental terrestrial POC additions. In both of our study lakes, the addition of maize POC increased the fraction of secondary production as based on Callo, primarily in crustaceans and some benthic macroinvertebrate groups, organisms that directly or indirectly use the detrital POC, or at least the microbes growing on terrestrial POC. This relatively higher production attributable to Callo in treatment sections was caused by the additional contribution of maize to consumer tissues, as calculated by mixing models. The combination of lower overall production rates in the treatment sections, but a higher production rate based on Callo suggest that crustaceans and benthic macroinvertebrates partly replaced Cauto by maize C as source of tissue production in response to the treatment. A similar replacement of autochthonous by allochthonous resources in the diet of zooplankton consumers at higher terrestrial subsidies, but accompanied by lowered zooplankton production, was recently shown across several lakes that differed in their DOC concentration (Kelly and others 2014). In contrast, weights of planktivorous juvenile fish have been found to be enhanced by organic matter export from forested catchments, suggesting that growth of these fish was directly subsidized by Callo via bacterial and zooplankton production (Tanentzap and others 2014). These latter two studies focused on allochthonous inputs of dissolved Callo (DOC), which may stimulate bacterial production, but may limit phytoplankton production by decreasing water transparency at high concentrations (Kelly and others 2014; Tanentzap and others 2014). In contrast, we observed that total ecosystem production was not subsidized by particulate Callo from maize, in either of our study lakes, because the total secondary production of benthic macroinvertebrates and crustaceans and the tertiary production by fish were higher in reference sections than in treatment sections of both lakes.
Our estimates of secondary production were based on allometric equations that used animal biomass, size, and lake temperature as input variables. Animal size and lake temperature were very similar in the lake sections. Accordingly, the differences in production rates between treatment and reference sections were primarily caused by differences in animal biomasses, but we preferred to calculate secondary production over biomasses due to the clear link of production to energy flow and C budgets (Dolbeth and others 2012; Kelly and others 2014). The differences in crustacean and macroinvertebrate biomasses between treatment and reference sections of both lakes could have been caused by differences in habitat quality, spatial aggregation, or predation mortality of organisms between both lake sections. For example, average annual odonate and gastropod biomasses were substantially higher in the reference than in the treatment sections of Schulzensee, attributable to the occurrence of single large individuals of these groups in one or two of the monthly samples. Crustacean biomasses were overall very low, presumably because of strong predation by young fish, which occurred at high densities after winter fish kills in both lakes (Hilt and others 2015). However, we were unable to quantify potential differences in young fish densities between the lake sections, which may have caused the higher crustacean biomasses and production rates in the reference sections. Therefore, the apparently higher estimates of secondary production in the reference sections should be interpreted with caution, but our results suggest that it is likely that production in the treatment sections did not increase and hence maize addition did not subsidize overall secondary production of consumers.
The allochthony of crustaceans was substantially lower than that of all other consumer groups, and the standard deviations of their allochthony estimates from mixing models included zero for all lake sections. We quantified the Callo and Cauto contributions to crustacean production from mixing models of the stable isotopes, which were based on seston samples from the open water column and included only phytoplankton and a POC mix as potential sources. We assumed that the composition of a seston sample reflects the quantitative contributions of Callo and Cauto to the lake-wide C balances. Furthermore, we assumed that the crustaceans did not select strongly for particular C sources but ingest phytoplankton and detritus according to their relative proportions in the seston (Berggren and others 2014). Our estimates of crustacean allochthony as based on the mixing model closely matched the proportions of Callo relative to total C available in the four lake sections because allochthony was higher in the treatment than in the reference sections, and overall higher in Schulzensee than in Gollinsee. The close correspondence between allochthonous contributions to lake-wide C budgets and consumer allochthony confirms that our assumptions on unselective feeding of crustaceans were reliable. Accordingly, because crustacean allochthony was higher in the treatment sections of both lakes, Callo replaced Cauto in the diet of crustaceans after maize addition. However, quantification of allochthony in crustaceans was further complicated by the fact that the phytoplankton δ13C values were also indirectly estimated from seston by a two end-member mixing model (Bade and others 2006; Taipale and others 2007). Therefore, it may be questioned whether our results unequivocally demonstrate an enhanced allochthony of crustaceans in response to maize addition. Numerous recent studies demonstrate that zooplankton can strongly rely upon terrestrial C sources (for example, Berggren and others 2014; Wilkinson and others 2014). There is a lively debate surrounding the question of how much autochthonous (algal) C, which is typically of high nutritional quality compared to terrestrial POC, is needed to support the growth and reproduction of crustaceans (for example, Brett and others 2009; Cole and others 2011; Francis and others 2011). Our results quantified the Callo and Cauto contributions to crustacean production only indirectly, but given the dominance of Cauto in the C balances of these eutrophic lakes, phytoplankton was likely the primary C source for crustaceans in both lakes and in both treatment and reference sections.
