Abstract
Experimental studies of how global changes and human activities affect plant diversity often focus on broad measures of diversity and discuss the implications of these changes for ecosystem function. We examined how experimental warming and grazing affected species within plant groups of direct importance to Tibetan pastoralists: medicinal plants used by humans and palatable plants consumed by livestock. Warming resulted in species losses from both the medicinal and palatable plant groups; however, differential relative vulnerability to warming occurred. With respect to the percent of warming-induced species losses, the overall plant community lost 27%, medicinal plants lost 21%, and non-medicinal plants lost 40% of species. Losses of palatable and non-palatable species were similar to losses in the overall plant community. The deep-rootedness of medicinal plants resulted in lowered sensitivity to warming, whereas the shallow-rootedness of non-medicinal plants resulted in greater sensitivity to warming; the variable rooting depth of palatable and non-palatable plants resulted in an intermediate response to warming. Predicting the vulnerability of plant groups to human activities can be enhanced by knowledge of plant traits, their response to specific drivers, and their distribution within plant groups. Knowledge of the mechanisms through which a driver operates, and the evolutionary interaction of plants with that driver, will aid predictions. Future steps to protect ecosystem services furnished by medicinal and palatable plants will be required under the novel stress of a warmer climate. Grazing may be an important tool in maintaining some of these services under future warming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global environmental changes and human activities are affecting the distribution and abundance of plant species, with potential implications for ecosystem function and human well-being. Most ecological experimental work on this topic falls into two categories. The first type of study investigates how human activities and global changes affect biodiversity (for example, Klein and others 2004; Walker and others 2006), but does not distinguish species according to their importance for human well-being. The second type of study examines the relationship between biodiversity and ecosystem function (Schwartz and others 2000; Loreau and others 2001; Tilman and others 2001; Zavaleta and Hulvey 2004; Hooper and others 2005). These experiments do not directly examine the relationship between global change drivers and total or plant group biodiversity; moreover, these studies tend to focus on processes such as primary production, nutrient cycling, and resistance to invasion (Kremen 2005; Kremen and Ostfeld 2005; Hooper and others 2005; Balvanera and others 2006). Neither type of study examines how global changes and human activities affect the diversity of species within plant groups of direct use to humans. The lack of controlled experiments in real ecosystems represents a gap in our understanding of which plant groups directly utilized by humans may be most vulnerable to which human activities and what factors contribute to their relative stability.
Here, we present results from a study of the independent and combined effects of two drivers, climate warming and grazing, on plant species distinguished by the products they provide to a Tibetan Plateau subsistence-oriented pastoral society. Recent climate warming has already been observed on the Tibetan Plateau (Thompson and others 1993, 2000); moreover, future warming there is predicted to be “greater than average” (Giorgi and others 2001). Concurrent with these climatic changes, land-use changes associated with privatization and sedentarization are reportedly increasing grazing pressures on the land (Miller 1999). Inversely, recent policies—driven by the perception that overgrazing is the main cause of rangeland degradation—are completely eliminating domestic grazing from these rangelands (Yeh 2005). We examined how climate warming and grazing altered the number of medicinal and palatable plant species. Rather than focusing on warming and grazing effects on overall plant diversity, or on plant groups that affect a given ecosystem function, we examined effects on the species within two plant groups which are of direct importance to pastoralists on the Tibetan Plateau.
Medicinal plants are an important group of plant species on the Tibetan Plateau. Traditional Tibetan medicine, and the plants and animals from which these medicines are derived, have long been an integral aspect of Tibetan life and society. In rural areas, Tibetan medicine is the most accessible form of health care and tends to serve the most impoverished Tibetans (Janes 1999; Salick and others 2006). As national and foreign demand for Tibetan medicinal products is ‘skyrocketing’ (Salick and others 2006), medicinal plants are becoming an important commodity (Janes 1999; Law and Salick 2005). Data regarding the harvest, trade, and use of Tibetan medicinal plants are scarce. In the Tibetan Autonomous Prefecture of NW Yunnan Province, non-timber forest products, which include medicinal plants, provide from 25 to 80% of Tibetan household cash incomes (Xu and Wilkes 2004; Salick and others 2005). In neighboring Nepal, 323,000 households (10% of all rural households) harvest an estimated 7,000–27,000 tons of medicinal plants annually (Olsen 2005).
