Abstract
Without canopy-opening fire disturbances, shade-tolerant, fire-sensitive species like red maple (Acer rubrum L.) proliferate in many historically oak-dominated forests of the eastern U.S. Here, we evaluate potential implications of increased red maple dominance in upland oak forests of Kentucky on rates of leaf litter decomposition and nitrogen (N) cycling. Over 5 years, we evaluated mass loss of leaf litter and changes in total N and carbon (C) within six leaf litter treatments comprised of scarlet oak, chestnut oak, and red maple, and three mixed treatments of increasing red maple contribution to the leaf litter pool (25, 50, and 75% red maple). Over a 1.5-year period, we conducted a plot-level leaf litter manipulation study using the same treatments plus a control and assessed changes in net nitrification, ammonification, and N mineralization within leaf litter and upper (0–5 cm depth) mineral soil horizons. Red maple leaf litter contained more “fast” decomposing material and initially lost mass faster than either oak species. All litter treatments immobilized N during initial stages of decomposition, but the degree of immobilization decreased with decreasing red maple contribution. The leaf litter plot-level experiment confirmed slower N mineralization rates for red maple only plots compared to chestnut oak plots. As red maple increases, initial leaf litter decomposition rates will increase, leading to lower fuel loads and more N immobilization from the surrounding environment. These changes may reduce forest flammability and resource availability and promote red maple expansion and thereby the “mesophication” of eastern forests of the U.S.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In temperate deciduous forests of the eastern U.S., “multiple interacting ecosystem drivers” (McEwan and others 2011) including decades of fire suppression, climate change, and changes in herbivore populations have facilitated the proliferation of shade-tolerant, fire-intolerant species into historically oak-dominated stands (Abrams 1992). In many areas, the “super-generalist” red maple (Acer rubrum L.) dominates the seedling and sapling pool beneath the oak (Querus spp.) overstory (Abrams 1998). Oak seedlings, which have relatively high light requirements and a conservative growth strategy, require periodic disturbances to open the canopy and promote height growth (Abrams 1992). In an undisturbed understory, shade-tolerant, fast-growing species like red maple outcompete oaks (Lorimer 1984). Although overstory oaks still dominate stands in eastern forests, many researchers predict a compositional shift following mortality of the current canopy dominants in the absence of successful restoration attempts (Goins and others 2013). Numerous attempts have been made to restore fire to these forests and halt compositional changes, but results are highly site-specific and largely inconclusive (Arthur and others 2012).
If oak forests shift to those dominated by red maple or other shade-tolerant species, there will be numerous consequences for forest ecosystem function. Wildlife populations will be negatively impacted by the loss of a mast species (McShea 2000; Rodewald and Abrams 2002), and changes to leaf litter characteristics that influence forest pond habitats (Rubbo and Kiesecker 2004) and ground-nesting sites for songbirds (Fox and others 2010). Changes in bark and canopy structure will affect stemflow and throughfall inputs, thus forest hydrology and nutrient cycling (Alexander and Arthur 2010). Forest flammability, and our ability to remove fire-sensitive species via prescribed fire, will decrease because of differences in leaf litter morphology and chemistry that render red maple leaf litter less flammable than that of oaks (Kreye and others 2013). As fires become less and less frequent, and fire-sensitive species grow to maturity, these forests may reach an irreversible state (that is, “tipping point”), where restoration via prescribed fire or other stand management techniques is impossible (Abrams 2005; Nowacki and Abrams 2008). Thus, understanding the influence of rapidly proliferating species like red maple on ecosystem structure and function is an essential part of predicting forest change under alternative states.
The primary objective of this research was to assess the impacts of increased red maple abundance in historically oak-dominated forests of the eastern U.S. on long-term (5 years) decomposition rates using a litter bag study and changes in forest floor nitrogen (N) cycling via a plot-level experimental manipulation of leaf litter species composition. We were interested in how increased red maple contribution to the leaf litter pool influenced decomposition because this is the major pathway by which organic material is removed from the ecosystem and nutrients are recycled; thus, changes in decomposition associated with shifts in forest composition can have cascading effects on forest floor fuel loads (Brooks and others 2004), C accumulation and storage (Cornwell and others 2008), and N availability (Ashton and others 2005; Hickman and others 2013).
We hypothesized that as red maple contribution to leaf litter increases, rates of decomposition and N cycling would increase due to presumably higher-quality leaf litter of red maple compared to that of two dominant oak species, chestnut (Q. montana Willd.) and scarlet oak (Q. coccinea Münchh.). We expected that there would be “non-additive” effects (Gartner and Cardon 2004) of adding red maple leaf litter to oak leaf litter that may reflect changes in the physical, chemical, or microbial characteristics of combined litter types compared to single litter types (Chapman and Newman 2010). Based on findings from this and related studies (Alexander and Arthur 2010; Kreye and others 2013), we constructed conceptual models illustrating the various mechanisms by which increased red maple dominance could influence forest flammability and resource availability, creating a positive feedback to its own growth and continued “mesophication” (Nowacki and Abrams 2008) of forests of the eastern U.S.
