Abstract
Improved management of carbon storage by terrestrial biomes has significant value for mitigating climate change. The carbon value of such management has the potential to provide additional income to rural communities and provide biodiversity and climate adaptation co-benefits. Here, we quantify the carbon stores in a 49,300-ha landscape centered on the cloud forest–grassland transition of the high Andes in Manu National Park, Peru. Aboveground carbon densities were measured across the landscape by field sampling of 70 sites above and below the treeline. The forest near the treeline contained 63.4 ± 5.2 Mg C ha−1 aboveground, with an additional 13.9 ± 2.8 Mg C ha−1 estimated to be stored in the coarse roots, using a root to shoot ratio of 0.26. Puna grasslands near the treeline were found to store 7.5 ± 0.7 Mg C ha−1 in aboveground biomass. Comparing our result to soil data gathered by Zimmermann and others (Ecosystems 13:62–74, 2010), we found the ratio of belowground:aboveground carbon decreased from 15.8 on the puna to 8.6 in the transition zone and 2.1 in the forest. No significant relationships were found between carbon densities and slope, altitude or fire disturbance history, though grazing (for puna) was found to reduce aboveground carbon densities significantly. We scaled our study sites to the study region with remote sensing observations from Landsat. The carbon sequestration potential of improved grazing management and assisted upslope treeline migration was also estimated. Afforestation of puna at the treeline could generate revenues of US $1,374 per ha over the project lifetime via commercialization of the carbon credits from gains in aboveground carbon stocks. Uncertainties in the fate of the large soil carbon stocks under an afforestation scenario exist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is now widely recognized that anthropogenic landuse can either contribute to climate change (through degradation) or mitigate climate change (through improved management of biosphere carbon stocks) (Denman and others 2007). The carbon stocks and fluxes in above- and belowground tropical forest components have been increasingly studied in attempts to understand their roles in the global carbon cycle (Houghton and others 1998; Malhi and Grace 2000) and their potential valuation in the newly created carbon markets and funds (Glenday 2006). Similarly, the size and dynamics of the above-, and, particularly, belowground carbon stocks of grasslands and pastures are being evaluated as important components of the global carbon cycle that are affected by human landuse (Scurlock and Hall 1998; Schuman and others 2002).
In this article, we focused our attention on an ecotone with poorly documented carbon stocks and high sensitivity to human and climate change pressure—the puna grassland–cloud forest transition of the high Andes. Tropical montane cloud forests (TMCFs) are most generally defined as forests “frequently covered by cloud or mists” (Stadtmuller 1987, p. 18) and represent about 2.5% of the world’s tropical forests (Bubb and others 2004). TMCFs are characterized by stunted, twisting trees, laden with ephiphytes and a species assemblage that is significantly different from the tropical forests of lower altitudes (Bruijnzeel and Veneklaas 1998).
The base of the clouds that provide TMCFs with a vital moisture source are anticipated to rise due to climate change, this drying coupled with rising temperatures makes the TMCFs a vulnerable biome (Foster 2001; Still and others 1999; Bush 2002). The predicted rate of climate change for this century will require species to migrate upslope at rates an order of magnitude greater than has been experienced in the past 50,000 years to remain in their climatic niche (Bush and others 2004). However, a second threat to some Andean TMCFs comes from the grazing and burning of puna grasslands above, which as well as degrading the aboveground vegetation and soil of the puna (Bustamante Becerra and Bitencourt 2007) are believed to contribute to an anthropogenically constrained upper limit of the treeline (Braun and others 2002; Sarmiento and Frolich 2002; Young and León 2007).
Higher elevation carbon stores are less studied than lowland areas, but are also under threat from human disturbance and could potentially benefit from an influx of carbon financing (Fehse and others 2002). Some studies have quantified aboveground, coarse root and soil carbon up altitudinal gradients of montane forests from low-altitude island mountains (Raich and others 1997, Hawaii; Weaver and Murphy 1990, Puerto Rico; Edwards and Grubb 1977, New Guinea) and also from larger mountain ranges (Moser and others 2008, Ecuador; Kitayama and Aiba 2002, Borneo; Delaney and others 1997, Venezuela). Some site-specific studies have quantified carbon stock changes when plantations or secondary succession occurs on former cropland or pasture (Cavelier 1995, Columbia; Farley and others 2004, Ecuador; Fehse and others 2002, Ecuador). Likewise, some studies focusing on the impact of landuse on the carbon stocks of high altitude grasslands have been conducted in the Andes (Pucheta and others 1998 and Adler and Morales 1999, both Argentina). However, an integrated carbon stock quantification study across the upper treeline of a TMCF has not yet been conducted.
Carbon credits can be generated through the voluntary carbon market for avoided deforestation, afforestation/reforestation and through better management of grasslands (Hamilton and others 2009). However, historically, the potential to sequester carbon in high-altitude regions has been neglected, as attention has focused on lowland biomes (Fehse and others 2002).
This study, from Manu National Park, Peru, using a combination of field and remotely sensed data aimed to (1) quantify, at the landscape-scale, the aboveground carbon stocks across the upper treeline of TMCFs into the puna grasslands—an ecotone facing pressure from climate change and landuse, (2) assess the impact of vegetation type, slope, altitude, grazing and fire on the distribution of carbon stocks, (3) compare results to below soil carbon data gathered by Zimmerman and others (2010) at the same sample points, and (4) identify possible management options and calculate their potential effects on the carbon stocks of the area.
Methods
Site Description
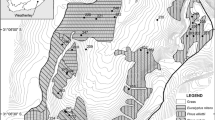
Manu National Park (a World Heritage Site) extends from the eastern slopes of the Andes into the lowland Peruvian Amazon (IUCN 2008). The park border is defined by the watershed separating the catchment basins of the Urubamba and Madeira rivers to the south and west, and the catchment of the Manu River in the lowlands. The National Park boundary divides ownership of the high Andean plateau between the communities to the west and the National Park to the east (Bustamante Becerra and Bitencourt 2007). The study area was at the southern extent of the Manu’s western border on the Paucartambo mountain range (Figure 1). The study area was defined as those areas above 3,000 m inside the park boundary, extending from the southwest boundary of the park to a northern limit at 12°21′36″S. An additional area of about 3,500 ha contiguous with the SW corner of the park in the Wayqecha Valley was also included, giving a surface area of the study area of approximately 49,300 ha.
The bottom left inset shows the location of the study area in central Peru. The top right inset shows the full study area running northwest to southeast along the southeastern border of Manu National Park. Dark gray areas were classified as forest and white areas as puna. The main image shows the distribution of all plots as black circles with a white border. The Manu National Park Border is shown as a black line that delimits the western extent of puna classified and goes northeast across the lowland Amazon.
