Abstract
Seafloor ecosystems play pivotal roles in biogeochemical cycling, but hypoxia (O2 < 2 mg l−1) is changing the way they contribute to ecosystem function on a global scale. A major issue for mitigation of eutrophication-driven hypoxia is the continued release of phosphorus and nitrogen from sediments. Nutrient cycling is affected by sediment characteristics, benthic communities, and oxygen conditions, but the context dependency of these processes in natural ecosystems is poorly known. The Baltic Sea is naturally a low-diversity system, where hypoxia has further decimated the benthic communities. To investigate how oxygen conditions affect the relationship between benthic fauna and nutrient fluxes across the sediment–water interface, we conducted macrofaunal sampling and measurements of benthic oxygen and nutrient fluxes (NO3 −, NO2 −, NH4 +, PO4 3−, SiO4) at 26 sites across the entire north–south salinity gradient of the Baltic Sea (>1200 km). This broad-scale sampling confirmed the strong salinity-driven diversity gradient and large spatial variations in oxygen conditions, which affected the status of the benthic communities. Benthic nutrient fluxes varied several orders of magnitude, both between sea areas and along gradients of hypoxia within areas. DistLM modeling indicated that benthic fauna can affect nutrient cycling, also under hypoxic conditions, with the invasive polychaete Marenzelleria spp. being particularly important. However, as the oxygen content decreases—even slightly—the subsequent changes in the faunal abundance and functional diversity may influence the nature and the rate of fluxes. These results imply that management targets for oxygen concentrations need to be raised to sustain healthy ecosystem functioning and to facilitate the recovery of large degraded sea areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the face of global species loss, understanding the influence of biodiversity on ecosystem functioning is becoming increasingly important (Cardinale and others 2012). Less attention, however, has been paid to how organisms’ contribution to ecosystem functioning changes as the environmental conditions deteriorate. Such degradation does not necessarily entail species loss, but rather changes in abundance, biomass, and dominance patterns (Chapin and others 1997). Indeed, exploring changes in natural communities across environmental disturbance gradients may yield significant insights into how species and their relative dominance may affect ecosystem functioning in a context-dependent manner (Dayton 1971; Polis and others 1996; Larsen and others 2005; Snelgrove and others 2014; Allgeier and others 2015).
Marine benthic communities are seriously threatened by the exponential spread of hypoxia (low oxygen concentrations, <2 mg l−1 O2) that has been observed worldwide since the 1960s as a consequence of increased nutrient loading to the coastal zone due to poor wastewater treatment and extensive agricultural practices with increased fertilizer use (Diaz and Rosenberg 2008; Rabalais and others 2010). As the spatial extent of these problems increases, they become more difficult to resolve and our focus needs to encompass both the nutrient loading and the ability of the receiving ecosystem to process, assimilate, or export these nutrients. One major issue for mitigation of eutrophication and hypoxia is the continued recycling of phosphorus and nitrogen from sediments. In particular, the benthic efflux of phosphate is enhanced under hypoxic conditions (Mortimer 1941; Ingall and others 1993; Slomp and others 2002; Reed and others 2011), which may fuel larger algal blooms and perpetuate the problem (Vahtera and others 2007). Although many links between hypoxia, degraded benthic fauna, and sediment nutrient release have been identified (Karlson and others 2007), we lack in our understanding of the relevant larger-scale feedback mechanisms, which may be particularly important for ecosystem resilience and remediation efforts (Kemp and others 2009; Norkko and others 2012; Carstensen and others 2014; Snelgrove and others 2014).
Soft-sediment habitats are important in the global cycles of elements and energy. For instance, approximately 30% of the primary production in the world’s oceans takes place over shelf and coastal sediments (Berger and others 1989) and up to 50% of this production may reach the sediments (Wollast 1991). The benthic macrofauna inhabiting these sediments provide important ecosystem services, by providing food for higher trophic levels, through their habitat engineering, and their effects on benthic–pelagic coupling and sediment biogeochemistry (Rhoads 1974). Bioturbation and bioirrigation (that is, sediment mixing and ventilation) by infaunal species enhance oxygen penetration into the sediments and thus influence all oxygen-dependent processes in the sediment, including organic matter mineralization through stimulation of microbial activity, and nutrient cycling (Levinton 1995; Aller and Aller 1998; Meysman and others 2006; Glud 2008). Eutrophication and hypoxia affect benthic communities in complex ways, with generally increasing biomasses at early stages of organic enrichment followed by community collapse when hypoxia and anoxia develop (Pearson and Rosenberg 1978; Grall and Chauvaud 2002; Levin and others 2009; Timmermann and others 2012). Given the role of infauna in affecting the flux of energy and matter across the sediment–water interface, it is likely that loss of diversity and in particular abundant key species with increasing hypoxia will adversely affect or fundamentally shift the nature of ecosystem functioning, for example, in terms of nutrient cycling (Conley and others 2009; Norkko and others 2013; Villnäs and others 2013). Therefore, the status (condition/diversity) of benthic communities should be important for ecosystem functioning, with a potentially diminished role for benthic macrofauna under stressful conditions.
The strength, direction, and relative importance of the relationship between benthic community structure and ecosystem function (measured, for example, as nutrient fluxes across the sediment–water interface) is, however, likely to be highly context dependent (Snelgrove and others 2014). A range of environmental factors such as temperature, wave exposure, salinity, sediment type, mineral content, and habitat heterogeneity are likely to influence these relationships (Norkko and others 2006; Hewitt and others 2008; Hiddink and others 2009; Godbold and others 2011). Nevertheless, the majority of studies of the relationship between benthic fauna (usually species diversity) and ecosystem function have been conducted either in the laboratory or at only one or a few field sites, encompassing only a limited range of environmental variability. The need to expand the spatial and temporal extent of studies to include larger ranges of environmental variables and stressors is starting to become recognized (Dyson and others 2007; Godbold and Solan 2009; Hiddink and others 2009; Braeckman and others 2014), with a particular need for field studies with natural communities, where the scale of individual species effects and health of individuals may vary (Volkenborn and others 2010; Villnäs and others 2013). Moreover, disturbance history is likely to affect sediment properties and the structure of resident communities due to non-random species loss targeting vulnerable species and causing dominance by more tolerant and rapid colonist species. As faunal effects on sediments and chemical fluxes are dependent on functional traits, such as mobility, bioturbation mode, burrowing depth, and feeding mode (Solan and others 2004), shifts in ecosystem function are likely to occur in areas subjected to different frequencies and extent of hypoxic disturbance (Levin and others 2001; Villnäs and others 2012, 2013). Functional redundancy in high-diversity systems may allow benthic communities to retain their role in ecosystem function (Yachi and Loreau 1999; Loreau and others 2001), but in a low-diversity system such as the Baltic Sea, functions are performed by a restricted range of species with little redundancy (Villnäs and Norkko 2011; Norkko and others 2013; Villnäs and others 2013). Therefore, we would predict that under good oxygen conditions the benthic fauna would be unaffected and would drive nutrient fluxes, whereas under poor conditions, environmental factors (particularly oxygen content) would drive fluxes and the decimated benthic fauna would have little effect on fluxes.