Methods
Study Area
Studies were conducted on Klaber (37°51′N, 83°37′W) and Whittleton (37°46′N, 83°39′W) Ridges in Menifee and Powell Counties of eastern Kentucky. These ridges were selected because of their ease of access and because extensive datasets exist for the area (Arthur and others 1998; Blankenship and Arthur 1999, 2006; Kuddes-Fischer and Arthur 2002; Alexander and Arthur 2010). Both ridges are non-contiguous and located in the Cumberland Ranger District of the Daniel Boone National Forest (DBNF) which is part of the Red River Gorge Geological Area, Cliff Section of the Cumberland Plateau (37°46′N, 83°39′W).
Ridgetops are xeric and consist of a diverse assemblage of tree species. Oaks [scarlet oak (Q. coccinea Münchh.), chestnut oak (Q. montana Willd.), white oak (Q. alba L.), and black oak (Q. velutina Lam.)] dominate the overstory, whereas red maple (Acer rubrum L.) dominates the midstory (Blankenship and Arthur 2006). Oak seedlings are dominated by chestnut and scarlet oak (1,800 and 900 stems ha−1, respectively). However, red maple seedling density is considerably greater (10,675 stems ha−1) (Green and others 2010). Pine species are also common and consist mostly of shortleaf pine (Pinus echinata Mill.) and pitch pine (P. rigida Mill.). Other occasional species include eastern white pine (P. strobus L.), blackgum (Nyssa sylvatica Marsh.), bigleaf magnolia (Magnolia macrophylla Michx.), eastern hemlock [Tsuga canadensis (L.) Carr.], sassafras [Sassafras albidum (Nutt.) Nees], and sourwood [Oxydendron arboreum (L.) DC.].
Soils are silt loam, moderately deep, well-drained and generally acidic (Hayes 1993). Climate is temperate, humid, and continental, with mean annual temperature of 12°C and annual precipitation (113 cm y−1) fairly well distributed throughout the year (Foster and Conner 2001). Study areas on each ridge encompassed about 15 ha with similar conditions in slope (<20% grade) and elevation (~400 m). These areas had minimum human influence and no recent fires (>20 year).
Leaf Litter Decomposition
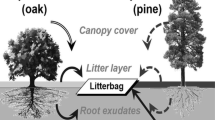
To determine if increases in red maple leaf litter alter decomposition rates due to species-specific litter differences, we implemented a traditional decomposition bag study. Treatments included leaf litter assemblages of the following: (1) chestnut oak (CO) only, (2) scarlet oak (SO) only, (3) red maple (RM) only, (4) RM (75%) + SO (12.5%) + CO (12.5%), (5) RM (50%) + SO (25%) + CO (25%), and (6) RM (25%) + SO (37.5%) + CO (37.5%). In September 2006, leaf litter collection nets (1 cm2 mesh) were suspended about 1 m above the forest floor at approximately 20 locations across both Klaber and Whittleton Ridges. Litter was collected weekly throughout leaf fall (November 2006). Fresh litter was immediately returned to the laboratory, air dried in cardboard boxes, and sorted by species. A total of 10 g (air-dry weight) of litter from each treatment was placed into a 20 × 20 cm plastic bag with a 1 × 2 mm mesh size (large enough to allow entry of small soil fauna and permit aerobic microbial activity) (Melillo and others 1982), tightly sealed, and labeled with a unique identification number.
Bags were installed in five blocks (~7.5 m radius, ~177 m2 area) located within each of Klaber and Whittleton Ridges, resulting in a randomized complete block experimental design nested within ridge (n = 10). In total, the sampling design consisted of 480 bags (2 ridges, 5 blocks, 6 treatments, 8 pick-ups). Blocks had similar red maple and oak litterfall and overstory influence (determined using basal area and leaf litter input data obtained along these ridges in summer 2005). Prior to installation, the Oi layer (that is, intact leaf litter) was carefully removed, and bags were arrayed at random in a grid on top of the Oe layer about 20 cm apart and at least 0.5 m distant from a tree bole. Bags were secured to the forest floor with metal pins, and their locations mapped to facilitate re-location. One bag per block was removed at time 0 (spring 2007), and then again at 3, 6, 9, 12, 18, 24, and 60 months (spring 2012) afterwards. Upon removal from the field, original leaf litter contents of each bag were returned to the laboratory, cleaned of external debris and invertebrates, dried at 60°C for 48 h, and weighed. Leaf litter decomposition rates were calculated using both single (Olson 1963) and double (Wider and Lang 1982) exponential decay models. The single decay model is as follows:
where M 0 is the absolute dry weight of litter remaining at time 0, M t is the absolute dry weight of litter remaining at time t, t is the time of incubation (in years), k is the decomposition rate constant. The double decay model is as follows:
where M 0, M t , and t represent the same values as above, M 1 and M 2 represent the proportion of labile and recalcitrant litter (thus, M 1 + M 2 = 1), respectively, and k 1 and k 2 are decay constants for each pool.