Rainfall averaged 1900–2500 mm annually across the study area, with a wet season from October to April. Although no site-specific data were available, TMCFs can also receive significant cloud water deposition inputs ranging from 0.2 to 4 mm day−1 (Bruijnzeel and Proctor 1995). Mean annual temperatures at 3,600 m were 11°C with frosts being common on clear nights. Various classifications for the altitudinal transition of vegetation types existed for this area (Terborgh 1977; Young and León 2000). For the purpose of this study, three land-cover classes were used: (1) puna, where grasses dominate (2) a transition zone, where a mixture of shrubs and grasses dominated, and (3) upper TMCFs, where closed canopy trees and bamboo dominated. Puna was predominant in the high elevation areas in the west of the study area from 4,061 m to the average treeline height of 3,450 m. The puna terrain was steeply rolling and mountainous, punctuated by occasional permanent and semi-permanent small lakes (up to 1 ha in area). Puna vegetation comprised grasses such as Jarava ichu Ruiz & Pav., Calamagrostis vicunarum (Wedd.) Pilg., Festuca dolichophylla J. Presl. with an average height of approximately 30 cm. Descending in altitude, the puna was replaced by a transition zone of 0.5–2.0 m high shrubs of genera including the dominants Baccharis (Asteraceae), Hypericum (Hypericaceae), Lycopodium (Lycopodiaceae), Hesperomeles (Rosaceae), Brachyotum (Melastomataceae), and Escallonia (Escalloniaceae). The shrubs had an average height of about 1 m. The transition zone was between 1 and 50 m in width, separating the puna from cloud forest, with terrain similar to that of the puna.
Below the transition zone, tree species from the Asteraceae, Cunoniaceae, Melastomataceae, Rosaceae, Rubiaceace, and bamboo in the genus Cusquea dominated to comprise the upper TMCFs (referred to hereafter as “forest”). Patches of primary succession indicated disturbance from landslides and some areas had suffered the impacts of fire started in the puna. The understory had few shrubs and herbaceous plants, and trees were festooned with vascular and non-vascular epiphytes (mainly bryophytes, ferns, bromeliaceae, ericaceae, and orchidaceae) with a high incidence of asteraceous vines. Most branches were covered in bryophytes. The forest terrain was steeply sloping with rocky outcrops in places.
A full description of the soil characteristics of the study site can be found in Zimmerman and others (2010). Forest soils were characterized by an organic forest floor layer (Oh) of an average 22 cm with a high fine root density, overlaying a humic Ah layer (11 cm) and a mineral B layer. Some mineral forest soil layers contain mixed mineral layers with a high stone content as a result of ancient landslides. Soil depths in the forest varied between 26 and 100 cm, with an average depth of 44 cm. Transition zone soils were shallower and had only a thin Oh layer of about 2 cm, followed by an average 17-cm thick A(h) layer. Soils in the transition zone were between 23 and 50 cm deep (36 cm on average). Puna soils had no Oh layers at all and consisted mainly of organic-rich A layers (19 cm on average) and stony B/C layers. The average soil profile in the puna was 33 cm deep.
The puna had been degraded due to grazing and related seasonal burning and was classified as a “Zone of Recovery” within Manu National Park; being identified as an area that had been, and continued to be, severely damaged and needed special management to recover (INRENA 2002).
Sampling Strategy
Aboveground carbon stocks were measured across the landscape with a stratified sampling regime, using methods adapted from De Castro and Kauffman (1998). Random geographical points were generated for the puna or transition zone close to the treeline based on a Landsat classification. At each point, a transect was established that descended 100 m in elevation into the forest and ascended in increments of 100 m in elevation across the puna to the highest point in the local landscape for a total of 55 points; 35 in the puna, 8 in the transition zone, and 12 in the forest (Figure 1).
Aboveground Carbon Estimation
At each sample point, tree, coarse woody debris (CWD), and bamboo biomass were measured in four 3 × 15 m plots arranged around the central, randomly generated point. Within each of these plots, a 1 × 5 m plot was used for shrub biomass measurement and a 0.5 × 0.5 m plot for litter/moss/grass (LMG) layer biomass determination. An additional LMG plot was located at the center of the arrangement. The methods used to estimate the dry biomass for each pool are described below. Dry vegetative biomass was assumed to contain 50% carbon (after Glenday 2006).
Trees
The diameter at breast height (dbh 1.3 m above ground height on uphill side of stem) and height were measured for all stems (living and dead) with a height greater than 2 m and a dbh of greater than 1 cm. Where trees did not grow straight or perpendicular to the ground, dbh was measured 1.3 m from the base along the main stem. The tree carbon data gathered in this study are referred to as data set 1 (DS1). Tree dbh and height measurements from plots comprising two additional data sets collected within the study area were also used, these are referred to as DS2 and DS3 (Table 1). In total, 5.3 ha of forest data were used for the tree biomass estimation from 27 sites.
Allometric equations were used to estimate biomass from the field measurements. Although species-specific equations were not available for the study area, Chave and others (2005) provided an equation based on a large pan-tropical multi-species data set for wet climates and recommended its use for montane forests (equation (1)). To compare results from other commonly used allometries, Chave and others’ (2005) dbh only, wet climate equation (2) was used. To allow further comparison, Brown’s (1997) dbh only, wet climate equation (3) was used to calculate aboveground tree biomass. Brown’s equation, like Chave and others, was based on a large pan-tropical multi-species data set which was not specifically designed for montane regions, and when elevation was considered, the study site met Brown’s definition of “wet.” Only trees with dbh greater than 5 cm were used in the biomass estimation due to limits in the applicability of allometric equations at lower dbh values.
where aboveground biomass (AGB) is in kg, ρ (wood density) is in g cm−3, D is dbh in cm, and H is in m.
Where the wood density was available (from Chave and others’ (2006) wood density database), genus (63% of individuals) or family (18%) values were used. Where there was no family level data available or the species was not known, the mean of the known trees, 0.571 g cm−3, was used.
Coarse Woody Debris
The length and maximum diameter of pieces of CWD within the plot were measured for all pieces larger than 10 cm in diameter. A reduced wood density of 0.31 g cm−3 was used for all dead trees based on a mean standing deadwood density measured in the study area. This value represented the mean value taken from unpublished data of CWD densities from two plots within the study area (L.E.O. Aragão, unpublished data). This value concurred with Wilcke and others (2005) who also recorded 0.31 g cm−3 as an average density for all CWD found above 2,000 m in a montane forest.