In this study, we investigate the relationship between benthic fauna and nutrient fluxes across the sediment–water interface, and how this may vary with hypoxia. We conducted a comprehensive set of benthic nutrient flux measurements across the entire salinity gradient of the open Baltic Sea, from the Arkona Basin in the south to the Bothnian Bay in the north. We also sampled along distinct hypoxic disturbance gradients within three different geographic areas with differing, salinity-driven natural background diversity. With this extensive field-sampling program, we tested the role of macrofaunal diversity, abundance, biomass, and bioturbation potential in ecosystem functioning in terms of nutrient fluxes across the sediment–water interface. We then identified likely relationships between benthic fauna and nutrient fluxes across gradients of hypoxia and how the history of hypoxic stress (that is, the status of the community) may affect these links.
Methods
Baltic Sea Benthic Communities
The Baltic Sea is one of the largest brackish-water ecosystems in the world (Figure 1), with strong north–south gradients in salinity, temperature, as well as sea-ice cover (ice cover each winter for 3–5 months in the north, whereas ice only rarely forms in the south). Eutrophication is one of the main problems in the Baltic (HELCOM 2009b). More or less permanently hypoxic (<2 mg l−1 O2) zones in the open Baltic Sea occupy up to 80,000 km2 and are largely devoid of any benthic macrofauna (Karlson and others 2002; Conley and others 2009; Carstensen and others 2014). Although salinity sets the limits for benthic macrofaunal diversity in the Baltic Sea and the number of functional groups is low (Bonsdorff and Pearson 1999; Villnäs and Norkko 2011), bottom-water hypoxia is currently the main factor structuring the benthic communities in the Baltic Proper and Gulf of Finland (Karlson and others 2002; Conley and others 2009). Hypoxia is not a problem in the Bothnian Bay and Bothnian Sea, since a sill prevents hypoxic water from entering from the south and there is no major problem with eutrophication. Nevertheless, signs of eutrophication are starting to be noticed also in the Bothnian Sea (Lundberg and others 2009).
Abundances and biomasses of Baltic open sea benthic communities are highly variable, both spatially and temporally (HELCOM 2009a). Macrofaunal diversity also increases with salinity from north to south, and species composition changes over time, both through natural changes in salinity and through the spread of introduced species (Villnäs and Norkko 2011). The most notable new soft-sediment invaders are the spionid polychaetes Marenzelleria spp., now occurring with at least three sibling species (Blank and others 2008), which have spread throughout the Baltic since the first record of M. viridis in the 1980s (Bick and Burckhardt 1989). It is now established as one of the dominant genera in benthic communities in the northern Baltic Sea (Kauppi and others 2015), with subsequent potential for significant ecosystem effects on nutrient cycling (Josefson and others 2012; Norkko and others 2012). Notably, Marenzelleria spp. is able to tolerate low oxygen concentrations (Schiedek 1997).
Area and Sampling
Sampling of benthic fauna and nutrient fluxes was conducted on-board R/V Aranda in conjunction with the annual Finnish/HELCOM benthic monitoring, covering all the major sub-basins of the open Baltic Sea. The sampling was done at 26 sites in late May–early June 2009, after the spring bloom (Figure 1). All sites had muddy sediment with water depths greater than 47 m. At each site, salinity and temperature depth-profiles were determined from CTD casts and bottom-water dissolved oxygen (DO) concentrations were determined by Winkler titration of water samples obtained from cores used for flux measurements (see below; Appendix S1). To utilize information on long-term patterns in oxygen conditions and benthic community status along the whole salinity gradient of the Baltic, sampling sites were chosen to coincide with HELCOM monitoring stations. To better investigate the interactions between benthic diversity, hypoxic stress, and ecosystem function, additional transects of multiple sampling sites were chosen to encompass gradients of increasing hypoxia in three areas with different salinity-driven background diversity (Gulf of Finland, Eastern Gotland Basin, Southern Baltic; one transect per area).
Flux Measurements
Oxygen and nutrient (NO3 −, NO2 −, NH4 +, PO4 3−, SiO4) fluxes across the sediment–water interface were estimated by on-board incubation of intact sediment cores. Cores were collected with a Gemax twincorer (internal diameter 90 mm), using split tubes, where the upper parts were used as flux chambers (30 cm sediment depth + 10 cm of overlying water). Five replicate cores were incubated per site and only cores with undisturbed sediment surfaces were accepted.
Core incubations started immediately after collection and water samples for oxygen and nutrient concentrations (NO3 −, NO2 −, NH4 +, PO4 3−, SiO4) were obtained at the start and the end of incubation, and the differences in concentration were used to calculate fluxes per surface area (µmol m−2 d−1, positive flux = flux out of the sediment). DO was determined by Winkler titration, whereas the nutrient samples were centrifuged (20 min, 2500 rpm, 4°C) and analyzed on-board with an autoanalyzer (Lachat QuickChem 8000). The cores were sealed with a lid containing a Teflon-coated magnetic stirring bar, which provided continuous gentle stirring by an external magnet. Cores were incubated 4 h in the dark at 4°C. At the end of incubation, each core was photographed to obtain a visual estimate of bioturbation depth. All cores were sieved to quantify benthic species richness and abundance (0.5-mm sieve, preserved in 70% ethanol). Two of the cores per site were sliced to estimate faunal depth distribution (top 3, 3–12, >12 cm), with the sections sieved separately. The remaining cores were sieved unsliced.
Benthic Community Structure and Bioturbation Potential
To provide an additional and more robust estimate of benthic species richness, abundance, and biomass, than gained from sieving of the above flux chambers (0.006 m2), benthic fauna were sampled at each site with a van Veen grab (0.1 m2, 3–5 replicate grabs per site, 1-mm sieve, preserved in 4% buffered formalin). To compare species and/or communities and their likely contribution to ecosystem function in terms of bioturbation, these data were also used to estimate the species-specific bioturbation potential at each site (Solan and others 2004):
where B 0.5i is the average individual biomass of a species at a site (in g wwt), square root transformed to linearize the effect of biomass, M i is the mobility of the species, and R i is the reworking mode of the species (both scored on a categorical 1–5 scale based on biological trait information, Solan and others 2004) (Appendix S2). Mobility was scored as 1 = grazers on the sediment surface, 2 = in a fixed tube, 3 = limited movement, 4 = slow movement through the sediment, and 5 = free movement via burrow system. Reworking was scored as 1 = epifauna bioturbating at the sediment–water interface, 2 = surficial modifiers, restricted to the top 1–2 cm of the sediment, 3 = head-down/head up feeders actively transporting sediment to/from the surface, 4 = biodiffusers causing a random diffusive transport of particles over short distances, and 5 = gallery diffusers. These species-specific data were further used together with abundance data to calculate the bioturbation potential of the whole benthic community (BPc) at each site.