The low allochthony of crustaceans and the questionable evidence for a response of crustacean allochthony to maize addition in the treatment sections corroborate our earlier analyses from this experiment that the pelagic habitats of both lakes did not receive much Callo from the maize leaves (Scharnweber and others 2014a). In contrast to the application of mixing models of stable isotopes as used here, we initially compared only the δ13C of producers and consumers in treatment and reference sections of both lakes, and found an enhancement of δ13C in benthic consumers from spring and summer samples, presumably caused by the ingestion of maize with its high δ13C (-13.5 ‰) (Scharnweber and others 2014a). The response to maize addition of benthic consumers was less clearly seen based on the results of mixing models in this study, although allochthony and Callo-based production were higher for some groups in the treatment than reference sections. A potentially weaker enhancement of allochthony documented here in comparison with the statistically significant enhancement of δ13C in several consumer groups (Scharnweber and others 2014a) is attributable to the fact that we included organisms sampled over the entire year in this study, in contrast to the focus on the short-term response of consumer allochthony in spring and summer as applied earlier (Scharnweber and others 2014a). This is an important difference because the enhanced δ13C signal was strongest in spring and summer, but declined in autumn in many consumers (Scharnweber and others 2014a). However, the differences between both approaches also indicate the general problems of mixing models based on two isotopes to disentangle contributions from Callo and Cauto to consumer tissues if several sources are included. In these cases, the posterior probability distributions of contributions from the sources can be flat and sometimes include zero (Scharnweber and others 2014b). The high uncertainty of the results from mixing models with respect to allochthony is also reflected by the high standard deviations of allochthony estimates in our data. Therefore, a statistically valid conclusion of enhanced allochthony in response to higher load of Callo from the terrestrial environment is possible only if there are major shifts in carbon and nitrogen isotope values of the consumers. A strong isotopic response is most likely if the terrestrial source has a δ13C signal strongly distinct from that of aquatic primary producers and if the quantity of terrestrial Callo is large compared to in-lake C fixation by algae. The amount of Callo added by maize leaves, although several times higher than the alder leaf litter fall, was still small relative to the autochthonous C fixation.
Ecological Efficiencies and C Balances
According to our C balances, Callo inputs represented a very low proportion of total organic C inputs in both lakes. Leaf litter from alder and DOC contributed about 2–4% of the overall C supply in the reference sections and even the addition of maize leaves to the treatment sections increased allochthony to only about 9–11% of total C inputs. These balances indicate that the C supply in these shallow, eutrophic lakes was dominated by C fixation from autochthonous primary production, primarily by algae (Brothers and others 2013a). This contrasts with balances from naturally unproductive lakes in which terrestrial DOC and POC can dominate in-lake C supply (Wilkinson and others 2013). The low relative supply of Callo in our eutrophic lakes supports the conclusion that increasing nutrient loading decouples lake C cycles from their terrestrial surroundings due to enhanced autochthonous primary production (Carpenter and others 2005). A stronger response of animal production rates to Callo additions seems possible in lakes with very low autochthonous productivity, because in these cases the amount of subsidies exceeds the amount of equivalent autochthonous resources (Marczak and others 2007). However, our results also demonstrate that individual consumer allochthony can still be high even in eutrophic lakes, and seems to be independent of quantitative C supply (see below).