Bamboo
The dbh was measured, and height was estimated for all bamboo stems within the plot. Five bamboo shoots were measured, dried, and weighed. They had a dbh range of 0.6 to 2.0 cm. A power–law relationship that related dbh to mass with R 2 of 0.96 was then determined to be:
where AGB is in kg, D is dbh in cm.
Six of the bamboo shoots (<5%) measured in the plots had a dbh greater than 2 cm. The biomass of these shoots was capped at the biomass for shoots of 2 cm dbh.
Shrubs
Shrub biomass was calculated within each plot by modeling the crown area as an ellipse and then converting the measure to biomass per ha using the linear regression equation in Flombaum and Sala (2007):
where AGB_ha−1 is in Mg ha−1, correction factor c has no units and is 2 for this site, Fraction_Shrub_Cover between 0 and 1.
Shrubs were defined as woody plants with a main stem larger than 1 cm in diameter and between 0.5 and 2.0 m in height. Equation (5) was calculated for shrub cover in the dry desert of Argentina using three shrub species. A correction factor of 2 was introduced to account for the mean shrub height of this study (1 m), being two times higher than in the data used to develop the equation. This methodology would have benefitted from better calibration with the species found in the study area and likely represented an underestimate of shrub biomass for this wetter region. However, shrub biomass was found to account for a small fraction of overall carbon stocks in the transition zone, suggesting uncertainty in shrub biomass had little effect on the conclusions of this study.
Litter, Moss, and Grass
All litter, moss, grass, herbaceous plants, and shrubs with diameters smaller than 1 cm and/or those with a diameter larger than 1 cm but height less than 50 cm were collected by clipping to ground level. The samples were bagged, dried at 60°C for 3 days, and weighed.
Roots
Coarse root biomass (>2 mm diameter) was not directly measured due to prohibitively high costs. Due to the effort of gathering root data, few studies were available in the literature, and none were found for the TMCFs of Peru. Therefore, Cairns and others’ (1997) commonly used, globally derived, mean root to shoot ratio of 0.26 was used to estimate the root biomass of the aboveground biomass of trees, shrubs, and bamboo. Mokany and others (2006) also presented 0.26 as the best linear regression fit in their synthesis of global forest root to shoot ratios. A study from a TMCF in Ecuador suggested root:shoot ratios increased with altitude as a higher proportion of carbon was allocated belowground. Leuschner and others (2007) reported this trend with a ratio of 0.43 at 3,060 m in Ecuadorian TMCF. Thus the 0.26 value used here may have underestimated root carbon stocks. A standard error equal to 20% of the mean was attributed to the value. Carbon from fine root biomass (<2 mm) was included in the soil carbon pool.
Soil
Zimmerman and others (2010) quantified the carbon stocks of the soil at the same sample points from which our aboveground data were gathered by taking equal interval samples down to the bedrock. A comparison between above- and belowground stocks is presented in the “Discussion” section below.
Topographic and Land-Use Variables
At each point, the maximum slope was recorded with a clinometer, and altitude was recorded from a handheld GPS unit. Evidence of past fires (fire damage, charcoal in soil profile) was noted. Cattle droppings were counted within four 5 × 25 m transects that extended out from the center of each random point in the puna and transition zone.
Aggregation and Extrapolation
An analysis of the difference in carbon densities between the three tree carbon data sets is presented. A total ecosystem carbon density (Mg C ha−1) for each land-cover class was estimated by summing the plot carbon densities for each of the five measured pools, root, and soil data. The standard error was calculated for each carbon pool from the variance between plots and propagated by quadrature when the pools were summed (for example, see Malhi and others 2008, for an example of error propagation). The standard error of the total carbon content of the study site was calculated by propagating the relative standard error of the area and the carbon densities of each land-cover class by quadrature. A t test was used to test for between-biome differences if the data were normally distributed and had similar variances; otherwise a Mann–Whitney rank sum test was run (using a significance level of P = 0.05). More than two groups were compared with a one-way ANOVA if they were normally distributed and had similar variances. Mean values are given with ± 1 standard error.
Remote Sensing Classification
Landsat (TM) images at 30 m spatial resolution were acquired for the study area (WRS-1, Path 4 / Row 69). The images, acquired on the 14 August 2007 Shuttle Radar Topography Mission (SRTM), 90 m resolution Digital Elevation Model (DEM) data, were received from the CGIAR consortium for spatial information (Jarvis and others 2006). All remote sensing and GIS processing were completed in IDRISI Andes V15. The surface area estimates of the study area were improved by incorporating slope into the area calculations (Jenness, 2004). The SRTM DEM was projected to 30 m resolution using the nearest neighbor method and then was used to calculate the slope in each pixel in degrees. The surface area of each pixel was then calculated as the planimetric area multiplied by 1/cos(slope angle).
Using the methodologies of Eastman (2006), unsupervised and supervised (maximum likelihood equal probability and probability weighted methods) classifications of the study area were attempted. It was only possible to distinguish between puna and forest at 30 m resolution; therefore, the narrow transition belt was incorporated into forest and puna totals. An accuracy assessment of the classifications was carried out in accordance with the methodology described by Eastman (2006). The kappa index of agreement (KIA) was calculated using the error matrix module in IDRISI to assess the accuracy of the classification (Congalton 1991). A KIA of 1 indicates a perfect match between the classification and the observed land cover, whereas 0 indicates no match (Campbell 1996). The areal extent of the land-cover class identified was corrected according to the results of the error matrix using the methodology described by Dymond (1992). The final estimates of area are presented as the mean ± two times the root mean square, within which the mean was “most likely to sit” according to Dymond (1992).
Results
Land Area Classification
The maximum likelihood probability weighted classifier was found to be the most accurate classification technique with a KIA score of 0.88. Although no agreement on a minimum acceptable KIA exists, values greater than 0.85 are generally perceived as demonstrating a “good” classification (Foody 2002). The maximum likelihood equal probability classifier and unsupervised classification had KIA scores of 0.872 and 0.765, respectively. According to the maximum likelihood probability weighted classifier, the study area contained 29,190 ± 1,260 ha of forest and 19,480 ± 1,060 ha of puna; 640 ± 572 ha were unidentified due to the topographic effect of shadows. The figures presented above are “surface area,” as opposed to “planimetric area.” The correction applied for slope yielded a 15% increase in forested areas and a 10% increase in puna area. Forest areas were increased more due to their prevalence on steeper slopes. Total land area in any one altitude band was found to decline with altitude, with the land area declining most above 3,600 m (Figure 2). Although the average treeline height was 3,450 m, puna was found at all altitudes in the study area and forest was classified up to 3,800 m. The highest forest site visited was 3,515 m.