To determine the C and N content of each leaf litter sample on each sample date, a 5 g subsample was ground to a fine powder and analyzed using a Leco CN 2000 Analyzer (Leco Corporation, St. Joseph, Michigan, USA). For each sample date, the percent of C and N remaining in relation to initial values was calculated as follows:
where C t0 and C t1 are proportion of C in the litter sample at time = 0 and on a given sampling date, and mass t0 and mass t1 are the dry weights of the litter sample at those times. Initial leaf litter lignin concentrations were determined using an Ankom fiber digester (Ankom Technology, Macedon, New York, USA).
Leaf Litter Effects on N Dynamics
We assessed the effects of increasing red maple leaf litter on soil N dynamics by manually manipulating leaf litter composition in the field. We used the same experimental design and leaf litter treatments described above for the decomposition experiment, with the addition of an unmanipulated control. In late August 2007, seven 1 m2 plots were delineated within each block adjacent to the leaf litter bag arrays and assigned at random to a leaf litter treatment or unmanipulated control. Plots were separated by at least 0.5 m and were placed more than 1 m from a tree bole. Vegetation within each plot was manually clipped to ground level and maintained in this state throughout the study to minimize any confounding effects due to nutrient uptake or input via plants. The Oi layer was removed by hand, and each plot was covered with 400 g (air-dry weight) of its randomly assigned litter treatment. This amount was based on an average pre-determined amount of litter received during litter fall in areas devoid of tree boles larger than 2 cm DBH within a 2 m radius. Plots were then covered with 1-cm2 square plastic mesh to prevent additional leaf litter entry but allow rainfall passage; mesh was adhered to the ground with landscape pins. During litter fall, plots were regularly cleared of outside litter.
After the litter manipulation plots were installed (August 2007), plots remained untouched for 9 months to allow decomposers time to begin utilizing the litter. At 9 months (April 2008), 11 months (June 2008), and 13 months (August 2008) afterwards, in situ N mineralization incubations were performed using the buried bag method (Smith and others 1977). In each plot, we collected four soil samples using a 4-cm diameter PVC corer. Any obvious sticks and/or invertebrates were removed from the leaf litter/organic horizons, and then this horizon and the upper mineral soil (0–5 cm) horizon were placed in separate polyethylene bags. Because we were interested in N mineralization rates in their natural in situ environment, we did not remove roots prior to incubations. Two replicates for each soil horizon were returned to the laboratory for analysis of initial inorganic N concentrations (NH4 +-N and NO3 −-N; μg N g−1 dry soil). In situ net N mineralization was determined by burying two mineral soil samples per plot at 5-cm depth below the Oa horizon and by placing two leaf litter/organic horizon samples per plot on top of the forest floor. Samples were left in place for 28 days. All soil samples were stored at 4°C and processed within 1 day of collection upon return to the laboratory. In the lab, initial and incubated leaf litter/O horizon samples were homogenized by hand, and mineral soils were sieved through a 2-mm mesh sieve to homogenize the sample and remove large rocks, debris, and root material. Removing this material allowed results to be standardized on a dry soil basis. A 10 g subsample was placed in extraction cups with 50 ml of 1 mol/l KCl and shaken for 1 h. The solution was filtered through No. 40 Whatman paper, and the supernatant analyzed colorimetrically for available inorganic N (NH4 +-N and NO3 −-N) with a Bran Luebbe Autoanalyzer III. Net ammonification and nitrification rates were calculated as the total NH4 +-N and NO3 −-N (μg N g−1 soil day−1), respectively, accumulated over the 28-day incubation period (final − initial concentration) divided by number of incubation days. Final and initial concentrations were derived from the means of the two soil samples taken from each soil horizon.
Statistical Analyses
Differences in leaf litter mass, C, and N remaining and C:N ratios among leaf litter treatments were analyzed as a two-way ANOVA with repeated measures (treatment = repeated unit) within a randomized complete block design, with treatment and time as fixed effects and ridge and block nested within ridge as random factors using PROC MIXED of SAS v. 9.2. Data from the initial retrieval (time = 0) were removed from the analysis of mass, C, and N remaining since at time = 0 the mean percent remaining is 100.0 for all treatments (Wider and Lang 1982).
Decay constants for the single and double exponential models were calculated using two approaches. First, an overall model was fit to the raw data of proportion mass remaining for each treatment across the blocks using both PROC NLMIXED of SAS v. 9.2 and SigmaPlot v. 12. This allowed computation of k values, proportion of labile and recalcitrant pools, AIC values for model comparisons (single vs. double exponential), R 2, and P values. Second, to determine if k values were significantly different among leaf litter treatments, individual models were fit to each treatment in each block to obtain a k value for each block. These numbers were used in an ANOVA with the same experimental design as described above to compare k values among treatments. k values obtained via both approaches were identical.