Palatable livestock forage is another important group of plant species on the Tibetan Plateau. Pastoralism has been the main form of subsistence for its inhabitants for millennia (Miller 1998). Indeed, the rangelands of the Tibetan Plateau are one of the most extensive grazing systems in the world. Herders have a diverse mix of livestock, including yak, sheep, and horses. From their livestock, herders obtain meat, dairy products, wool for clothing and housing, fuel for heating and cooking, transportation, income and capital. Across the Plateau, supplemental feeding is low; rather, livestock primarily rely on the natural vegetation to meet their energetic and nutritional requirements (Miller 1999). Thus, the natural livestock forage on the Tibetan Plateau is the foundation for many of the important products on which the Tibetan pastoralists survive.
We sought to understand medicinal and palatable forage plant species’ responses to warming and grazing using plant traits. Plant traits influence both how species respond to environmental drivers and affect ecosystem processes (Hooper and others 2005). Plant functional response types refer to plants that share traits for responding to environmental change, whereas plant functional effect types refer to plants that have a similar effect on a given ecosystem function, such as net primary productivity (Leishman and Westoby 1992; Lavorel and others 1997; Walker and others 1999; Diaz and Cabido 2001; Hooper and others 2005). The plant species within the medicinal and palatable forage plant groups are each analogous to a functional effects type in that plant species within each group serve a common role in the ecosystem (with respect to use by humans, for medicinal plants, and to use by the livestock on which humans depend, for palatable forage plants). We were interested in whether plant species within the medicinal and palatable forage plant groups (that is, those that serve a broad, but common role in the system) also share traits by which they respond to environmental and land-use drivers and whether this mediates that group’s response to the drivers. Current literature on the topic would suggest that having more plant response traits represented within a functional effect type (or, in this case, the medicinal or palatable plant group) would make that plant group more resistant or resilient to an environmental driver (Chapin and others 1997; Walker and others 1999; Yachi and Loreau 1999; Diaz and Cabido 2001; Lavorel and Garnier 2002; Hooper and others 2005).
The plant traits we examined were life history, growth form, rooting depth, and flowering phenology. All of these traits have been shown to influence plant responses to environmental factors and disturbances (Grime 1977; Golluscio and Sala 1993; Chapin and others 1996; Lavorel and others 1997; Price and Waser 1998; De Valpine and Harte 2001). We anticipated the perennial species would be less sensitive to warming than the annual/biennial species, because perennial species have belowground reserves from which to better tolerate or recover from environmental stresses. Similarly, we predicted that deep-rooted species would be less sensitive to warming than shallow-rooted species for the same reason described for perennial versus annual/biennial species. In addition, the microclimate and resources experienced by shallow-rooted species would likely be more impacted by warming than those experienced by deep-rooted species. Because we found that forb aboveground net primary productivity (ANPP) was resistant, whereas graminoid ANPP was sensitive to warming (Klein and others 2007), we predicted that forb species numbers would be more resistant, whereas graminoid species numbers would be more sensitive to warming. Finally, we anticipated that the early to mid-season flowering species would be more responsive to warming than the late-flowering species, as was found in another high altitude warming study (Price and Waser 1998).
We posed the following questions: what are the independent and combined effects of experimental warming and grazing on the number of medicinal and palatable plant species? Is one plant group relatively more vulnerable to species losses than the other? Are plant traits associated with particular plant use groups? If so, do the traits possessed by species within these plant groups or characteristics of the drivers mediate the plant group responses?