Differences in the Allometric Equations
Equation (1) (Chave equation with height) was found to be the most conservative estimator of tree carbon (>10 cm dbh), with an equally weighed mean of all plots being 50 ± 6 Mg C ha−1, whereas equation (2) (Chave without height) and equation (3) (Brown) estimated 99 ± 11 and 91 ± 11 Mg C ha−1, respectively. The biomass estimates for individual trees from the three equations diverged with increasing dbh (Figure 3). Equation (1) predicted consistently lower values than equations (2) and (3) and was used for all subsequent calculations.
A comparison between the carbon stock estimation for each individual tree measured in the study using Chave and others’ (2005) dbh, wood density (WD) and height, wet allometric equation (1) for tree carbon and two alternative equations; Chave and others’ (2005) dbh and WD only, wet (equation (2)) and Brown (1997) dbh only, wet (equation (3)).
Variation Between the Tree Carbon Pool Data Sets
When the three tree carbon pool data sets (>10 cm dbh only) were considered separately, DS1’s carbon density of 35 ± 9 Mg C ha−1 was lower than DS2’s and DS3’s with 63 ± 8 and 59 ± 6 Mg C ha−1, respectively (Table 2). The mean tree carbon density, with all data points considered equally, was 50 ± 6 Mg C ha−1, weighting by plot area increased the mean to 59 ± 4 Mg C ha−1. The distribution of carbon contribution from the different dbh classes of the data sets showed that DS1 had the lowest carbon stocks in every category (Figure 4). The largest differences between data sets were found in the above 50 cm dbh class where the DS1 mean of 2.3 Mg C ha−1 was significantly less than the mean of data sets 2 and 3 with means of 11.1 and 7.2 Mg C ha−1, respectively.
Carbon Density Determination for Each Land-Cover Class
Using equation (1) and a mean tree carbon value of all sampled forest plots, the mean aboveground carbon density in the forest was 63 ± 5 Mg C ha−1 (Table 3). This was significantly (P < 0.05) greater than the carbon densities of the transition zone 16.9 ± 2.2 Mg C ha−1 and puna 7.5 ± 0.7 Mg C ha−1. The forest’s aboveground carbon stock was dominated by trees with more carbon found in the litter/moss/grass layer than in the CWD pool. The LMG layer contained the largest fraction of aboveground carbon in both the transition zone and puna. The transition zone had more than double the carbon in the LMG layer than the puna, which accounts for the majority of the difference between the two. The shrubs, although a dominant visual feature of the transition zone, contributed relatively little to total aboveground biomass, even if the allometric relationship we employed underestimated biomass by a factor of three.
The Total Carbon Stock of the Study Area
The carbon stock of the study area gradually decreased from 3,000 m to 3,600 m (Figure 5) as both land area decreases (Figure 2) and the prevalence of puna relative to forest increased steadily. Belowground carbon stocks were seen to represent an increasing proportion of the total stock as altitude increased, again due to the transition to puna with a higher belowground:aboveground carbon stock ratio (Figure 5). When Zimmerman and others’ data are considered, in total 75% of the carbon in the study area was stored belowground in roots and soil. Given that there was no relationship between slope and altitude, the total aboveground carbon stock was calculated by scaling the mean carbon densities of puna and forest by the spatial area covered when corrected for slope at 30 m resolution. The total carbon stock 8.2 ± 0.6 Tg comprised 2.3 ± 0.2 Tg from the puna and 5.9 ± 0.5 Tg from the forest.
Discussion
The Effect of Topography and Land-Use Variables
Aboveground biomass was not significantly related to slope, topographical position, or altitude within any of the land-cover classes, with the exception of the positive correlation between aboveground carbon density and altitude of the transition zone (P < 0.05). This correlation could be due to a third factor; higher treelines existed in places that were less affected by fire.
Of the 27 puna points where grazing was analyzed, 69% had evidence of grazing (at least 1 dropping found), whereas one of the five transition points assessed was grazed (Table 4). Puna points with evidence of grazing had a mean aboveground carbon density of 6.5 ± 0.3 Mg C ha−1, significantly different from 8.9 ± 0.6 Mg C ha−1 for those without evidence (P < 0.05). There was a negative relationship between the cattle dropping count and the aboveground carbon density of puna; however, the relationship was not significant (P > 0.15). Evidence of past fires was found at 26% of puna sites and in 25% of the forest sites from DS1. Our sampling did not find a significant difference in the aboveground carbon densities of those sites with historical evidence of fire and those without (within the same land-cover class). Our methodology would not capture data related to carbon losses due to the transition from a higher-to-lower carbon density land-cover class.
Puna sites with evidence of past fires were found to have a mean soil carbon density of 96 ± 11 Mg C ha−1 compared to 123 ± 11 Mg C ha−1 for those without (Zimmerman and others 2010). Those sites with evidence of grazing had a mean soil carbon density of 111 ± 10 Mg C ha−1 compared to 124 ± 14 Mg C ha−1 without (Zimmerman and others 2010). However, there was not a statistically significant difference between either of these relationships. Further analysis of the Zimmerman and others data revealed that, when only the top 10 cm of soil was analyzed, the soil carbon density in grazed sites of 56 ± 2 Mg C ha−1 was significantly higher than in ungrazed areas 48 ± 2 Mg C ha−1 (P < 0.05). This appeared to be a compaction effect because there was a corresponding significant increase in bulk density of grazed sites (0.55 ± 0.02 g cm−3 vs. 0.45 ± 0.02 g cm−3, P < 0.05), yet no significant difference in carbon concentrations (both 14 ± 1 %). Forest sites affected by fire had higher mean carbon stocks 148 ± 25 Mg C ha−1 compared to non-affected sites 120 ± 40 Mg C ha−1; however, this difference was not significant, primarily due to the small sample size and large variation between sites (Zimmerman and others 2010).
Comparisons with Other Studies
Zimmerman and others (2010) found that soil carbon densities were not significantly different between forest, transition zone, and puna (118 ± 15, 147 ± 14, and 119 ± 8 Mg C ha−1, respectively). Using Zimmerman and others’ data, the ratio of belowground:aboveground carbon (all pools) decreased from 15.8 on the puna to 8.6 in the transition zone and 2.1 in the forest. There were no significant relationships between aboveground and belowground carbon densities within land-cover classes.