The estimated total ecological efficiencies (~1.2–5.4%) indicated that the animal consumers we considered incorporated only a small proportion of total C inputs. Furthermore, allochthonous ecological efficiencies were likewise low (2.1–8.2%). Even when acknowledging that C incorporation into animal tissues is lower than the actual C consumption due to losses via egestion and respiration, the overall C supply substantially exceeded the C ingested by the consumers considered here. Similarly low efficiencies (~3%) for the incorporation of Callo have been found in unproductive Scandinavian lakes (Karlsson and others 2012). Interestingly, the allochthonous efficiency was higher in Schulzensee (than in Gollinsee), that is, that a greater fraction of terrestrial POC was processed in the lake where submerged macrophytes create a distinct littoral zone which may trap POC. Similar conclusions have been drawn based on the comparison of δ13C consumer data between both lakes (Scharnweber and others 2014a).
Estimates from C mass balance and ecosystem C budgets demonstrate that a substantial portion of Callo is permanently buried in the sediments (82% in Gollinsee, 34% in Schulzensee), and a considerable fraction of C is respired (Brothers and others 2013b). The high ecosystem respiration rates are dominated by respiration of benthic bacteria (Brothers and others 2013b), thus bacterial metabolism presumably contributes greatly to the processing of Callo and Cauto (Cole and others 2006; Jones and others 2012). However, the allochthony of the microbial food web is only incompletely understood in our lakes. It is known that pelagic bacterial activities were stimulated as a result of the leaching process from the maize leaves (Attermeyer and others 2013). Thus, an indirect stimulation of animal production appears likely as bacterial production can be enhanced by Callo (Kritzberg and others 2004; Berggren and others 2010; Attermeyer and others 2013) and this higher production is at least partly transferred to production of animal consumers. However, less than 30% of the leaf C (Petersen and Cummins 1974; Benfield 1996) is leached and thus accessible to the pelagic food web, leaving the majority of the leaf POC to subsidize benthic food webs. Furthermore, this indirect transfer of the leached POC to the pelagic food webs is dampened by the energy losses during metabolism within the microbial food web (Kritzberg and others 2005; Karlsson 2007). In a well-studied plankton food web, high ecological efficiencies of around 30% were found only when accounting for all trophic groups including bacteria, heterotrophic flagellates, ciliates, rotifers, and crustaceans (Gaedke and Straile 1994; Boit and Gaedke 2014). This was not feasible in this study due to the lack of information on stable isotopes of the small organisms. However, substantial energy losses within the pelagic food web of our lakes are likely because the biomass of crustacean zooplankton was low, but ciliate biomass was extremely high due to shifts in fish community composition after severe winter kills (Hilt and others 2015). Accordingly, parts of crustacean zooplankton may be considered secondary consumers because they ingested ciliates instead of phytoplankton, and hence their production rates can be considered tertiary production.
The two major terrestrial POC sources in our experiment, leaves from alder and maize, differed with respect to the correspondence between contributions to C balances versus consumer allochthony. Whereas the allochthony of consumers originating from maize was similar to the contribution of maize POC to the lake C balances (~5–8% for both), alder POC caused a much higher allochthony in all animal consumers (22–35%) than reflected by the low contribution of alder to C balances (~2%). The disproportionate incorporation of Callo from alder into the tissue of mostly littoral macroinvertebrates may be caused by the high local supply of leaf litter in the littoral habitats of both lakes (Marcarelli and others 2011; Attermeyer and others 2013), an effect that may be even more pronounced if submerged macrophytes trap POC as in Schulzensee (Scharnweber and others 2014a). After the autumnal litter fall, alder leaves are processed by shredders and microbial communities until the subsequent summer (Scharnweber and others 2014a). Furthermore, we estimated relatively low C:N ratios in alder leaves (about 16:1) which are probably of similar quality for consumers as littoral Cauto sources, for example Ceratophyllum (about 14:1) or periphyton (about 9:1). In turn, mixing models indicated that maize leaves (with a C:N ratio of 41:1) contributed only 3–13% to the diet of crustaceans, benthic macroinvertebrates and indirectly to fish in both lakes, despite their high local availability. Accordingly, there was no quantitative correspondence between C sources of consumers and the contributions of these sources to C balances. We thus conclude that the quantity of resources is less important than their differing nutritional quality and the food web pathways by which they are channeled to consumers (Marcarelli and others 2011).