Methods
Site Description and Experimental Design
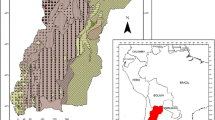
We established our experiment at four sites within the northeastern region of the Tibetan Plateau (latitude 37°37′ N, longitude 101°12′ E) (Figure 1). Two sites were situated in meadow habitat along the valley floor at 3,200 m and two sites were situated in shrubland habitat on the higher slopes encircling the valleys at 3,240 m elevation. These two habitat types comprise approximately 35% of the area of the Tibetan Plateau. Abiotic and biotic site characteristics have been documented by Klein and others (2004, 2005, 2007). We fenced each of the four 30 × 30 m sites within which we laid out 16 plots in a 4 × 4 matrix (for a total of 64 plots) (Figure 1). Within each site, we established a complete factorial experimental design where we simulated warming using open top chambers (OTCs) and the defoliation effects of grazing through selective clipping. We placed the conical OTCs on the plots in September 1997. The OTCs, which were 1.5-m diameter and 40-cm high, remained on the plots year-round. The chambers elevated growing season averaged daily air temperature by 0.6–2.0°C. In some sites and years, OTCs increased growing season averaged soil temperature, measured at 12-cm depth, by 0.3–1.9°C. The OTC effects on soil moisture, measured at 12-cm depth, depended on habitat, grazing history, and the presence or absence of clipping (Klein and others 2005). We began the defoliation treatments in the spring of 1998. In the winter-grazed meadows, we clipped the plots prior to initiation of growth in the early spring. In the summer-grazed shrublands, we clipped the plots in mid-July. We clipped plots to approximately 3 cm in height, which is the height of the vegetation outside of our fenced plots in the sites with a high grazing history. The simulated grazing was selective in that we did not clip plants that yak and sheep completely avoid (Oxytropis spp., and Stellera chamaejasme). Moreover, we plucked the shrub leaves and stem tips to simulate sheep browsing. To examine how well clipping simulated the effects of actual grazing, in 2000 we established four replicated “grazing control” (GC) plots situated outside of the fenced areas in all four sites. We established these plots more than 5 m but less than 15 m away from the fence to eliminate any “fence” effect but to be representative of the plots within the fenced area. Pairs of GC plots were approximately 2 m apart from each other.
Left side: study site region. The asterisk indicates the approximate location of the experiment. Right side: experimental design. We conducted this study at summer-grazed shrubland and winter-grazed meadow. Within each habitat, we identified “low” and “high” grazing intensity history sites, for a total of four study sites. In each site, we fenced a 30 × 30 m area, within which we established a fully factorial experimental design, with “OTCs (warming)” and “clipping” as our main treatments. We had four replicates per treatment.
Medicinal and Palatable Forage Plant Groups
We identified all of the plant species present within the study plots and characterized them with respect to two main categories: medicinal properties and palatability (Supplementary Appendix A). Plants were characterized as either medicinal (possessing medicinal properties) or non-medicinal (lacking medicinal properties). Some of the plants that possess medicinal properties were not necessarily utilized as such by the local pastoral community in our study region; however, we categorized these as medicinal plants because they possess medicinal properties which are utilized in other regions of the Himalaya and the Tibetan Plateau. Plants were also characterized as either palatable or non-palatable to the main domestic livestock in the region: yak, sheep, and horses. Non-palatable plants are those that the livestock tend to avoid. There is certainly a range of palatability along the palatable and non-palatable continuum. However, because livestock rely on the natural vegetation (and receive minimal supplemental feed), they eventually graze both the more and less palatable plants and tend to avoid only the non-palatable plant species. Each plant species was assigned to each of the two plant groups. Assignment into the medicinal or non-medicinal group was independent of assignment into the palatable or non-palatable group. For example, a given plant species was characterized as medicinal or non-medicinal and as palatable or non-palatable.
Although our two plant groups—medicinal and palatable plants—are broadly defined, this categorization is an important first step toward evaluating potential future changes to plant species that are of particular importance to human communities. Moreover, certain technical barriers, such as the complexity of Tibetan medicine and its lack of direct comparison to Western medicine (Adams 2001) preclude our further dividing medicinal plants into, for example, groups according to uses for different ailments. Thus, the “substitution approach” of Kremen and others (1998) is beyond the scope of this project. Furthermore, by using these broader plant groupings, we can discuss potential implications for a broader set of users. For example, in the rural areas of Tibet, there is a high degree of variation in plant use by medicinal practitioners due to different traditions (with different medical lineages dominating in different parts of Tibet), environmental variation across the region, and different types of afflictions in different regions (Salick and others 2006). Thus, we have categorized medicinal plants as those plants that have potential medicinal uses broadly across the Plateau. Moreover, because the relative species composition of herds can vary over small distances on the Plateau (Ekvall 1968; Miller 1999), the broader category of “palatable plants,” versus more narrow distinctions according to uses by different animal species, is more generalizable across the region.