We calculated predicted mass loss rates of litter mixtures for each retrieval time using actual values of mass loss for monocultures and percent of litter of each species within mixtures at the start of the study. We compared predicted and actual mass loss using paired t tests for each mixture and retrieval date. Differences in initial litter lignin, %C, %N, lignin:N, C:N ratios, and N mineralization rates were compared using a one-way ANOVA (fixed effect = litter treatment) and the same experimental design as described above.
All variables were tested for normality and homogeneity of variance prior to analyses and were transformed using logarithmic or square-root transformations when they did not meet these underlying assumptions. F values were computed based on Type III sums of square, and degrees of freedom were estimated using Satterthwaite’s approximation. For all significant factors and interactions (P < 0.05), least squares means were compared via a post-hoc Fischer’s LSD test to determine differences among means at α = 0.05. All P values reported are those generated from transformed data, but means and standard errors are presented for untransformed data.
Results
Initial Litter Chemistry
The initial chemistry of red maple leaf litter was distinct from that of both chestnut and scarlet oak (Table 1). Lignin concentration was significantly lower in red maple leaf litter (10.57 ± 0.35%) compared to that of chestnut (19.91 ± 1.14%) and scarlet (14.75 ± 0.68%) oak leaf litters. Nitrogen concentration was lowest in red maple leaf litter (0.43 ± 0.03%) compared to all other treatments. Lignin:N was highest in chestnut oak litter (36.29 ± 2.30) and lowest in red maple leaf litter (25.97 ± 2.37), but lignin:N concentration was not significantly different between red maple and scarlet oak (28.18 ± 1.80) leaf litter. Leaf litter C:N ratios were relatively high for all litter treatments (>89), but were highest for red maple leaf litter (116.37 ± 9.72).
Mass Loss
Initial rates of mass loss increased with increasing red maple contribution to the leaf litter pool (Figure 1A; Table 2). Only 56% of red maple leaf litter mass remained after 1 year, which was significantly lower than chestnut oak (74% remaining; P < 0.0001), scarlet oak (69% remaining; P < 0.0001), and all other litter treatments (61–68%; P < 0.05). From 24 to 60 months, percent mass remaining of all treatments was similar. As expected, the amount of C remaining mirrored trends in mass remaining (Figure 1B). Mass remaining of treatments containing leaf litter mixtures did not differ (P > 0.05 for all comparisons) from that predicted using mass loss rates of individual species (not shown).
A double exponential decay model predicted mass loss better than a single exponential decay model for each leaf litter treatment except chestnut oak, but the double exponential model provided only a marginally better fit (ΔAICc ~5–10) for scarlet oak and 25% red maple leaf litter (Table 2). This suggests that leaf litter treatments generally underwent two phases of decomposition during the 5-year study period, an initial “fast” phase due to decomposition of labile material and subsequent “slow” phase due to decomposition of recalcitrant material. Red maple, 75% red maple, and 50% red maple leaf litter treatments had 23–28% leaf litter in the labile pool. The labile pool of the red maple treatment decomposed the fastest (k = 3.18) and was significantly higher than that of scarlet oak and 25% red maple. A double exponential model could not be fit to mass loss of chestnut oak leaf litter, which suggests that all of its litter is in the recalcitrant pool. Most of scarlet oak (88%) and 25% red maple (82%) leaf litter were in the recalcitrant pool.
N Remaining and C:N
Across all treatments, percent N remaining increased during the first 18 months, suggesting immobilization; however, this increase was substantially and significantly greater for red maple leaf litter compared to other litter treatments (Figure 1C). At 18 months, red maple leaf litter had 173% N remaining compared to only about 132% remaining for other leaf litter treatments (P < 0.001 for all comparisons). Amount of N gained from time = 0 to that at peak immobilization was 1.4–1.5 times higher for red maple (2.4 ± 0.3 mg N g−1 dry wt litter) compared to chestnut (1.7 ± 0.3 mg N g−1 dry wt litter) and scarlet (1.6 ± 0.3 g N g−1 dry wt litter) oak. From 24 to 60 months, %N remaining declined across all treatments but did not drop below original values until 60 months. This decline was most pronounced for chestnut and scarlet oak (with only ~60 %N remaining at 60 months), whereas red maple still had 92% of N remaining at study cessation. Values at 60 months were significantly lower (P < 0.0001) than original values for all treatments. C:N ratios declined with time for all treatments (Figure 1D), but the decline was greatest for red maple leaf litter, which had an initial C:N of 116.37 ± 9.72 (significantly higher than other treatments) and a final value of 21.43 ± 0.72, similar to the other species and species mixes.