Conclusions
Terrestrial C sources are generally considered a resource subsidy to lakes, but dissolved and particulate fractions subsidize different consumers. We have shown here that the experimental addition of terrestrial POC may have increased the allochthonous reliance of secondary production. Whereas this result may be debatable for pelagic crustaceans due to methodological reasons, there was clearer evidence for a higher Callo reliance of some littoral macroinvertebrates in response to maize addition. This process has been called a “benthic shortcut” (Attermeyer and others 2013) because it transfers terrestrial POC directly to benthic animals (rather than through microbes), and ultimately to higher trophic levels. The effect of this allochthonous C input on consumer productivity was weak presumably because both lakes are characterized by a dominance of autochthonous productivity. In contrast, about 10% of C from maize leaves was leached immediately after introduction and stimulated pelagic bacterial production (Attermeyer and others 2013), confirming the subsidy effect of terrestrial DOC on bacteria (Kritzberg and others 2004; Berggren and others 2010). However, a significant transfer of DOC leachate into higher pelagic consumer levels was not observed, as evidenced by the low allochthony of crustaceans. The contrasting consequences of DOC and POC subsidies on aquatic food-webs underlie the ‘buffering’ effect of the large detrital POC pools in lakes which accumulate organic C derived from discontinuous supply and provide a constantly available resource to both benthic and pelagic consumers (Moore and others 2004). By calculating partial flow food webs and ecological efficiencies, we also demonstrated that the relative inputs of different Callo and Cauto sources do not predict the quantitative proportions of C incorporation into animal consumer tissues in these lakes. A low allochthony of animal consumers, predicted from the dominant supply of Cauto in productive lakes, was not confirmed. These results suggest that the C flows in productive lakes can become quantitatively decoupled from their terrestrial surroundings, but the supply of relatively high-quality Callo sources may nevertheless facilitate a strong qualitative land-water coupling.
References
Abelho M. 2001. From litterfall to breakdown in streams: a review. ScientificWorldJournal 17:656–80.
Attermeyer K, Premke K, Hornick T, Hilt S, Grossart HP. 2013. Ecosystem-level studies of terrestrial carbon reveal contrasting bacterial metabolism in different aquatic habitats. Ecology 94:2754–66.
Babler AL, Pilati A, Vanni MJ. 2011. Terrestrial support of detritivorous fish populations decreases with watershed size. Ecosphere . doi:10.1890/ES11-00043.1.
Bade DL, Carpenter SR, Cole JJ, Hanson PC, Hesslein RH. 2004. Controls of delta C-13-DIC in lakes: geochemistry, lake metabolism, and morphometry. Limnol Oceanogr 49:1160–72.
Bade DL, Pace ML, Cole JJ, Carpenter SR. 2006. Can algal photosynthetic inorganic carbon isotope fractionation be predicted in lakes using existing models? Aquat Sci 68:142–53.
Bartels P, Cucherousset J, Gudasz C, Jansson M, Karlsson J, Persson L, Premke K, Rubach A, Steger K, Tranvik LJ, Eklov P. 2012. Terrestrial subsidies to lake food webs: an experimental approach. Oecologia 168:807–18.
Benfield EF. 1996. Leaf breakdown in stream ecosystems. In: Hauer FR, Lamberti GA, Eds. Methods in stream ecology. San Diego: Academic Press. p 579–89.