Statistical Analysis
Non-metric multidimensional scaling (nMDS PRIMER 6, Clarke and Gorley 2006) on the Bray–Curtis similarity index, data square root transformed to down-weigh dominance, was used to illustrate area-specific patterns in abundance, biomass, and bioturbation potential (BPc) across the Baltic Sea (using data from van Veen grabs). Dummy species were included in the analyses to enable inclusion of anoxic sites with no fauna.
To explore differences in sediment oxygen consumption and nutrient fluxes between areas and the importance of oxygen conditions for the observed patterns, scales of spatial variability in flux measurements were analyzed using a mixed model and taking oxygen concentrations into account as a regulating factor. The potential for assumptions related to normality, independence, and homogeneity of variances was investigated. Examining the standard deviation among replicated flux measurements versus their means demonstrated that the variability of nutrient fluxes scaled with the mean, whereas variability of oxygen fluxes remained constant over the entire span of flux measurements. Consequently, nutrient fluxes were log-transformed using log10(x + c), where c = 1000 was chosen to ensure positive values for the transformation as some flux measurements were negative (that is, influx). The mixed model included three fixed effects and three random effects to describe variations in the transformed flux measurements (f(x); identity for oxygen and log10(x + c) for nutrients):
The fixed effects described the variation between sea areas (a i ; i denotes levels of sea area), a common linear dependency with the oxygen concentration (b 0 × o ijk ; b 0 is the slope), and sea area-specific linear relationships to oxygen (b i × o ijk ; b i are area-specific slopes). The random effects described the variation between sites within sea areas (S(a) j(i); j denotes levels of sites), variations in the oxygen dependency between sites (B j(i) × o ijk ; B j(i) are site-specific slopes), and variation between replicated flux measurements at a given site (e ijk ; k denotes the replications). The average oxygen concentration over the incubation period (o ijk ) was used in the mixed model. Interaction terms and main effects not significant at the α = 0.05 level were removed iteratively by backward-elimination and the model re-estimated until only significant effects were retained. Contrasts on parameter estimates were used to test differences between sea areas and significance of specific parameter estimates.
The importance of environmental factors (depth, oxygen content measured in the bottom water in the flux cores, and salinity and temperature derived from CTD measures at the site scale) and biological factors (species abundances, species biomass, and species bioturbation potential) in predicting oxygen and nutrient fluxes was investigated multivariately using the distance-based linear model option DistLM in PERMANOVA + for PRIMER (Anderson and others 2008). Flux data (NOx, NH4 +, PO4 3−, SiO4, O2) were normalized (that is, set to run between 0 and 1 to ensure equal importance of all fluxes) then used to construct a single matrix of dissimilarities between samples based on pairwise Euclidean distances. Two such matrixes were obtained, one based on all individual replicates and one on site means. Five sets of DistLM models were run, with predictors being environmental variables and either species-specific abundances (from (i) core replicates, (ii) core means per site, (iii) means per site based on van Veen grabs), (iv) biomass or (v) BPc. The different models using the core macrofaunal data (i and ii) as predictors were run to determine whether directly linking resident macrofauna to fluxes (i) or averaging the between-core variability of both fauna and fluxes (ii) increased explanatory power. The comparison of those results with the results including the van Veen abundances as predictors (iii) allowed for a broader description of macrofaunal communities and a focus on the abundance of larger organisms (>1 mm). The comparison between results from including the van Veen abundances versus biomasses as predictors indicates whether abundance estimates are sufficient or whether biomass needs to be incorporated when explaining fluxes. Finally, comparisons between results from including the van Veen abundances, biomasses, or the calculated BPc as predictors allowed us to assess whether BPc was a good surrogate across species for the effect of bioturbation activity on nutrient and oxygen fluxes. We also ran an analysis that included geographical location, in case any faunal predictive power we observed in the models was simply a surrogate for environmental conditions. This analysis revealed that “location” removed explanatory power as much from oxygen and salinity as it did from the fauna (~5% for fauna and oxygen, and ~6% for salinity), suggesting that faunal predictive power was not simply a surrogate for location, so analyses including location are not reported further.
Forward selection was used to develop models using AIC as the selection criterion. Given the potential overriding influence of oxygen on both fauna and fluxes, forward selection was utilized to ensure that oxygen effects on the fauna were first accounted for, before assessing the explanatory power of the fauna on nutrient fluxes. Because temperature and salinity were highly correlated (Pearson’s r = 0.97), the forward-selection procedure was not automated, and the order in which variables were introduced was varied to ensure collinearity did not drive the model results. Transformations were considered for all variables, but only proved necessary for BPc (Log10(BPc + 1)) and some van Veen abundances (Macoma balthica, Marenzelleria spp., Scoloplos armiger, and Bylgides sarsi).
In a meta-analysis of oxygen thresholds based on the physiological response of marine organisms, worldwide Vaquer-Sunyer and Duarte (2008) conclude that the commonly used 2 mg l−1 oxygen threshold for severe hypoxia is too low, because it only describes ‘dead zones’ and that the safe limit for well-functioning systems is likely to be around 4.6 mg l−1 (that is, 3.22 ml l−1, because 1 mg l−1 = 0.7 ml l−1), which was the 90th percentile median lethal concentration derived from the meta-analysis. To investigate the influence of oxygen on the benthic community, we divided sites into ‘good’ (O2 > 3.22 ml l−1) and ‘poor’ oxygen conditions. Note that site HAS was classified as ‘poor’ in the analyses, although the measured O2 concentration was 3.83 ml l−1. This site was thought to have experienced a recent injection of ‘new’ oxygenated water (potentially brought in by an internal wave) as the sediment was still laminated and there was virtually no macrofauna present. The site is close to the halocline and permanently hypoxic water. Only 4 of the 12 ‘good’ sites are located south of the Åland Islands (BY2, LF1, EGB1, GOF2). The DistLM models were then run separately on the ‘good’ and the ‘poor’ sites to determine how community composition and functional diversity above and below the 3.22 ml l−1 O2 threshold-influenced fluxes. One-way analysis of similarities (ANOSIM, PRIMER 6) was also used to determine the significance of differences in benthic community abundance and biomass between oxygen conditions.