N Cycling Following Leaf Litter Manipulation
Leaf litter manipulation plots with red maple leaf litter tended to have lower rates of N mineralization within the leaf litter/organic horizon compared to controls, and at times, lower rates than chestnut and/or scarlet oak (Figure 2). Treatment differences were driven by differences in ammonification rates, as nitrification rates never differed significantly among treatments, and the magnitude of these differences varied across the growing season. In spring, red maple leaf litter plots had lower N mineralization rates (0.70 ± 0.29 μg-N g−1 dry soil day−1) than control plots (2.22 ± 0.49 μg-N g−1 dry soil day−1; P = 0.001) and marginally lower rates than chestnut oak (1.59 ± 0.27 μg-N g−1 dry soil day−1; P = 0.055) plots. In mid-summer, both red maple and 75% red maple plots had lower N mineralization rates (1.32 ± 0.27 and 1.36 ± 0.34 μg-N g−1 dry soil day−1) more than 2× slower than control, scarlet oak, and 25% red maple plots. In late summer, red maple N mineralization (−0.17 ± 0.24 μg-N g−1 dry soil day−1) was substantially less than all other treatments, but there were no significant differences among treatment plots (P = 0.32). N cycling in the upper mineral soils varied by season, but leaf litter treatment had no significant effect on nitrification, ammonification, or N mineralization (Figure 3).
Net nitrification, ammonification, and N mineralization for organic material underneath various litter treatments during three separate in situ (28 days) buried bag incubations throughout spring and summer 2008 in the Daniel Boone National Forest, Kentucky. Different letters indicate significant differences among leaf litter types for a given parameter.
Net nitrification, ammonification, and N mineralization for upper mineral soils (0–5 cm) underneath various litter treatments during three separate in situ (28 days) buried bag incubations throughout spring and summer 2008 in the Daniel Boone National Forest, Kentucky. Different letters indicate significant differences among leaf litter types for a given parameter.
Discussion
Our findings suggest that increased red maple leaf litter contribution to eastern forests of the U.S. will lead to faster initial rates of decomposition, more N immobilization, and less leaf litter on the forest floor. In the first 2 years of decomposition, decomposition rates increased with increasing red maple leaf litter contribution. Higher initial decomposition rates in red maple leaf litter compared to other treatments appeared to reflect higher levels of fast-decomposing, labile C fractions. Although red maple leaf litter had higher concentration of total C and higher C:N ratio, there was less lignin, a lower lignin:N ratio, and a rapid decrease in C:N ratios within the first 3 months. These characteristics indicate that most C in red maple leaf litter is labile. Despite these differences in initial chemistry, adding red maple leaf litter to oak leaf litter did not have “non-additive” effects on mass loss, which suggests that consequences of this shift on decomposition rates should be predictable from studies of individual species (Ball and others 2008).
Because new leaf litter inputs occur annually, differences in initial rates of leaf litter decomposition between oaks and red maple could dramatically alter leaf litter accumulation on the forest floor. For example, if leaf litter inputs remained constant (~400 g m−2 y−1) over a 5-year period, and mass loss rates of new and old leaf litter were considered, approximately 130 g m−2 less leaf litter would accumulate on the forest floor beneath a red maple forest than beneath one comprised of 25% red maple and a mixture of chestnut and scarlet oak (that is, similar to today’s forests at our study sites). If leaf litter inputs were to increase with increasing red maple dominance, changes in leaf litter accumulation would be even more pronounced.
During the first 2 years of decomposition, all leaf litter treatments immobilized N, but the degree of immobilization was substantially higher in red maple leaf litter and decreased with decreasing red maple contribution. Red maple leaf litter had the lowest initial N concentration and the greatest increase in N remaining during the 5-year study, a pattern commonly found across ecosystems regardless of climate (Parton and others 2007). Most decomposition in terrestrial ecosystems is accomplished by fungi (de Boer and others 2005), whose C:N ratio is typically 14:1 (Perry and others 2008), which is considerably lower than that observed for red maple leaf litter (~116:1). Thus, decomposers must acquire N from the surrounding environment to utilize carbon from decomposing litter and meet their growth requirements (Boberg and others 2014). Ball and others (2008), also working in Coweeta, North Carolina, measured lower initial N content in red maple leaf litter compared to chestnut oak and higher rates of N immobilization. In this study, most immobilization occurred during the first 2 years, when labile, “fast-decomposing” C pools were likely most available. Blair (1988), working in Coweeta, North Carolina, also found red maple to have a higher concentration of labile (that is, water-soluble) fractions in leaf litter (47.8%) compared to chestnut oak (25.2%), which also coincided with higher rates of N immobilization through the second year of decomposition. The finding that red maple immobilizes N despite having a lower lignin:N ratio than either oak supports the idea that litter traits like fraction of labile C and N concentration influence patterns of N cycling (Prescott 2010).