The carbon density of trees larger than 5 cm dbh (54 ± 6 Mg C ha−1) in this study was comparable with those of Moser and others (2008), who recorded 56.1 Mg C ha−1 for all trees with dbh above 3 cm in a site selected as an example of closed canopy upper montane forest at 3,060 m in Ecuador. Secondary successional forest stands, of Alnus acuminata (Betulaceae) and Polylepis incana (Rosaceae) at 3,200 m in Ecuador where upper TMCF had previously been deforested, had higher carbon densities of 120 and 183 Mg C ha−1, respectively (Fehse and others 2002). In Borneo, Kitayama and Aiba (2002) report tree carbon densities of 18 and 107 Mg C ha−1 from two plots in “Subalpine forest /scrub” (3,100 m) as well as 60 and 150 Mg C ha−1 from two plots in “Upper Montane Rainforest” (2,700 m).
The wide range of carbon values found in the literature accurately reflected the range of carbon densities that were found in forested high elevation landscapes. The landscape average, however, reflected myriad factors, including succession after disturbances, environmental factors such as depth to water table and local orographic effects and their relative abundances across the landscape. Extrapolations to the landscape, a central goal of ecosystem ecology and required for use in ecosystem services markets, must weigh the relative importance of these factors across the landscape. We used a random sampling approach, which is labor intensive but eliminated many sources of bias due to site selection. Further work should combine randomized sampling schemes with remote sensing efforts to produce accurate landscape-level carbon stock estimates for high elevation systems.
Grazed puna sites which had 6.5 ± 0.3 Mg C ha−1 in aboveground carbon densities were comparable with the regularly burned and grazed Ecuadorian paramo grasslands with aboveground densities of 4.0–4.2 Mg C ha−1 found at 3,750 m and 4,000 m sites, respectively (Ramsay and Oxley 2001). The grazed sites had an aboveground carbon density 27% lower than the ungrazed sites, similar to the difference of 33% found between grazed sites and those excluded from grazing for 2 years in the Argentinean montane grasslands studied by Pucheta and others (1998).
The lack of slope or altitudinal effects on carbon densities for either the puna or forest sites suggested other factors such as micro-climate or landuse (both current and historical) were more important factors in stock distribution. Fire is known to have an immediate impact of reducing carbon stocks through combustion (Van Der Werf and others 2003). Although the results presented here did not show any relationship between past fires and reduced carbon stocks, our sampling did not aim to thoroughly capture the range of fire impact experienced by the land-cover classes, which would be necessary to draw conclusions on the effects of fire.
Uncertainties
Uncertainties can be classified as either random or systematic. Random uncertainties, caused by spatial variation, can largely be addressed through an appropriate random (or random stratified) sampling protocol, and are appropriately quantified for our soil, litter layer, and dead wood sampling. Chave and others (2004) found that when extrapolating forest inventory measurement to the landscape scale, the largest contributors to uncertainty were the choice of allometric equation, the size of study plots, and their representativeness of plots in the landscape.
The choice of allometric equation significantly affected the aboveground carbon results. Equation (1), which used tree height and dbh, gave significantly lower carbon estimates of the tree carbon pool than the other two equations which used dbh only. The estimation of tree heights in TMCFs where trees do not always grow straight was difficult. However, Williams and Schreuder (2000) conclude that, as long as height estimates are correct ±40%, they still improve allometric equation accuracy. Systematic errors in height measurement that would explain the significant difference in results from the with- and without-height equations are unlikely. Thus, it was thought the difference in results was due to the sample of trees used to generate equations (2) and (3) having a different dbh to height relationship for use in these upper TMCFs. Although no testing of the allometric equations with actual tree biomass data was possible, equation (1) was the most accurate equation in a study by Moser and others (2008) when compared to biomass data available from three wind thrown trees in an Ecuadorian TMCF.
Chave and others (2004) recommended at least 5 ha of sampling for a landscape scale assessment of forest carbon to ensure a representative sample of the landscape was taken. This study used a combination of large (1 ha) plots (DS3) and smaller plots (DS1 and DS2), that totaled 5.3 ha. DS1, which had the smallest and only completely random distribution of plots recorded the lowest carbon densities. The largest difference between the carbon content of the DS1 forest plots and the others was in the larger than 50 cm dbh class of trees. This could have been because sites for DS2 and DS3 were non-random and were selected as good examples of forest, avoiding bamboo patches and large clearings resulting from landslides. Another reason could have been the non-normal distribution of large but rare trees (Chave and others 2003), which a small number of small sampling plots below the recommended minimum plot size of 0.25 ha were likely to miss. Within each data set and considering all data together, no significant relationship was found between the distance from treeline or altitude and total carbon density. Although a decrease in total carbon density of forest may have been expected at the treeline approaches (Moser and others 2008), the narrow altitudinal range of plots (3,000–3,625 m) and observed differences in treeline forest structure (from gradual thinning through a transition zone to abrupt mature forest–puna transitions) explain the lack of correlation. Therefore, results from DS1 were likely to be an underestimate of the true mean carbon density of the forest at the treeline. With the possibility that results from DS2 and DS3 were overestimates, a mean of DS1-3 was considered an appropriate best estimation.
The methodology was designed to capture all significant carbon stocks in the landscape; however, some small pools were not quantified. A study of elfin forest in Ecuador (Moser and others 2008) found that trees 3–5 cm dbh contributed 9% of biomass. However using equation (1) beyond its recommended range of dbh values to include trees 2–4.99 cm dbh for DS1 and DS2, we found they contributed less than 2% of the total above ground biomass (<1 Mg C ha−1). Epiphytes such as lichens, mosses, and bromeliads were not counted but were not expected to contribute significantly to carbon stocks (Edwards and Grubb 1977). It was noted that the landscape contained sporadic lakes and bogs smaller than 1 ha in size, whose belowground carbon stocks could be significant but required more sampling.
Potential to Increase Carbon Stocks
Here, we quantified the potential volume of carbon sequestration resulting from projects to reduce degradation of puna from overgrazing and facilitating the upslope migration of the treeline in response to climate change. Conservative carbon sequestration totals under the scenarios were calculated by subtracting the current carbon densities plus one standard error from the expected carbon density minus one standard error.
Controlled Grazing
The results presented here are consistent with evidence that over-grazing of high altitude grasslands can reduce aboveground carbon densities (Pucheta and others 1998; Adler and Morales 1999). Therefore, improved grazing management schemes in such areas could lead to carbon sequestration benefits.
If the percentage of grazed sites (67%) was representative of the extent of grazed land within the study area, then approximately 13,000 ha were affected by grazing. If the mean carbon densities of these grazed areas were raised to the non-grazed mean values then with conservative gains of 1.5 Mg C ha−1 aboveground 0.019 Tg C would be sequestered. Due to the variation between sites, a more intense and focused study on the effects of grazing on soil carbon in the study area is needed before any significant trends can be identified.