Results
Environmental Variables
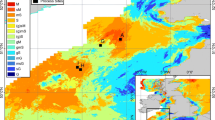
Both bottom-water salinity and temperature followed the natural decreasing gradient from south to north (Appendix S1). DO concentrations were generally low in the deeper parts of the Baltic Proper (Appendix S1; Figure 2) and there was no oxygen and no macroscopic life below 80–90 m depth. In most of the deeper areas, there was toxic H2S in the bottom water. Oxygen conditions were good (that is, above the 3.22 ml l−1 threshold defined by Vaquer-Sunyer and Duarte (2008) at the southernmost site (BY2), in the shallower parts of the Eastern Gotland Basin (EGB1, LF1) and Gulf of Finland (GOF2), and in the entire Gulf of Bothnia. These oxygen conditions were also mirrored in the bottom-water nutrient concentrations (Appendix S1), with higher PO4 3− and NH4 + concentrations at sites with low or no oxygen.
Bottom-water salinity, oxygen, and total number of species at the sites from south to north (see Figure 1 for site locations). The horizontal dotted line at 3.22 ml l−1 indicates the threshold between good and poor oxygen conditions used in the analyses. To provide a baseline for assessing the current status of the benthic communities, the infauna reference value for the average number of species in the different sea areas has also been plotted (Villnäs and Norkko 2011).
Benthic Fauna
The reference value for the average number of species in each sea area (Villnäs and Norkko 2011) follows the decreasing salinity gradient from south to north. The current status of the benthic communities in terms of species diversity was markedly below this reference value at almost all sites except the Bothnian Sea and Bothnia Bay, with the total number of species at each site closely following the bottom-water oxygen conditions (Figure 2). To further explore the current disturbance status at each site (‘history of hypoxic stress’), we related the number of species at each site to the infauna reference values for these specific sea areas (Villnäs and Norkko 2011). The resulting relationship (number of species divided by the infauna reference value) is independent of the natural diversity gradient in the Baltic, but was strongly influenced by the oxygen conditions at the site (linear regression, r 2 = 0.73, P < 0.0001).
The most abundant taxa were Marenzelleria spp. (most likely both M. neglecta and M. arctia), Macoma balthica, Monoporeia affinis, and Scoloplos armiger (Appendix S2). In terms of biomass, Macoma balthica dominated (biomass data not shown). Seven of the 26 sites had no fauna or very little fauna (on average less than one individual per van Veen grab), reconfirming the poor state of the benthic communities of the open Baltic Sea.
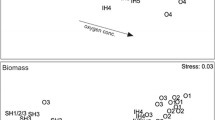
There was a clear gradient in community structure from south to north (Figure 3A, B), and a clear effect of oxygen conditions on both abundance and biomass within the sea areas. There were strong differences in benthic community abundance and biomass between good and poor oxygen conditions (that is, above and below the 3.22 ml l−1 threshold defined by Vaquer-Sunyer and Duarte (2008), ANOSIM, Abundance; Global R = 0.504, P < 0.001, Biomass: Global R = 0.354, P < 0.001). These differences in community structure translated into region-specific differences in the bioturbation potential (Figure 3C), suggesting differences in the potential contribution of benthic communities to ecosystem function.
Multidimensional Scaling (MDS) plot of community A abundance, B biomass, and C bioturbation potential at the sampling sites in the different sea areas (van Veen data). The areas separate along an axis from south (Southern Baltic Proper) to north (Gulf of Bothnia), whereas within areas sites are separated along gradients from poor to good oxygen conditions (that is, above and below the 3.22 ml l−1 threshold defined by Vaquer-Sunyer and Duarte (2008), except in the Gulf of Bothnia, where all oxygen conditions are good and the sites instead describe a north–south gradient.
Over 80% of the fauna was found in the top 3 cm of the sediment, indicating that the fauna through its bioturbation and bioirrigation has the potential to affect sediment nutrient dynamics and nutrient fluxes at the sediment–water interface. There was also a positive correlation between bioturbation potential and the visually assessed bioturbation depth (Pearson r = 0.67, P = 0.0002).
Nutrient Fluxes
Overall, the fluxes of oxygen and nutrients across the sediment–water interface varied markedly between sea areas and between sites within sea areas (Table 1) and were clearly influenced by bottom-water oxygen conditions, with generally higher efflux of PO4 3− and NH4 + at sites with low or no oxygen (Figure 4; Appendix S3). The NH4 + flux declined strongly with increasing oxygen concentrations and this relationship was general across all sea areas. The PO4 3− flux similarly decreased with increasing oxygen conditions, although only fluxes in the Gulf of Finland were significantly different from zero (P = 0.0001). NO2 − + NO3 − and SiO4 fluxes were not significantly related to oxygen, but significant differences between sea areas were found (Table 1). The highest influx of NO2 − + NO3 − and highest efflux of SiO4 were found in the Gulf of Finland, having fluxes about three times higher than in other sea areas (Figure 4). There was also a significant influx of NO2 − + NO3 − in the Southern Baltic (P = 0.0078), whereas in the Gotland Basin and Gulf of Finland NO2 − + NO3 − fluxes were not significantly different from zero (P = 0.3847 and P = 0.7045, respectively). Effluxes of SiO4 were significantly different from zero in all basins (P < 0.0001 for all) with similar rates in Gotland Basin, Gulf of Bothnia, and Southern Baltic (Figure 4). The influx (that is, consumption) of O2 naturally increased with oxygen concentrations in the bottom water, but the influx was largest in the Gotland Basin, followed by the Southern Baltic and lower, albeit similar (P = 0.4294), O2 fluxes in the Gulf of Bothnia and Gulf of Finland. For the nutrient fluxes, the variance between sites (on the log-scale) was 3–4 times larger than the variance between replicates.
Nutrient and oxygen fluxes versus oxygen concentrations in the bottom water for the different sea areas. Areas-specific relationships from the mixed models (Table 1) are shown as follows: Gotland Basin = thin solid line, Gulf of Bothnia = dotted line, Gulf of Finland = even dashed line, Southern Baltic = uneven dashed line. Thick solid line when a general relationship with oxygen was found (NH4 flux only). For nutrient fluxes, the estimated relationships were back-transformed using the results from the mixed models.
The total % variability in oxygen and nutrient flux explained by the predictors (depth, oxygen concentration, salinity, temperature, species-specific abundances) across all sites (DistLM analysis) was lower for the model containing individual core data (i) than site averages (ii) by ~15% (Table 2). The increase obtained was due to smoothing of both the flux and the faunal data, as both the physical factors measured at the site scale (oxygen and temperature) and fauna increased in their explanatory power (Table 2). Increasing the scale over which the fauna were measured from the core to the van Veen grab increased the total variance and faunal variance explained by 10% (Table 2). Using biomass instead decreased the total explanatory power by 4%.