Berggren M, Strom L, Laudon H, Karlsson J, Jonsson A, Giesler R, Bergstrom AK, Jansson M. 2010. Lake secondary production fueled by rapid transfer of low molecular weight organic carbon from terrestrial sources to aquatic consumers. Ecol Lett 13:870–80.
Berggren M, Ziegler SE, St-Gelais NF, Beisner BE, del Giorgio PA. 2014. Contrasting patterns of allochthony among three major groups of crustacean zooplankton in boreal and temperate lakes. Ecology 95:1947–59.
Boit A, Gaedke U. 2014. Benchmarking successional progress in a quantitative food web. PLoS One. doi:10.1371/journal.pone.0090404.
Brauns M, Gücker B, Wagner C, Garcia XF, Walz N, Pusch MT. 2011. Human lakeshore development alters the structure and trophic basis of littoral food webs. J Appl Ecol 48:916–25.
Brett MT, Kainz MJ, Taipale SJ, Seshan H. 2009. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proc Natl Acad Sci USA 106:21197–201.
Brothers SM, Ahilt S, Meyer S, Köhler J. 2013a. Plant community structure determines primary productivity in shallow, eutrophic lakes. Freshw Biol 58:2264–76.
Brothers SM, Hilt S, Attermeyer K, Grossart HP, Kosten S, Lischke B, Mehner T, Meyer N, Scharnweber K, Köhler J. 2013b. A regime shift from macrophyte to phytoplankton dominance enhances carbon burial in a shallow, eutrophic lake. Ecosphere 4:137. doi:10.1890/ES13-00247.1.
Carpenter SR. 1989. Replication and treatment strength in whole-lake experiments. Ecology 70:453–63.
Carpenter SR, Cole JJ, Pace ML, Van de Bogert M, Bade DL, Bastviken D, Gille CM, Hodgson JR, Kitchell JF, Kritzberg ES. 2005. Ecosystem subsidies: terrestrial support of aquatic food webs from C-13 addition to contrasting lakes. Ecology 86:2737–50.
Cole JJ, Carpenter SR, Kitchell J, Pace ML, Solomon CT, Weidel B. 2011. Strong evidence for terrestrial support of zooplankton in small lakes based on stable isotopes of carbon, nitrogen, and hydrogen. Proc Natl Acad Sci USA 108:1975–80.
Cole JJ, Carpenter SR, Pace ML, Van de Bogert MC, Kitchell JL, Hodgson JR. 2006. Differential support of lake food webs by three types of terrestrial organic carbon. Ecol Lett 9:558–68.
Dolbeth M, Cusson M, Sousa R, Pardal MA. 2012. Secondary production as a tool for better understanding of aquatic ecosystems. Can J Fish Aquat Sci 69:1230–53.
Dumont HJ, Vandevelde I, Dumont S. 1975. Dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from plankton, periphyton and benthos of continental waters. Oecologia 19:75–97.
Francis TB, Schindler DE, Holtgrieve GW, Larson ER, Scheuerell MD, Semmens BX, Ward EJ. 2011. Habitat structure determines resource use by zooplankton in temperate lakes. Ecol Lett 14:364–72.
Fraser CM. 1916. Growth of the spring salmon. Transactions of the Pacific Fisheries Society Seattle, pp. 29–39.
Gaedke U. 1992. The size distribution of plankton biomass in a large lake and its seasonal variability. Limnol Oceanogr 37:1202–20.
Gaedke U, Straile D. 1994. Seasonal changes of trophic transfer efficiencies in a plankton food web derived from biomass size distributions and network analysis. Ecol Model 75:435–45.
Haney JF, Hall DJ. 1973. Sugar-coated Daphnia: a preservation technique for Cladocera. Limnol Oceanogr 18:331–3.
Hilt S, Wanke T, Scharnweber K, Brauns M, Syvaranta J, Brothers S, Gaedke U, Kohler J, Lischke B, Mehner T. 2015. Contrasting response of two shallow eutrophic cold temperate lakes to a partial winterkill of fish. Hydrobiologia 749:31–42.