We assumed that the number of species within a plant-use group represents the robustness of that plant-use group. That is, the greater the number of species within the medicinal or palatable plant group, the greater the magnitude of the product provided by that plant-use group. For medicinal plants, the more medicinal species present, the more potential uses of these plant species and the more potential ailments they can treat. Losing one species could represent losing one treatment. There may be some redundancy in terms of different species treating similar ailments, but we assumed little overlap. Similarly, Dobson and others (2006) asserted that for provisioning ecosystem services such as pharmaceutical drugs, the loss of an individual species results in the loss of a “unit” of ecosystem service. That is, a linear decrease in a service occurs as each species is lost (Dobson and others 2006). For palatable plants, overall ANPP would also be a good indicator of the magnitude of the goods provided by that plant-use group. Because we wanted to compare the responses of medicinal and palatable plant groups to the treatments, we used the common metric of species numbers within a plant-use group. For palatable plants, we also discuss the results with respect to ANPP.
Plant Traits
We characterized plants with respect to four plant traits: life history (perennial, annual/biennial), growth form (forb, shrub, grass, sedge), rooting depth (shallow, deep), and flowering phenology (early blooming—May/June, average blooming—July/August, late blooming—September) (Supplementary Appendix A). Deep-rooted plants were defined as plants whose majority of roots penetrate to greater than 10 cm into the soil, whereas shallow-rooted plant roots were defined as plants whose majority of roots are limited to the top 0–10 cm of the soil. For the analyses, we combined grasses and sedges into the graminoid growth form. Experts in medicinal and rangeland plants at the Northwest Plateau Institute of Biology, Chinese Academy of Sciences, conducted the plant-use and plant trait categorizations.
Statistical Analyses
We conducted a split-plot, repeated measures ANOVA using SAS 9.1 (released 2002–2003) to identify significant treatment effects on species numbers. The whole plot component consisted of habitat, the split-plot component consisted of the individual plots within each site, the treatments were warm and clip and the repeated measure was year. We used a first-order autoregressive covariance structure for the repeated measure model. We had a balanced study design and used the Tukey test to adjust for multiple comparisons. Our treatments were: T CONTROL = (no OTC, no clip); T WARM = (+OTC, no clip); T CLIP = (no OTC, +clip); T WARM*CLIP = (+OTC, +clip). If a warm × clip interaction was absent, we refer to an overall “warm effect” = [(T WARM + T WARM*CLIP)/2 − (T CONTROL + T CLIP)/2]. This describes the average effects of warming, in both the presence and absence of clipping. If, however, a warm × clip interaction was present, we describe the effects of “warm (no clip)” = (T WARM − T CONTROL) and “warm (+clip)” = (T WARM*CLIP − T CLIP) separately. We follow the same convention for the clipping effects.
We also conducted a split-plot, repeated measures ANOVA with the same structure as described above, but where the treatment was warm × clip. This captures the “combined effects of warming and clipping” = (T WARM*CLIP − T CONTROL). If no warm × clip interaction was present, the combined treatments were strictly additive, where (T WARM*CLIP − T CONTROL) = [(T WARM − T CONTROL) + (T CLIP − T CONTROL)]. That is, the combined treatment effect was equal to the sum of the separate warm (no clip) and clip (no warm) effects. If an interaction was present, then (T WARM*CLIP − T CONTROL) = [(T WARM − T CONTROL) + (T CLIP − T CONTROL) + Interaction Effect]. Here, the combination of warm and clip interacted to create an effect that was not represented by the sum of the individual warm (no clip) and clip (no warm) effects. Rather, the combined effect and the strictly additive effect differed by the sign and magnitude of the interaction effect.