Increasing Forest Cover
Barriers to succession of forests onto pastures include the impact of grazing (Cavelier 1995), competition with dense grasses, and the lack of seed dispersal (Holl and others 2000). Furthermore, the rate of TMCF’s natural succession onto pastures is low, even when a source of seed dispersal was close, with a canopy dominated by shade tolerant species possibly taking several hundred years to establish (Golicher and Newton 2007). Therefore, to facilitate the upslope migration of TMCF species, assisted afforestation through seeding, or planting shrubs or native tree seedlings could be carried out (see Holl and others 2000). Projects with a primary objective of carbon sequestration and revenue generation such as monoculture plantations of Pinus or Eucalyptus species have questionable benefits for biodiversity and water supplies (Cavelier 1995; Hofstede and others 2002) and thus regeneration projects are considered more appropriate for protected areas such as Manu National Park.
Raising the mean puna aboveground and root carbon densities to that of the forest would sequester 62.4 Mg C ha−1. Afforesting the entire puna area would sequester 1.1 Tg C, however, 60% of these gains could be made with afforestation below 3,600 m (Figure 6). Rates of TMCF forest recovery are known to be relatively low (Golicher and Newton 2007) and with a space-by-time substitution experiment on ex-cropland at 1850 m in Mexico, Del Castillo and Blanco-Macías (2007) were able to show secondary succession of TMCF took up to 100 years to reach maturity and maximum carbon stocks. Strategies to speed-up the rate of forest regeneration should be central to any management effort, such as planting rapidly growing nurse trees that are attractive to avian seed dispersers.
Estimated maximum potential gains in aboveground (AG) and root carbon under an afforestation of puna scenario using the mean carbon densities found in this study (black) on top of current stocks (white). And the estimated gains in soil carbon based on an assumption of no change in the existing puna soil carbon stock and a gain of 20 cm of Oh layer (striped) using a mean of the carbon stock in the 0–20 cm layer of forest soils in the study area.
The Importance of the Soil Carbon Stock
Given the size of the carbon stock in the puna soils relative to the aboveground stock of the forest (about twice as large), the fate of this stock under an afforestation scenario has important implications for the net carbon budget of the area (Jackson and others 2002; González-Espinosa and others 2007). Soil carbon stocks of puna and forest were found to be equal; therefore, with simple assumptions that these stocks were in equilibrium, and a new equilibrium would be reached after afforestation with native species, no net change in soil carbon would be expected. However, the distribution of carbon through the soil profile was not the same in forest and puna soils, with forests being characterized by a 22-cm Oh layer and an 11-cm Ah layer, whereas the puna soils had only a 19-cm Ah layer. Therefore, under an optimistic scenario, with no losses to the current puna Ah layer and an accumulation of 20 cm of Oh layer, carbon stocks could increase by 65 Mg C ha−1 (data from Zimmermann and others 2010). When added to the 77.3 Mg C ha−1 that could be stored in aboveground and coarse root biomass, a total 2.3 Tg C could be sequestered in the 49,300-ha study area (Figure 6).
The assumption of no change in soil carbon stocks under an afforestation scenario was supported by Paul and others (2002) who found pasture to forests conversions experienced a loss in soil carbon over the first 5 years but a recovery to pre-afforestation levels after 30 years. Evidence from meta-analyses showed no significant change in soil carbon when broad leaf forests were established or secondary succession occured on pasture (Guo and Gifford 2002). Del Castillo and Blanco-Macías (2007) found after 100 years, abandoned cropland under secondary succession of TMCF accumulated an O horizon (including litter) of 15–30 cm, suggesting gains in Oh carbon stocks could be achieved in at least this amount of time.
Given the relative size of the carbon stock in high elevation pastures, best practice management that reduces carbon loss from soils should be executed (Paul and others 2002). High monitoring costs may discourage monitoring soil carbon in afforestation schemes on high altitude pastures (Robertson and others 2004); however, they may be necessary for truly conservative carbon stock change estimates and may reveal positive gains if the loss of Ah layer carbon is less than that of Oh layer accumulation.
Conclusion
Carbon sequestration projects now have the potential to earn financing through sales on the voluntary and regulated carbon markets. The sequestration of 1.5 Mg C ha−1 across the 18,000 ha of puna in the study area through improved grazing management, assuming a carbon price of US $3.4 per t CO2e (the average price for agricultural carbon projects in 2008, Hamilton and others 2009) would earn only USD 65,000. This is very unlikely to cover project development, monitoring, and verification costs. However, assisted migration of the treeline may not be possible without relieving grazing and fire pressure on the treeline. If only the aboveground and coarse root carbon stocks were considered, and the average carbon price for afforestation conservation projects of US $7.5 per t CO2e were achieved (Hamilton and others 2009), this would represent an income of US$ 1,374 per ha over the project lifetime for afforestation of puna. If soil carbon gains occurred and were monitored, this number could increase by up to a factor of 2.
There are still considerable uncertainties over the feasibility of such a project including whether sufficient management capacity exists within the park to implement such a project; whether the local communities could be sufficiently engaged and suitably compensated to ensure the project’s success. Likewise the impacts on water yield in the catchment through altered transpiration, interception, evaporation, and cloud water deposition require careful consideration (Buytaert and others 2007; Farley and others 2005). However, Manu National Park presents a real opportunity where assisted migration of the treeline through afforestation combined with grazing management could both increase carbon stocks and increase the chances of endemic species survival in the face of climate change.
References
Adler PB, Morales JM. 1999. Influence of environmental factors and sheep grazing on an Andean grassland. J Range Manag 52:471–81.
Braun G, Mutke J, Reder A, Bartbloft W. 2002. Biotope patterns, phytodiversity and forestline in the Andes, based on GIS and remote sensing data. In: Korner C, Spehn E, Eds. Mountain biodiversity—a global assessment. London: Parthenon Publishing Group. p 75–89.
Brown S. 1997. Estimating biomass and biomass change of tropical forests: a primer. FAO Forestry Paper No. 134, 55 pp
Bruijnzeel LA, Proctor J. 1995. Hydrology and biogeochemistry of tropical montane cloud forests: what do we really know? In: Hamilton LS, Juvik JO, Scatena FN, Eds. Tropical montane cloud forests. Ecological Studies, vol 110. New York: Springer. pp. 38–78.
Bruijnzeel LA, Veneklaas EJ. 1998. Climatic conditions and tropical montane forest productivity: the fog has not lifted yet. Ecology 79:3–9.