Regardless of which measure of fauna was placed into the model, faunal measures were always selected as predictors (Table 2), contributing between 35 and 46% of the total explanatory power for abundance measures, 43% for biomass but only 9% when incorporated into the BPc. Interestingly, using BPc rather than individual faunal information resulted in a further decrease in explanatory power of 23%. Oxygen was always selected as a predictor and contributed 49–66% of the total explanatory power (see Table 2 for actual % explained). Salinity was also important but less so than any of the individual faunal data.
Comparisons between the results obtained from the classification into ‘poor’ and ‘good’ oxygen conditions revealed that the importance of factors for predicting flux variability between sites differed between the two conditions (Figure 5; Table 3). Surprisingly, even in poor oxygen conditions, faunal abundance was an important predictor of nutrient fluxes (2 species explaining 58% of the variation) and oxygen by itself did not explain much (6%). In good oxygen conditions, the effect of oxygen was not significant; fauna were the most important predictors (explaining 69% of the variation), with salinity and temperature being selected last and explaining a further 17%. Marenzelleria abundance was selected as a predictor for both poor and good oxygen conditions, and explained approximately the same amount of variation in both. Bylgides was a statistically significant predictor in poor but not in good conditions. Unsurprisingly, there were more species that were selected as statistically significant predictors in good conditions (Macoma, Mysis, and Pygospio).
Graphical representation by dbRDA of the relationships between important predictors (water column oxygen and salinity, average van Veen abundances) and Euclidean distances of the average nutrient and oxygen fluxes at the sites. The closer sites are to each other, the more similar are their fluxes. Arrows indicate the direction of the effect that increases in the variable has on fluxes and the arrow length indicates relative importance on the 2 axes. Squares are sites with good oxygen status, and triangles are sites with poor oxygen status (see text for definition).
Discussion
It is well known that hypoxia decimates benthic macrofauna communities and equally well known that rates and pathways of benthic nutrient cycling change when oxygen concentrations decline. However, the interaction between these two, that is, whether the decimated benthic community is able to affect nutrient cycling under hypoxic conditions is not well known. As highlighted by Snelgrove and others (2014), it is critical that we analyze how biodiversity–ecosystem function relationships change along environmental stress gradients, as empirical evidence is lagging very far behind theory in this regard. We utilized well-known gradients in diversity and community structure in the Baltic Sea to study the links between fauna and function in terms of benthic nutrient fluxes under different environmental conditions and levels of hypoxic stress. Thus, we encompassed the variability and gradients in biodiversity, trophic state in terms of nutrient loadings, and the disturbance gradients more or less across the entire Baltic Sea, that is, at a much larger spatial scale than previously studied. Some species occur in a range of different environments, but their contribution to ecosystem functioning may not be equally important under all conditions. As predicted, the benthic fauna was severely reduced in abundance and diversity by the more or less permanent hypoxia in all sea areas south of the Gulf of Bothnia, and spatial differences in nutrient fluxes were strongly associated with oxygen conditions as well as differences in faunal composition. Fauna significantly contributed to the variability in fluxes, in both good and poor oxygen conditions (that is, below the oxygen threshold of 3.22 ml l−1). Thus, contrary to predictions, it is probable that the fauna still affected nutrient fluxes even when the oxygen conditions had deteriorated, although the species important for driving the variation differed, and the influence of the fauna was lower under poor oxygen conditions.
Although species loss per se may not be the driver of changes in ecosystem functioning, the loss of certain previously abundant functional groups due to widespread hypoxia could have important implications for ecosystem functioning (Levin and others 2001; Norkko and others 2013). Severe hypoxia may decimate sensitive native species, which potentially may be replaced by more tolerant species, for example, the invasive polychaete Marenzelleria spp. In fact this deep-burrowing species had an important effect on nutrient flux both above and below the oxygen threshold. Bioturbation and bioirrigation by Marenzelleria spp. may significantly increase phosphorus retention in sediments and thus over time decrease the nutrient loading from the sediments (Norkko and others 2012). The spread of Marenzelleria spp. may also bring about a change in functioning because this polychaete buries settling phytodetritus deeper into the sediment, compared with, for example, shallow-dwelling Monoporeia affinis, which slows down decomposition and oxygen consumption (Josefson and others 2012). Our findings suggest that Marenzelleria may be significantly affecting sediment biogeochemistry and benthic fluxes as suggested by previous work (Josefson and others 2012; Norkko and others 2012) and that Marenzelleria may have a positive effect on ecosystem function with regard to oxygen-dependent biota. Although invasions often may have detrimental effects on the ecosystems (for example, Vitousek and others 1997), Marenzelleria in the present area seems to be an example of the opposite effect, acting as a “rescuer” in areas where native fauna has been eliminated due to deteriorating environmental conditions, such as low oxygen. Thus, in a low-diversity system such as the Baltic Sea, dominance by one or a few functionally important species may be more important than diversity (Chapin and others 1997; Norkko and others 2013). Also other studies suggest that dominance effects may be important. Allgeier and others (2015) compared the role of fish communities for biochemical processes in six coastal ecosystem types (incl. coral reefs) across the Northern Antilles, and found that although species richness is important, conserving the mean maximum fish biomass may be equally important for maintaining ecosystem functioning.
Despite the general concern regarding biodiversity loss and concurrent loss of ecosystem functionality, a central empirically unresolved question is how much of the change is (a) due to species loss, (b) a direct effect of environmental change, and (c) caused by the interaction between these two (Hillebrand and Matthiessen 2009). This also applies to the role of soft-sediment communities when environmental conditions deteriorate; evidence from real-world studies is still lacking. In this study, we adopted an observational approach and used existing gradients in diversity (salinity driven) and disturbance (eutrophication-induced hypoxia) at the scale of the entire Baltic Sea to investigate the context dependency of these BEF relationships and found that the potential role of benthic fauna changed with increasing hypoxia. Similarly, Hiddink and others (2009) studied BEF relationships in the northern Irish sea and found that the relationship between species richness and some (but not all) measures of ecosystem functioning (including nitrogen fluxes, depth of the RPD layer, macrofaunal biomass) was moderated by wave-induced shear stress, that is, a natural gradient of disturbance. In the Arabian Sea, oxygen minimum zone macrofauna affected organic matter processing also at very low oxygen concentrations (<0.16 ml l−1), but with differences among sites and with shifts in the species important at certain oxygen thresholds (Woulds and others 2007). Also the type of sediment affects BEF relationships. Braeckman and others (2014) studied sedimentary biogeochemical cycling at ten coastal stations in the Southern North Sea and found that functional biodiversity was more important in fine sandy sediments, compared to permeable and muddy sediments. This is logical given the higher diversity in these fine sandy sediments. Contrary to our findings, they found that the bioturbation potential was more useful than abundance or biomass for explaining fluxes (Braeckman and others 2014).