Jones SE, Solomon CT, Weidel BC. 2012. Subsidy or subtraction: how do terrestrial inputs influence consumer production in lakes? Freshw Rev 5:37–49.
Karlsson J. 2007. Different carbon support for respiration and secondary production in unproductive lakes. Oikos 116:1691–6.
Karlsson J, Ask J, Jansson M. 2008. Winter respiration of allochthonous and autochthonous organic carbon in a subarctic clear-water lake. Limnol Oceanogr 53:948–54.
Karlsson J, Berggren M, Ask J, Byström P, Jonsson A, Laudon H, Jansson M. 2012. Terrestrial organic matter support of lake food webs: evidence from lake metabolism and stable hydrogen isotopes of consumers. Limnol Oceanogr 57:1042–8.
Kelly PT, Solomon CT, Weidel BC, Jones SE. 2014. Terrestrial carbon is a resource, but not a subsidy, for lake zooplankton. Ecology 95:1236–42.
Kritzberg ES, Cole JJ, Pace ML, Graneli W, Bade DL. 2004. Autochthonous versus allochthonous carbon sources of bacteria: results from whole-lake C-13 addition experiments. Limnol Oceanogr 49:588–96.
Kritzberg ES, Cole JJ, Pace MM, Graneli W. 2005. Does autochthonous primary production drive variability in bacterial metabolism and growth efficiency in lakes dominated by terrestrial C inputs? Aquat Microb Ecol 38:103–11.
Larsen S, Andersen T, Hessen DO. 2011. Climate change predicted to cause severe increase of organic carbon in lakes. Glob Change Biol 17:1186–92.
Lee RM. 1920. A review of the methods of age and growth determination of juvenile fish in the littoral area of a shallow lake. Fish Investig Lond Ser 4:32.
Marcarelli AM, Baxter CV, Mineau MM, Hall RO. 2011. Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92:1215–25.
Marczak LB, Thompson RM, Richardson JS. 2007. Meta-analysis: trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology 88:140–8.
Masese FO, Abrantes KG, Gettel GM, Bouillon S, Irvine K, McClain ME. 2015. Are large herbivores vectors of terrestrial subsidies for riverine food webs? Ecosystems 18:686–706.
Mehner T, Ihlau J, Dörner H, Hölker F. 2005. Can feeding of fish on terrestrial insects subsidize the nutrient pool of lakes? Limnol Oceanogr 50:2022–31.
Moore JC, Berlow EL, Coleman DC, de Ruiter PC, Dong Q, Hastings A, Johnson NC, McCann KS, Melville K, Morin PJ, Nadelhoffer K, Rosemond AD, Post DM, Sabo JL, Scow KM, Vanni MJ, Wall DH. 2004. Detritus, trophic dynamics and biodiversity. Ecol Lett 7:584–600.
Nakano S, Murakami M. 2001. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA 98:166–70.
Parnell AC, Phillips DL, Bearhop S, Semmens BX, Ward EJ, Moore JW, Jackson AL, Grey J, Kelly DJ, Inger R. 2013. Bayesian stable isotope mixing models. Environmetrics 24:387–99.
Petersen RC, Cummins KW. 1974. Leaf processing in a woodland stream. Freshw Biol 4:343–68.
Phillips DL, Koch PL. 2002. Incorporating concentration dependence in stable isotope mixing models. Oecologia 130:114–25.
Plante C, Downing JA. 1989. Production of freshwater invertebrate populations in lakes. Can J Fish Aquat Sci 46:1489–98.
Polis GA, Anderson WB, Holt RD. 1997. Towards an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316.
Post DM. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–18.
R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Ricker WE. 1975. Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Can 191:1–382.
Rudnick S, Lewandowski J, Nützmann G. 2015. Investigating groundwater–lake interactions by hydraulic heads and a water balance. Groundwater 53:227–37.
Scharnweber K, Syväranta J, Hilt S, Brauns M, Vanni MJ, Brothers SM, Köhler J, Knezevic-Jaric J, Mehner T. 2014a. Whole-lake experiments reveal the fate of terrestrial particulate organic carbon in benthic food webs of shallow lakes. Ecology 95:1496–505.