Bubb P, May I, Miles L, Sayer J. 2004. Cloud forest agenda. Cambridge: UNEP-WCMC. p 34.
Bush MB. 2002. Distributional change and conservation on the Andean flank: a palaeoecological perspective. Glob Ecol Biogeogr 11:463–73.
Bush MB, Silman MR, Urrego DH. 2004. 48,000 years of climate and forest change in a biodiversity hot spot. Science 303:827–9.
Bustamante Becerra JA, Bitencourt MD. 2007. Ecological zoning of an Andean grasslands (puna) at the manu biosphere reserve, Peru. Int J Environ Sustain Dev 6:357–72.
Buytaert W, Iñiguez V, Bièvre BD. 2007. The effects of afforestation and cultivation on water yield in the Andean páramo. For Ecol Manag 251:22–30.
Cairns MA, Brown S, Helmer EH, Baumgardner GA. 1997. Root biomass allocation in the world’s upland forests. Oecologia 111:1–11.
Campbell JB. 1996. Introduction to remote sensing. 2nd edn. New York (NY): Guilford.
Cavelier J. 1995. Reforestation with the native tree Alnus acuminata: effects on phytodiversity and species richness in an upper montane rain forest area of Colombia. In: Hamilton LS, Juvik JO, Scatena FN, Eds. Tropical montane cloud forests. New York (NY): Springer. p 125–37.
Chave J, Condit R, Lao S, Caspersen JP, Foster RB, Hubbell SP. 2003. Spatial and temporal variation of biomass in a tropical forest: results from a large census plot in Panama. J Ecol 91:240–52.
Chave J, Condit R, Aguilar S, Hernandez A, Lao S, Perez R. 2004. Error propagation and scaling for tropical forest biomass estimates. Philos Trans R Soc B 359:409–20.
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Fölster H, Fromard F, Higuchi N, Kira T. 2005. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99.
Chave J, Muller-Landau HC, Baker TR, Easdale TA, Steege H, Webb CO. 2006. Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol Appl 16:2356–67.
Congalton RG. 1991. A review of assessing the accuracy of classifications of remotely sensed data. Remote Sens Environ 37:35–46.
De Castro EA, Kauffman JB. 1998. Ecosystem structure in the Brazilian Cerrado: a vegetation gradient of aboveground biomass root mass and consumption by fire. J Trop Ecol 14:263–83.
Del Castillo RF, Blanco-Macías A. 2007. Secondary succession under a slash-and-burn regime in a tropical montane cloud forest: soil and vegetation characteristics. In: Newton AC, Ed. Biodiversity loss and conservation in fragmented forest landscapes: the forests of montane Mexico and temperate South America. Wallingford: CABI Publishing. p 158–80.
Delaney M, Brown S, Lugo AE, Torres-Lezama A, Bello Quintero N. 1997. The distribution of organic carbon in major components of forests located in five life zones of Venezuela. J Trop Ecol 13:697–708.
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, da Silva Dias PL, Wofsy SC, Zhang X. 2007. Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignorand M, Miller HL, Eds. Climate change 2007: the physical science basis contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. New York, Cambridge: Cambridge University Press.
Dymond JR. 1992. How accurately do image classifiers estimate area? Int J Remote Sens 13:1735–42.
Eastman J. 2006. IDRISI Andes—guide to GIS and image processing. Clark Labs [online]. http://planetuwcacza/nisl/Gwen’s%20Files/GeoCourse/Resource%20Mapping/Andes%20Manualpdf. Accessed 14 Feb 2008.
Edwards PJ, Grubb PJ. 1977. Studies of mineral cycling in a montane rain forest in New Guinea I: the distribution of organic matter in the vegetation and soil. J Ecol 65:943–69.
Farley KA, Kelly EF, Hofstede RGM. 2004. Soil organic carbon and water retention after conversion of grasslands to pine plantations in the Ecuadorian Andes. Ecosystems 7:729–39.
Farley KA, Jobbagy EG, Jackson RB. 2005. Effects of afforestation on water yield: a global synthesis with implications for policy. Glob Change Biol 11:1565–76.
Fehse J, Hofstede R, Aguirre N, Paladines C, Kooijman A, Sevink J. 2002. High altitude tropical secondary forests: a competitive carbon sink? For Ecol Manag 163:9–25.
Flombaum P, Sala OE. 2007. A non-destructive and rapid method to estimate biomass and aboveground net primary production in arid environments. J Arid Environ 69:352–8.
Foody G. 2002. Status of land cover classification accuracy assessment. Remote Sens Environ 80:185–201.
Foster P. 2001. The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Sci Rev 55:73–106.
Glenday J. 2006. Carbon storage and emissions offset potential in an East African tropical rainforest. For Ecol Manag 235:72–83.
Golicher D, Newton AC. 2007. Applying succession models to the conservation of tropical montane forest in biodiversity loss and conservation. In: Newton AC, Ed. Fragmented forest landscapes: the forests of montane Mexico and temperate South America. Wallingford: CABI Publishing. p 200–20.
González-Espinosa M, Ramírez-Marcial N, Newton A, Rey-Benayas JM, Camacho-Cruz A, Armesto JJ, Lara A, Premoli AC, Williams-Linera G, Altamirano A, Alvarez-Aquino C, Cortés M, Galindo-Jaimes L, Muñiz MA, Núñez M, Pedraza RA, Rovere AE, Smith-Ramírez C, Thiers O, Zamorano C. 2007. Restoration of forest ecosystems in fragmented landscapes of temperate and montane tropical Latin America. In: Newton AC, Ed. Biodiversity loss and conservation in fragmented forest landscapes: the forests of montane Mexico and temperate South America. Wallingford: CABI Publishing. p 355–69.
Guo LB, Gifford RM. 2002. Soil carbon stocks and land use change: a meta analysis. Glob Change Biol 8:345–60.
Hamilton K, Sjardin M, Shapiro A, Marcello T. 2009. Fortifying the foundation: state of the voluntary carbon markets 2009. Ecosystem Marketplace and New Carbon Finance.
Hofstede RGM, Groenendijk JP, Coppus R, Fehse JC, Sevink J. 2002. Impact of pine plantations on soils and vegetation in the Ecuadorian High Andes. Mt Res Dev 22:159–67.
Holl KD, Loik ME, Lin EHV, Samuels IA. 2000. Tropical montane forest restoration in Costa Rica: overcoming barriers to dispersal and establishment. Restor Ecol 8:339–49.
Houghton RA, Davidson EA, Woodwell GM. 1998. Missing sinks feedbacks and understanding the role of terrestrial ecosystems in the global carbon balance. Glob Biogeochem Cycles 12:25–34.