The role of biodiversity in the maintenance of ecosystem functioning may thus be context dependent across disturbance regimes, but the nature of this dependency differs between types of ecosystems and over time. There is an urgent need for empirical studies of these BEF relationships under a range of environmental conditions out in the real world. It is clear that benthic oxygen and nutrient fluxes will differ not only between sites but also between seasons, driven by temperature, macrofaunal diversity, and food availability. In addition, mineralization rates, diversity, and food supply will respond to, for example, climate change on different time scales, and temporal sampling of different types of sites is necessary to detect change, as shown for the Canadian Arctic Shelf (Link and others 2013).
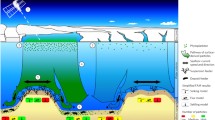
Soft-sediments play an important role in global biogeochemical cycles, for example, through functioning as either sinks or sources of carbon and other nutrients (burial vs. regeneration) (Wollast 1991). Many of the processes at the sediment–water interface are highly redox dependent and affected by sediment characteristics, including organic content and mineral content (for example, Fe oxides) (Jilbert and others 2011). In some areas, nutrients have accumulated in the sediments because of decades of excessive nutrient loading from land and organic enrichment, and under hypoxic conditions more phosphate and ammonia is released from the sediments (Mortimer 1941; Ingall and others 1993; Slomp and others 2002; Jäntti and Hietanen 2012). This is obvious, for example, in the Gulf of Finland. Fe oxide contents of the sediments vary, which may give different locations a higher or lower capability to either bind phosphate or to release it rapidly under hypoxia (Jilbert and others 2011). The mineral distribution is, however, not well mapped for the Baltic Sea. The nutrient cycling is thus affected by organic matter deposition, increasing hypoxia as well as the degree of macrofaunal activity, potentially through complex feedback mechanisms. For example, the high bioturbation potential of a healthy community will increase oxygen penetration and thus the capacity of sediments to bind more phosphate, which would reduce the flux of phosphate out of the sediment (Norkko and others 2012). Similarly, fauna can stimulate coupled nitrification–denitrification and thus removal of nitrogen from the system (Tuominen and others 1998).
The fact remains that real-world examples of how seafloor biodiversity affects ecosystem functioning, and its context dependency, are scarce. Although it may be difficult to assign causality in field studies, relevant field measurements involving natural communities are imperative (Villnäs and others 2012, 2013). We found that the variance in oxygen and nutrient fluxes explained increased with the scale over which the fauna were measured, from the core (0.006 m2) to the van Veen grab (0.1 m2), despite the smaller sieve size used for the cores (0.5 vs. 1.0 mm). The larger area encompassed contains a better representation of the whole benthic community, in particular less numerous animals with larger body size, and likely also of the ‘biogeochemical memory’ of the sediment (Quintana and others 2013), thus representing also the history of hypoxic stress and consequent changes in, for example, redox conditions in the sediment. Hiddink and others (2009) also call for research including more realistic conditions and varying levels of disturbance to advance our understanding of biodiversity–ecosystem functioning relationships. Although many of the mechanisms operating at different spatial and temporal scales need to be untangled, for further analyses of survey and monitoring data in the Baltic, we propose to use the relationship between total number of species found at each site and the infauna reference value for each sea area (Villnäs and Norkko 2011) to make a prediction about the strength of the effect of fauna on nutrient fluxes. Although the reference value is based on the average regional diversity, the measure does give an indication of the condition of the community: A low value, which is likely under hypoxic conditions, would imply a weaker faunal influence, although a high value would imply a stronger faunal influence on nutrient dynamics.
The exact feedback mechanisms between hypoxia, benthic macrofauna, and the internal nutrient loading from the sediments are still not known and abrupt changes in benthic communities are often observed. Predicting the thresholds or tipping points will thus be an important focus for future studies (Thrush and others 2009). The major management implication of our findings is that benthic macrofauna has the potential to influence nutrient cycling even in hypoxic sediments, but that nutrient loading from land needs to be reduced to such a low level that oxygen conditions improve and benthic fauna subsequently can recover and increase its positive role. As shown in a modeling study by Timmermann and others (2012), benthic biomass production can increase significantly if oxygen levels are increased not only to 2 mg l−1, but rather to 4 mg l−1 (equalling 1.4 and 2.8 ml l−1, respectively, since 1 mg l−1 = 0.7 ml l−1). This implies that the currently used oxygen target is too low. Under slightly better oxygen conditions the positive feedback on nutrient dynamics from the benthic communities would help overcome hysteresis and speed recovery of the ecosystem from hypoxic disturbance.
Through positive feedback, natural communities can help maintain a healthy ecosystem, by contributing to ecosystem functioning even as environmental conditions deteriorate.
Exploring the contribution of biodiversity, with an emphasis on species identities, functional traits, and dominance patterns, across gradients of disturbance and in different types of heterogeneous habitats is thus of central importance for understanding the context dependency of these relationships and for management purposes that are by definition broad scale.
References
Aller RC, Aller JY. 1998. The effect of biogenic irrigation intensity and solute exchange on diagenetic reaction rates in marine sediments. J Mar Res 56:905–36.
Allgeier JE, Layman CA, Mumby PJ, Rosemond AD. 2015. Biogeochemical implications of biodiversity and community structure across multiple coastal ecosystems. Ecol Monogr 85:117–32.
Anderson MJ, Gorley RN, Clarke KR. 2008. PERMANOVA + for PRIMER. Guide to software and statistical methods. Plymouth: PRIMER-E Ltd.
Berger WH, Smetacek VS, Wefer G. 1989. Ocean productivity and paleoproductivity—an overview. In: Berger WH, Smetacek VS, Wefer G, Eds. Productivity of the ocean: present and past. New York: Wiley. p 1–34.
Bick A, Burckhardt R. 1989. Erstnachweis von Marenzelleria viridis (Polychaeta, Spionidae) für den Ostseeraum, mit einem Bestimmungsschlüssel der Spioniden der Ostsee. Mitteilungen aus dem Zoologischen Museum in Berlin 65:237–47.
Blank M, Laine AO, Jürss K, Bastrop R. 2008. Molecular identification key based on PCR/RFLP for three polychaete sibling species of the genus Marenzelleria, and the species’ current distribution in the Baltic Sea. Helgol Mar Res 62:129–41.
Bonsdorff E, Pearson TH. 1999. Variation in the sublittoral macrozoobenthos of the Baltic Sea along environmental gradients: a functional-group approach. Aust J Ecol 24:312–26.