Scharnweber K, Vanni MJ, Hilt S, Syväranta J, Mehner T. 2014b. Boomerang ecosystem fluxes: organic carbon inputs from land to lakes are returned to terrestrial food webs via aquatic insects. Oikos 123:1439–48.
Stock BC, Semmens BX. 2013. MixSIAR GUI User Manual, version 2.1.2.
Stockwell JD, Johannson OE. 1997. Temperature-dependent allometric models to estimate zooplankton production in temperate freshwater lakes. Can J Fish Aquat Sci 54:2350–60.
Taipale S, Kankaala P, Jones RI. 2007. Contributions of different organic carbon sources to Daphnia in the pelagic foodweb of a small polyhumic lake: results from mesocosm (DIC)-C-13-additions. Ecosystems 10:757–72.
Taipale S, Kankaala P, Tiirola M, Jones RI. 2008. Whole-lake dissolved inorganic C-13 additions reveal seasonal shifts in zooplankton diet. Ecology 89:463–74.
Tanentzap AJ, Szkokan-Emilson EJ, Kielstra BW, Arts MT, Yan ND, Gunn JM. 2014. Forests fuel fish growth in freshwater deltas. Nat Commun 5:4077.
Vander Zanden MJ, Chandra S, Park SK, Vadeboncoeur Y, Goldman CR. 2006. Efficiencies of benthic and pelagic trophic pathways in a subalpine lake. Can J Fish Aquat Sci 63:2608–20.
Vander Zanden MJ, Gratton C. 2011. Blowin’ in the wind: reciprocal airborne carbon fluxes between lakes and land. Can J Fish Aquat Sci 68:170–82.
Wetzel RG. 2001. Limnology. 3rd edn. London: Elsevier Academic Press.
Wilkinson GM, Carpenter SR, Cole JJ, Pace ML. 2014. Use of deep autochthonous resources by zooplankton: results of a metalimnetic addition of 13C to a small lake. Limnol Oceanogr 59:986–96.
Wilkinson GM, Pace ML, Cole JJ. 2013. Terrestrial dominance of organic matter in north temperate lakes. Glob Biogeochem Cycles 27:43–51.
Acknowledgments
We thank A. Türck, C. Helms, J. Schreiber, S. Schuchort, S. Oksanen, and T. Wanke for their help in the field. We further acknowledge discussion and contributions by M. Gessner, R. Jones, S. Devlin, A. Vogt, K. Kuntze, M. Graupe, A. Busse, D. Thompson, S. Schmidt-Halewicz, N. Walz, P. Casper, K. Premke, G. Nützmann, J. Rääpysjärvi, and M. Kaupenjohann. Two anonymous reviewers provided comments, which helped improving the text. We thank K. Metzdorf (Technoplan Zelte und Planen GmbH) for lake divisions. R. Mauersberger (Förderverein Feldberg-Uckermärkische Seen e.V.) and R. Tischbier (Stiftung Pro Artenvielfalt) kindly provided background information and access to the lakes. This study was financed by the TERRALAC-project (http://terralac.igb-berlin.de) of the Wissenschaftsgemeinschaft Leibniz (WGL). J. Syväranta and M.J. Vanni were supported by the IGB Fellowship program in Freshwater Science and K. Scharnweber was further supported by the German Academic Exchange Service (DAAD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
TM & SH: Conceived of study. KA, MB, SB, JD, BL, NM, KS, JS: Performed research. TM, KA, MB, SB, JD, UG, HPG, JK, BL, NM, KS, JS, MJM, SH: Analyzed data. MB, JS: Contributed new methods or models. TM together with all co-authors: Wrote the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehner, T., Attermeyer, K., Brauns, M. et al. Weak Response of Animal Allochthony and Production to Enhanced Supply of Terrestrial Leaf Litter in Nutrient-Rich Lakes. Ecosystems 19, 311–325 (2016). https://doi.org/10.1007/s10021-015-9933-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-015-9933-2