INRENA. 2002. Instituto Nacional de Recursos Naturales Plan Maestro—Proyecto Aprovechamiento y Manejo Sostenible de la Reserva de Biosfera y Parque Nacional del Manu (Pro-Manu) Peru: INRENA.
IUCN. 2008. Protected areas and world heritage Peru—Manu National Park. UNESCO IUCN WHC [online] UNEP. http://wwwunep-wcmcorg/sites/wh/pdf/Manupdf. Accessed 08 Jan 2009.
Jackson RB, Banner JL, Jobbagy EG, Pockman WT, Wall DH. 2002. Ecosystem carbon loss with woody plant invasion. Nature 418:623–6.
Jarvis A, Reuter H, Nelson A, Guevara E. 2006. Hole-filled seamless SRTM data v3. Cali: International Center for Tropical Agriculture.
Jenness JS. 2004. Calculating landscape surface area from digital elevation models. Wildl Soc Bull 32:829–39.
Kitayama K, Aiba SI. 2002. Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorus pools on Mount Kinabalu Borneo. J Ecol 90:37–51.
Leuschner C, Moser G, Bertsch C, Röderstein M, Hertel D. 2007. Large altitudinal increase in tree root/shoot ratio in tropical mountain forests of Ecuador. Basic Appl Ecol 8:219–30.
Malhi Y, Grace J. 2000. Tropical forests and atmospheric carbon dioxide. Trends Ecol Evol 15:332–7.
Malhi Y, Aragão LEO, Metcalfe DB, Paiva R, Quesada CA, Almeida S, Anderson L, Brando P, Chambers JQ, da Costa ACL, Hutyra LR, Oliveira P, Patiño S, Pyle EH, Robertson AL, Teixeira LM. 2008. Comprehensive assessment of carbon productivity allocation and storage in three Amazonian forests. Glob Change Biol 15:1255–74.
Mokany K, Raison J, Prokushkin A. 2006. Critical analysis of root:shoot ratios in terrestrial biomes. Glob Change Biol 12:84–96.
Moser G, Roderstein M, Soethe N, Hertel D, Leuschner C. 2008. Altitudinal changes in stand structure and biomass allocation of tropical mountain forests in relation to microclimate and soil chemistry. Ecol Stud 198:229–42.
Paul KI, Polglase PJ, Nyakuengama JG, Khanna PK. 2002. Change in soil carbon following afforestation. For Ecol Manag 168:241–57.
Pucheta E, Cabido M, Díaz S, Funes G. 1998. Floristic composition biomass and aboveground net plant production in grazed and protected sites in a mountain grassland of central Argentina. Acta Oecol 19:97–105.
Raich JW, Russell AE, Vitousek PM. 1997. Primary productivity and ecosystem development along an elevational gradient on Mauna Loa Hawaii. Ecology 78:707–21.
Ramsay PM, Oxley ERB. 2001. An assessment of aboveground net primary productivity in Andean grasslands of Central Ecuador. Mt Res Dev 21:161–7.
Robertson K, Loza-Balbuena I, Ford-Robertson J. 2004. Monitoring and economic factors affecting the economic viability of afforestation for carbon sequestration projects. Environ Sci Policy 7:465–75.
Sarmiento FO, Frolich LM. 2002. Andean cloud forest tree lines: naturalness agriculture and the human dimension. Mt Res Dev 22:278–87.
Schuman GE, Janzen HH, Herrick JE. 2002. Soil carbon dynamics and potential carbon sequestration by rangelands. Environ Pollut 116:391–6.
Scurlock JMO, Hall DO. 1998. The global carbon sink: a grassland perspective. Glob Change Biol 4:229–33.
Stadtmuller T. 1987. Cloud forests in the humid tropics—a bibliographic review. Turrialba: CATIE.
Still CJ, Foster PN, Schneider SH. 1999. Simulating the effects of climate change on tropical montane cloud forests. Nature 398:608–10.
Terborgh J. 1977. Bird species diversity on an Andean elevational gradient. Ecology 58:1007–19.
Van Der Werf GR, Randerson JT, Collatz GJ, Giglio L. 2003. Carbon emissions from fires in tropical and subtropical ecosystems. Glob Change Biol 9:547–62.
Weaver PL, Murphy PG. 1990. Forest structure and productivity in Puerto Rico’s Luquillo Mountains. Biotropica 22:69–82.
Wilcke W, Hess T, Bengel C, Homeier J, Valarezo C, Zech W. 2005. Coarse woody debris in a montane forest in Ecuador: mass C and nutrient stock and turnover. For Ecol Manag 205:139–47.
Williams MS, Schreuder HT. 2000. Guidelines for choosing volume equations in the presence of measurement error in height. Can J For Res 30:306–10.
Young KR, León B. 2000. Biodiversity conservation in Peru’s eastern montane forests. Mt Res Dev 20:208–11.
Young KR, León B. 2007. Tree-line changes along the Andes: implications of spatial patterns and dynamics. Philos Trans R Soc B 362:263–72.
Zimmermann M, Meir P, Silman MR, Fedders A, Gibbon A, Malhi Y, Urrego DH, Bush MB, Feeley KJ, Garcia KC, Dargie GC, Farfan WR, Goetz BP, Johnson WT, Kline KM, Modi AT, Rurau NMQ, Staudt BT, Zamora F. 2010. No differences in soil carbon stocks across the tree line in the Peruvian Andes. Ecosystems 13:62–74.
Acknowledgments
We thank the Blue Moon Fund for support. We especially thank Manu National Park and the Peruvian Instituto Nacional de Recusos National (INRENA) and the Amazon Conservation Association (ACCA) for permission to work in Manu National Park and at the Wayquecha field station, respectively. The guards at Manu National Park and Luis Imunda provided essential logistical support and advice. Five hard-working undergraduate students from Wake Forest University and Flor Zamora, Percy Chambi and Nelson Cahuana from Universidad Nacional de San Antonio Abad del Cusco, were essential for the completion of this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
AG, MRS, YM, JBF, and PM conceived of or designed this study; AG, MRS, MZ, GCD, WRF, and KCG performed research; AG analyzed data; and AG, MRS, YM, and JBF wrote the paper.
Rights and permissions
About this article
Cite this article
Gibbon, A., Silman, M.R., Malhi, Y. et al. Ecosystem Carbon Storage Across the Grassland–Forest Transition in the High Andes of Manu National Park, Peru. Ecosystems 13, 1097–1111 (2010). https://doi.org/10.1007/s10021-010-9376-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-010-9376-8