Braeckman U, Foshtomi MY, Van Gansbeke D, Meysman F, Soetaert K, Vincx M, Vanaverbeke J. 2014. Variable importance of macrofaunal functional biodiversity for biogeochemical cycling in temperate coastal sediments. Ecosystems 17:720–37.
Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava DS, Naeem S. 2012. Biodiversity loss and its impact on humanity. Nature 486:59–67.
Carstensen J, Conley DJ, Bonsdorff E, Gustafsson BG, Hietanen S, Janas U, Jilbert T, Maximov A, Norkko A, Norkko J, Reed DC, Slomp CP, Timmermann K, Voss M. 2014. Hypoxia in the Baltic Sea: biogeochemical cycles, benthic fauna and management. Ambio 43:26–36.
Chapin FSIII, Walker BH, Hobbs RJ, Hooper DU, Lawton JH, Sala OE, Tilman D. 1997. Biotic control over the functioning of ecosystems. Science 277:500–4.
Clarke KR, Gorley RN. 2006. PRIMER v6: user manual/tutorial. Plymouth: PRIMER-E.
Conley DJ, Björck S, Bonsdorff E, Carstensen J, Destouni G, Gustafsson BG, Hietanen S, Kortekaas M, Kuosa H, Meier HEM, Müller-Karulis B, Nordberg K, Norkko A, Nürnberg G, Pitkänen H, Rabalais NN, Rosenberg R, Savchuk OP, Slomp CP, Voss M, Wulff F, Zillén L. 2009. Hypoxia-related processes in the Baltic Sea. Environ Sci Technol 43:3412–20.
Dayton PK. 1971. Competition, disturbance and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr 41:351–89.
Diaz RJ, Rosenberg R. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321:926–9.
Dyson KE, Bulling MT, Solan M, Hernandez-Milian G, Raffaelli DG, White PCL, Paterson DM. 2007. Influence of macrofaunal assemblages and environmental heterogeneity on microphytobenthic production in experimental systems. Proc R Soc B 274:2547–54.
Glud RN. 2008. Oxygen dynamics of marine sediments. Mar Biol Res 4:243–89.
Godbold JA, Bulling MT, Solan M. 2011. Habitat structure mediates biodiversity effects on ecosystem properties. Proc R Soc B 278:2510–18.
Godbold JA, Solan M. 2009. Relative importance of biodiversity and the abiotic environment in mediating an ecosystem process. Mar Ecol Prog Ser 396:273–82.
Grall J, Chauvaud L. 2002. Marine eutrophication and benthos: the need for new approaches and concepts. Glob Change Biol 8:813–30.
HELCOM. 2009a. Biodiversity in the Baltic Sea—an integrated thematic assessment on biodiversity and nature conservation in the Baltic. Baltic Sea Environment Proceedings No. 116B.
HELCOM. 2009b. Eutrophication in the Baltic Sea—an integrated thematic assessment of the effects of nutrient enrichment and eutrophication in the Baltic Sea region. Baltic Sea Environment Proceedings No. 115B.
Hewitt JE, Thrush SF, Dayton PD. 2008. Habitat variation, species diversity and ecological functioning in a marine system. J Exp Mar Biol Ecol 366:116–22.
Hiddink JG, Davies TW, Perkins M, Machairopoulou M, Neill SP. 2009. Context dependency of relationships between biodiversity and ecosystem functioning is different for multiple ecosystem functions. Oikos 118:1892–900.
Hillebrand H, Matthiessen B. 2009. Biodiversity in a complex world: consolidation and progress in functional biodiversity research. Ecol Lett 12:1405–19.
Ingall ED, Bustin RM, Van Cappellen P. 1993. Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochim Cosmochim Acta 57:303–16.
Jäntti H, Hietanen S. 2012. The effects of hypoxia on sediment nitrogen cycling in the Baltic Sea. Ambio 41:161–9.
Jilbert T, Slomp CP, Gustafsson BG, Boer W. 2011. Beyond the Fe-P-redox connection: preferential regeneration of phosphorus from organic matter as a key control on Baltic Sea nutrient cycles. Biogeosciences 8:1699–720.
Josefson AB, Norkko J, Norkko A. 2012. Burial and decomposition of plant pigments in surface sediments of the Baltic Sea—role of oxygen and benthic fauna. Mar Ecol Prog Ser 455:33–49.
Karlson K, Bonsdorff E, Rosenberg R. 2007. The impact of benthic macrofauna for nutrient fluxes from Baltic Sea sediments. Ambio 36:161–7.
Karlson K, Rosenberg R, Bonsdorff E. 2002. Temporal and spatial large-scale effects of eutrophication and oxygen deficiency on benthic fauna in Scandinavian and Baltic waters—a review. Oceanogr Mar Biol Annu Rev 40:427–89.
Kauppi L, Norkko A, Norkko J. 2015. Large-scale species invasion into a low-diversity system: spatial and temporal distribution of the invasive polychaetes Marenzelleria spp. in the Baltic Sea. Biol Invasions 17:2055–74.
Kemp WM, Testa JM, Conley DJ, Gilbert D, Hagy JD. 2009. Temporal responses of coastal hypoxia to nutrient loading and physical controls. Biogeosciences 6:2985–3008.
Larsen TH, Williams NM, Kremen C. 2005. Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol Lett 8:538–47.
Levin LA, Boesch DF, Covich A, Dahm C, Erséus C, Ewel KC, Kneib RT, Moldenke A, Palmer MA, Snelgrove P, Strayer D, Weslawski JM. 2001. The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 4:430–51.
Levin LA, Ekau W, Gooday AJ, Jorissen F, Middelburg JJ, Naqvi SWA, Neira C, Rabalais NN, Zhang J. 2009. Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 6:2063–98.
Levinton J. 1995. Bioturbators as ecosystem engineers: control of the sediment fabric, inter-individual interactions, and material fluxes. In: Jones CG, Lawton JH, Eds. Linking species and ecosystems. New York: Chapman and Hall. p 29–38.
Link H, Piepenburg D, Archambault P. 2013. Are hotspots always hotspots? The relationship between diversity, resource and ecosystem functions in the Arctic. PLoS One 8:e74077.
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–8.
Lundberg C, Jakobsson B-M, Bonsdorff E. 2009. The spreading of eutrophication in the eastern coast of the Gulf of Bothnia, northern Baltic Sea—an analysis in time and space. Estuar Coast Shelf Sci 82:152–60.
Meysman FJR, Middelburg JJ, Heip CHR. 2006. Bioturbation: a fresh look at Darwin’s last idea. Trends Ecol Evol 21:688–95.