Initial C:N and lignin:N values for red maple found in this study differ from those reported in other studies, largely because N concentration of red maple leaf litter in our stands (0.43%) was often half of that reported in other studies (Table 3). Of the studies reporting red maple leaf litter N concentration, values ranged from a low of 0.43% (this study) to a high of 1.0% in Tunk Mountain, Maine. The most similar values were reported by Washburn and Arthur (2003) working on a nearby stand and by Blair and Crossley (1988) working in North Carolina, also in the Central Appalachia hardwood region on a ridgetop site. Differences in leaf litter N concentration within and among study sites may reflect differences in N inputs to the system associated with N deposition or differences in site quality (for example, soil temperature, moisture) that affect the ability of microbes to break down organic material and make N biologically available.
In studies where both red maple and an oak species were evaluated from the same site, leaf litter N concentration was always lower in red maple litter than that of oaks. This corresponds to previous studies which have found that foliar N concentration is lower in red maple than in co-occurring species (Abrams 1998). Red maple has been shown to have low foliar N requirements and less N for growth than many other trees from the eastern U.S. (Abrams 1998).
The leaf litter plot-level manipulation experiment confirmed the idea that red maple leaf litter immobilizes more N from the environment than other leaf litter treatments. Although not as obvious as in the decomposition study, N mineralization generally was slowest for red maple only plots compared to those with chestnut oak. Differences among treatments were probably not as obvious as those found in the decomposition study because samples contained leaf litter mixed with remnant Oe and Oa layers, and even a small amount of material with a higher lignin:N ratio can lower N mineralization rates (Finzi and Canham 1998). Slower N mineralization rates underneath red maple compared to chestnut oak were also measured by Washburn and Arthur (2003) working on nearby study sites. This finding contrasts with that of Finzi and others (1998) who measured higher N mineralization rates in organic soils underneath red maple in Connecticut compared to northern red oak (Q. rubra L.) and American beech (Fagus grandifolia Erhr.). In their study, the forest floor underneath red maple had a lower C:N ratio compared to other treatments.
The absence of a litter treatment effect on mineral soil N cycling likely occurred because of the short-term nature of this study (<1.5 year) and/or because of soil characteristics that limit vertical transfer of leaf litter-derived material. Although a previous study of coppice poplars (Populus x euramericana) in Central Italy showed that organic C compounds from leaf litter leachates or microbial exudates can be translocated into underlying soils within relatively short periods (<1 year) (Rubino and others 2010), work in temperate forests similar to those in this study showed no effect of recent (<4 years old) leaf litter inputs on C transport to mineral soils (Kramer and others 2010). The efficiency of vertical transport of C and nutrients to mineral soils depends on a variety of other factors, such as soil texture and other physical and chemical properties of the soil (Cotrufo and others 2013). Thus, the extent to which vertical transport of leaf litter-derived materials affects microbial processes like N mineralization likely varies across forest types and over time.
Implications
Red maple has key characteristics that may alter ecosystem function of previously oak-dominated ecosystems of the eastern U.S. and reinforce its continued propagation via a variety of feedback mechanisms (Figure 4). Findings from this study and that of Blair and Crossley (1988) suggest that leaf litter of red maple decomposes faster than that of oak. Thus, as red maple contribution to the leaf litter pool increases, higher rates of litter decomposition will lead to reduced fine fuels on the forest floor. Fires in eastern forests are often surface fires that spread by consumption of the leaf litter layer, so less litter on the forest floor will inevitably reduce forest flammability. Red maple leaf litter also tends to absorb more moisture and dry slower than oak litter, so the likelihood of fires igniting will decrease (Kreye and others 2013). Red maples may further decrease forest flammability through large inputs of stemflow near their boles which could create zones of high moisture and low fuel bed continuity (Alexander and Arthur 2010). Because red maple is less fire tolerant than oaks, these various characteristics that reduce forest flammability may serve as positive feedbacks to increase red maple proliferation (Figure 4A).
Conceptual models depicting mechanisms by which decreased fire activity and increased red maple abundance could influence (A) forest flammability and (B) resource availability, potentially creating a positive feedback to red maple growth and survival, reduced fire across the landscape, and continued “mesophication” of eastern forests. Dotted lines indicate mechanisms measured in this and a related study (Alexander and Arthur 2010).
Another mechanism by which red maple could alter ecosystem function and promote its own dominance is by altering resource availability (Figure 4B). Red maples have wider canopies than co-occuring oaks with greater canopy volume, which restricts throughfall inputs of water and nutrients to plants growing on the underlying forest floor (Alexander and Arthur 2010). In regions where red maple leaf litter has a high fraction of labile C and low lignin concentration, such as that found in this study, N immobilization in the forest floor will also decrease N availability for plant uptake. Red maples have high drought tolerance and low foliar nutrient requirements (Abrams 1998); thus, red maple seedlings/saplings distributed across the forest floor will likely be less impacted by reduced resources than co-occurring species with higher resource requirements. Decreased water or nutrient availability to the forest floor may also be inconsequential for mature red maple trees, which funnel 2–3× more nutrient-rich stemflow to a narrow zone surrounding the bole than either chestnut or scarlet oak and have higher N mineralization rates in a localized area near the stem (Alexander and Arthur 2010). For trees without a high funneling capacity and for small plants with high N requirements or low nutrient use efficiency, decreased N availability resulting from increased red maple abundance could further limit growth on sites already low in nutrient availability. Again, if these changes in the amount and distribution of resources favor red maple, a positive feedback to reduced forest flammability and increased red maple dominance will occur because of red maple’s “mesophying” properties.
The results of this and previous studies clearly indicate that red maple is unlike co-occurring oak species in many ways. Red maple can influence its surrounding environment via a suite of mechanisms with different effects across the forest setting. Decreased fuel loads and higher fuel moisture associated with increased red maple cover could decrease forest flammability, whereas decreased N availability could hinder growth of plants with higher N requirements than red maples. All these changes could feed back to exacerbate red maple proliferation and the “mesophication” of eastern forests.
References
Aber JD, Melillo JM, McClaugherty CA. 1990. Predicting long-term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Can J Bot 68:2201–8.
Abrams MD. 1992. Fire and the development of oak forests. BioScience 42:346–53.
Abrams MD. 1998. The red maple paradox. BioScience 48:355–64.
Abrams MD. 2005. Prescribing fire in eastern oak forests: is time running out? North J Appl For 22:190–6.
Alexander HD, Arthur MA. 2010. Implications of a predicted shift from upland oaks to red maple on forest hydrology and nutrient availability. Can J For Res 40:716–26.
Arthur MA, Alexander HD, Dey DC, Schweitzer CJ, Loftis DL. 2012. Refining the oak-fire hypothesis for management of oak-dominated forests of the eastern United States. J For 110:257–66.
Arthur MA, Paratley RD, Blankenship BA. 1998. Single and repeated fires affect survival and regeneration of woody and herbaceous species in an oak-pine forest. J Torrey Bot Soc 125:225–36.
Ashton IW, Hyatt LA, Howe KM, Gurevitch J, Lerdau MT. 2005. Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecol Appl 15:1263–72.
Ball BA, Hunter MD, Kominoski JS, Swan CM, Bradford MA. 2008. Consequences of non-random species loss for decomposition dynamics: experimental evidence for additive and non-additive effects. J Ecol 96:303–13.
Blair JM, Crossley DA Jr. 1988. Litter decomposition, nitrogen dynamics and litter microarthropods in a Southern Appalachian Hardwood Forest 8 years following clearcutting. J Appl Ecol 25:683–98.
Blair JM. 1988. Nutrient release from decomposing foliar litter of three tree species with spicial reference to calcium, magnesium and potassium dynamics. Plant Soil 110:49–55.
Blankenship BA, Arthur MA. 1999. Prescribed fire affects eastern white pine recruitment and survival on eastern Kentucky ridgetops. South J Appl For 23:144–50.
Blankenship BA, Arthur MA. 2006. Stand structure over 9 years in burned and fire-excluded oak stands on the Cumberland Plateau, Kentucky. For Ecol Manag 225:134–45.
Boberg JB, Finlay RD, Stenlid J, Ekblad A, Lindahl BD. 2014. Nitrogen and carbon reallocation in fungal mycelia during decomposition of boreal forest litter. PLoS One 9:e92897.
Brooks ML, D’Antonio CM, Richardson DM, Grace JB, Keeley JE, DiTomaso JM, Hobbs RJ, Pellant M, Pyke D. 2004. Effects of invasive alien plants on fire regimes. BioScience 54:677–88.
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF. 2000. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–65.
Chapman SK, Newman GS. 2010. Biodiversity at the plant–soil interface: microbial abundance and community structure respond to litter mixing. Oecologia 162:763–9.
Cornwell WK, Cornelissen JH, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N et al. 2008. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–71.
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E. 2013. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–95.
de Boer W, Folman LB, Summerbell RC, Boddy L. 2005. Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29:795–811.
Delaney MT, Fernandez IJ, Simmons JA, Briggs RD. 1996. Red Maple and White Pine litter quality: initial changes with decomposition. http://digitalcommons.library.umaine.edu/aes_techbulletin/38/. Accessed 17 Jan 2014.
Finzi AC, Van Breemen N, Canham CD. 1998. Canopy tree–soil interactions within temperate forests: species effects on soil carbon and nitrogen. Ecol Appl 8:440–6.
Finzi AC, Canham CD. 1998. Non-additive effects of litter mixtures on net N mineralization in a southern New England forest. For Ecol Manag 105:129–36.
Finzi AC, Schlesinger WH. 2002. Species control variation in litter decomposition in a pine forest exposed to elevated CO2. Glob Change Biol 8:1217–29.
Foster S, Conner G. 2001. Kentucky Climate Center. Department of Geography and Geology, Western Kentucky University.
Fox VL, Buehler CP, Byers CM, Drake SE. 2010. Forest composition, leaf litter, and songbird communities in oak-vs. maple-dominated forests in the eastern United States. For Ecol Manag 259:2426–32.