Mortimer CH. 1941. The exchange of dissolved substances between mud and water in lakes. J Ecol 29:280–329.
Norkko A, Hewitt JE, Thrush SF, Funnell GA. 2006. Conditional outcomes of facilitation by a habitat-modifying subtidal bivalve. Ecology 87:226–34.
Norkko A, Villnäs A, Norkko J, Valanko S, Pilditch CA. 2013. Size matters: implications of the loss of large individuals for ecosystem function. Sci Rep 3:2646.
Norkko J, Reed DC, Timmermann K, Norkko A, Gustafsson BG, Bonsdorff E, Slomp CP, Carstensen J, Conley DJ. 2012. A welcome can of worms? Hypoxia mitigation by an invasive species. Glob Change Biol 18:422–34.
Pearson TH, Rosenberg R. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr Mar Biol Annu Rev 16:229–311.
Polis GA, Holt RD, Menge BA, Winemiller KO. 1996. Time, space, and life history: influences on food webs. In: Polis GA, Winemiller KO, Eds. Food webs: integration of patterns and dynamics. New York: Chapman and Hall. p 435–60.
Quintana CO, Kristensen E, Valdemarsen T. 2013. Impact of the invasive polychaete Marenzelleria viridis on the biogeochemistry of sandy marine sediments. Biogeochemistry 115:95–109.
Rabalais NN, Díaz RJ, Levin LA, Turner RE, Gilbert D, Zhang J. 2010. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 7:585–619.
Reed DC, Slomp CP, Gustafsson BG. 2011. Sedimentary phosphorus dynamics and the evolution of bottom water hypoxia: a coupled benthic-pelagic model of a coastal system. Limnol Oceanogr 56:1075–92.
Rhoads DC. 1974. Organism-sediment relations on the muddy sea floor. Oceanogr Mar Biol Annu Rev 12:223–300.
Schiedek D. 1997. Marenzelleria cf. viridis (Polychaeta: Spionidae)—ecophysiological adaptations to a life in the coastal waters of the Baltic Sea. Aquat Ecol 31:199–210.
Slomp CP, Thomson J, de Lange GJ. 2002. Enhanced regeneration of phosphorus during formation of the most recent eastern Mediterranean sapropel (S1). Geochim Cosmochim Acta 66:1171–84.
Snelgrove PVR, Thrush SF, Wall DH, Norkko A. 2014. Real world biodiversity–ecosystem functioning: a seafloor perspective. Trends Ecol Evol 29:398–405.
Solan M, Cardinale BJ, Downing AL, Engelhardt KAM, Ruesink JL, Srivastava DS. 2004. Extinction and ecosystem function in the marine benthos. Science 306:1177–80.
Thrush SF, Hewitt JE, Dayton PD, Coco G, Lohrer AM, Norkko A, Norkko J, Chiantore M. 2009. Forecasting the limits of resilience: integrating empirical research with theory. Proc R Soc B 276:3209–17.
Timmermann K, Norkko J, Janas U, Norkko A, Gustafsson BG, Bonsdorff E. 2012. Modelling macrofaunal biomass in relation to hypoxia and nutrient loading. J Mar Syst 105:60–9.
Tuominen L, Heinänen A, Kuparinen J, Nielsen LP. 1998. Spatial and temporal variability of denitrification in the sediments of the northern Baltic Proper. Mar Ecol Prog Ser 172:13–24.
Vahtera E, Conley DJ, Gustafsson BG, Kuosa H, Pitkänen H, Savchuk OP, Tamminen T, Viitasalo M, Voss M, Wasmund N, Wulff F. 2007. Internal ecosystem feedbacks enhance nitrogen-fixing cyanobacteria blooms and complicate management in the Baltic Sea. Ambio 36:186–94.
Vaquer-Sunyer R, Duarte CM. 2008. Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci 105:15452–7.
Villnäs A, Norkko A. 2011. Benthic diversity gradients and shifting baselines: implications for assessing environmental status. Ecol Appl 21:2172–86.
Villnäs A, Norkko A, Norkko J, Lukkari K, Hewitt J. 2012. Impacts of increasing hypoxic disturbance on benthic biodiversity and ecosystem functioning. PLoS One 7:e44920.
Villnäs A, Norkko J, Hietanen S, Josefson AB, Lukkari K, Norkko A. 2013. The role of recurrent disturbances for ecosystem multifunctionality. Ecology 94:2275–87.
Vitousek PM, D’antonio CM, Loope LL, Rejmanek M, Westbrooks R. 1997. Introduced species: a significant component of human-caused global change. N Z J Ecol 21:1–16.
Volkenborn N, Polerecky L, Wethey DS, Woodin SA. 2010. Oscillatory porewater bioadvection in marine sediments induced by hydraulic activities of Arenicola marina. Limnol Oceanogr 55:1231–47.
Wollast R. 1991. The coastal organic carbon cycle: fluxes, sources, and sinks. In: Mantoura RFC, Martin JM, Wollast R, Eds. Ocean margin processes in global change. New York: Wiley. p 365–81.
Woulds C, Cowie GL, Levin LA, Andersson JH, Middelburg JJ, Vandewiele S, Lamont PA, Larkin KE, Gooday AJ, Schumacher S, Whitcraft C, Jeffreys RM, Schwartz M. 2007. Oxygen as a control on seafloor biological communities and their roles in sedimentary carbon cycling. Limnol Oceanogr 52:1698–709.
Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci 96:1463–8.
Acknowledgments
This study was funded by the Academy of Finland (project numbers 114076 and 110999), the BONUS + project HYPER, the DEVOTES project (EU Grant agreement no. 308392), the Walter and Andrée de Nottbeck Foundation (Senior Research Fellowship to JH), the Kone Foundation, and the University of Helsinki (3-year grant to JN). We thank the scientific crew on R/V Aranda for invaluable help with sampling and laboratory analyses (J Bruun, K Hyvärinen, S Hyvärinen, M Jaale, P Jämsen, P Kosloff, I Lastumäki, J Pitkänen, J Rapo, I Remonen, H Söderman, S Toijanaho, P Varmanen, J Ögård) and SF Thrush, A Villnäs, and two reviewers for constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
JN and AN designed the study; JN, JG, AN, and AJ conducted the field sampling; JG conducted laboratory analyses; JH, JN, AN, and JC conducted the numerical analyses; JN, AN, and JH wrote the manuscript; and all authors contributed to revisions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Norkko, J., Gammal, J., Hewitt, J.E. et al. Seafloor Ecosystem Function Relationships: In Situ Patterns of Change Across Gradients of Increasing Hypoxic Stress. Ecosystems 18, 1424–1439 (2015). https://doi.org/10.1007/s10021-015-9909-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-015-9909-2