Gartner TB, Cardon ZG. 2004. Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–46.
Goins SM, Chapman JI, McEwan RW. 2013. Composition shifts, disturbance, and canopy-accession strategy in an oldgrowth forest of Southwestern Ohio, USA. Nat Areas J 33:384–94.
Green SR, Arthur MA, Blankenship BA. 2010. Oak and red maple seedling survival and growth following periodic prescribed fire on xeric ridgetops on the Cumberland Plateau. For Ecol Manag 259:2256–66.
Hayes RA. 1993. Soil survey of Powell and Wolfe counties, Kentucky. http://agris.fao.org/agris-search/search.do?recordID=US9522838. Accessed 13 June 2014.
Hickman JE, Ashton IW, Howe KM, Lerdau MT. 2013. The native–invasive balance: implications for nutrient cycling in ecosystems. Oecologia 173:319–28.
Kramer C, Trumbore S, Fröberg M, Cisneros Dozal LM, Zhang D, Xu X, Santos GM, Hanson PJ. 2010. Recent (<4 year old) leaf litter is not a major source of microbial carbon in a temperate forest mineral soil. Soil Biol Biochem 42:1028–37.
Kreye JK, Varner JM, Hiers JK, Mola J. 2013. Toward a mechanism for eastern North American forest mesophication: differential litter drying across 17 species. Ecol Appl 23:1976–86.
Kuddes-Fischer LM, Arthur MA. 2002. Response of understory vegetation and tree regeneration to a single prescribed fire in oak-pine forests. Nat Areas J 22:43–52.
Lorimer CG. 1984. Development of the red maple understory in northeastern oak forests. For Sci 30:3–22.
McEwan RW, Dyer JM, Pederson N. 2011. Multiple interacting ecosystem drivers: toward an encompassing hypothesis of oak forest dynamics across eastern North America. Ecography 34:244–56.
McShea WJ. 2000. The influence of acorn crops on annual variation in rodent and bird populations. Ecology 81:228–38.
Melillo JM, Aber JD, Muratore JF. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–6.
Nowacki GJ, Abrams MD. 2008. The demise of fire and “mesophication” of forests in the eastern United States. BioScience 58:123–38.
Olson JS. 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–31.
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC et al. 2007. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–4.
Perry DA, Oren R, Hart SC. 2008. Forest ecosystems. Baltimore, MD: JHU Press.
Prescott CE. 2010. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–49.
Rodewald AD, Abrams MD. 2002. Floristics and avian community structure: implications for regional changes in eastern forest composition. For Sci 48:267–72.
Rubbo MJ, Kiesecker JM. 2004. Leaf litter composition and community structure: translating regional species changes into local dynamics. Ecology 85:2519–25.
Rubino M, Dungait JAJ, Evershed RP, Bertolini T, De Angelis P, D’Onofrio A, Lagomarsino A, Lubritto C, Merola A, Terrasi F et al. 2010. Carbon input belowground is the major C flux contributing to leaf litter mass loss: evidences from a<sup>13</sup>C labelled-leaf litter experiment. Soil Biol Biochem 42:1009–16.
Rustad LE, Cronan CS. 1988. Element loss and retention during litter decay in a red spruce stand in Maine. Can J For Res 18:947–53.
Smith SJ, Young LB, Miller GE. 1977. Evaluation of soil nitrogen mineralization potentials under modified field conditions. Soil Sci Soc Am J 41:74–6.
Washburn CS, Arthur MA. 2003. Spatial variability in soil nutrient availability in an oak-pine forest: potential effects of tree species. Can J For Res 33:2321–30.
Wider RK, Lang GE. 1982. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636–42.
Acknowledgments
This research was supported by the Joint Fire Science Program (01-3-3-14, 04-2-1-06) and would not have been possible without the field and laboratory help of Millie Hamilton, Jessi Lyons, Jamison Paul, Gretchen Carmean, Autumn Foushee, Michael Mahala, Stephen Bell, Amy Herberg, Adam Sovkoplas, Elizabeth Carlisle, and many others. We are also grateful to our collaborators at the U.S. Forest Service who assisted with field set-up and data collection, and to Megan Poulette, Matt Weand, and Ryan McEwan who provided insightful comments and suggestions throughout this study. This study (#14-09-011) is connected with a project of the Kentucky Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
HDA designed the study, performed research, analyzed data, and wrote the manuscript and MAA contributed to research design, assisted in data interpretation, and critically reviewed manuscript.
Rights and permissions
About this article
Cite this article
Alexander, H.D., Arthur, M.A. Increasing Red Maple Leaf Litter Alters Decomposition Rates and Nitrogen Cycling in Historically Oak-Dominated Forests of the Eastern U.S.. Ecosystems 17, 1371–1383 (2014). https://doi.org/10.1007/s10021-014-9802-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-014-9802-4