Because the number of species within each plant-use group varied, we compared relative responses by converting the results (number of species) into the percent of species change (% species relative to pre-treatment) within each plant-use group. With respect to the warming treatment, we refer to this value as “percent of warming-induced species change” = [(“number of species in warm plots” − “number of species in non-warm plots”)/“number of species in non-warm plots”] × 100. The number of species in the non-warm plots was from the same year as the number of species in the warm plots. We also conducted a split-plot, repeated measures ANOVA to examine treatment effects on the proportion of species represented within each plant-use group relative to the proportion of species in the non-plant-use group (for example, medicinal versus non-medicinal plants). With respect to medicinal plants, we refer to this as “proportion of medicinal plants” = (“number of medicinal plant species present”/“total number of plant species present”). Both the “percent of treatment-induced species change” and the treatment effects on the “proportion of species within a plant group” allowed us to compare treatment effects on plant groups despite differences in the initial number of species present in each group. More species within a plant-use group represent a more robust plant-use group. Therefore, a larger % treatment-induced species loss or proportional decline in a plant-use group indicates a greater vulnerability of that plant-use group to the treatment. To examine whether significant associations existed between plant-use groups and plant traits, we conducted a Chi-square (χ2) goodness-of-fit analysis. We examined all possible two-way pairings of plant-use groups with plant-trait groups using the crosstabs statistic in SPSS 11.5. We present the Pearson χ2 statistic.
Results
Treatment Effects on Species Richness Within Medicinal and Palatable Plant Groups
Both medicinal and palatable plant groups experienced species losses with warming. The warming-induced species loss was independent of habitat. For both plant groups, there was a significant warm × year interaction, with no warming effect in 1998 and significant effects thereafter (Table 1). Medicinal plants lost 4.0 species in 1999 (P = 0.001), 5.0 species in 2000 (P < 0.0001), and 5.7 species in 2001 (P < 0.0001), for an average loss of 4.9 species over the 3 years (Figure 2A). Palatable plants lost 4.4 species in 1999 (P < 0.0001), 5.5 species in 2000 (P < 0.0001), and 6.1 species in 2001 (P < 0.0001), for an average loss of 5.3 species over the 3 years (Figure 2B).
Warming effects (solid line) and clipping effects (dashed line) on (A) medicinal plant species numbers and (B) palatable plant species numbers from 1998 to 2001. Since warm × clip interactions were absent, for each year we present the “overall warming effect” = [(T WARM + T WARM*CLIP)/2 − (T CONTROL + T CLIP)/2] and the “overall clipping effect” = [(T CLIP + T WARM*CLIP)/2 − (T CONTROL + T WARM)/2]. Asterisks represent a significant treatment effect at P ≤ 0.05 for that year. ANOVA results are presented in Table 1.
Clipping accurately simulated grazing effects on species numbers within the medicinal and palatable plant-use groups (Supplementary Appendix B). There were no significant differences between the number of species in clipped and grazed plots for both medicinal and palatable plants (Figure 3). Species within both plant-use groups were resistant to or enhanced by simulated grazing; this effect was independent of habitat (Table 1). Clipping, which had no significant effect on medicinal plant species in all years (Figure 2A), initially increased palatable plants by 2.3 species in 1998 (P = 0.04), with no significant effects thereafter (Figure 2B).
Mean number of species plus standard error for (A) medicinal plant species and (B) palatable plant species in clipped plots (white bar with cross hatch) and grazed plots (black bar with cross hatch) in 2000 and 2001. Grazed plots were outside of the fenced experimental plots and were grazed by yak and sheep. Repeated measures ANOVA revealed no significant difference in species numbers between clipped and grazed plots for both plant-use groups (Supplementary Appendix B).
The combined warm and clip treatment interacted with year to affect species numbers, but was independent of habitat (Table 2). For medicinal plants, there was no combined treatment effect in 1998. Medicinal plants then declined by 4.6 species in 1999 (P = 0.02), 3.3 species in 2000 (P = 0.07), and 3.8 species in 2001 (P = 0.04), for an average loss of 3.9 species over the 3-year period (Figure 4A). Combined treatments marginally increased palatable plants by 1.4 species in 1998 (P = 0.06). Palatable plants then declined by 4.8 species in 1999 (P = 0.03), 4.9 species in 2000 (P = 0.03), and 6.4 species in 2001 (P = 0.006), for an average decline of 5.4 species over the 3-year period (Figure 4B). For both medicinal and palatable plant species, the combined effects of warming and clipping (T WARM*CLIP − T CONTROL) initially resulted in more species losses as compared to the additive treatment effect [(T WARM − TCONTROL) + (T CLIP − T CONTROL)]. However, by the fourth year of the study, the combined treatment effect resulted in fewer species losses than the additive treatment effect (Figure 4). In 2001, medicinal plants lost 3.8 species under the combined model and 5.9 species under the additive model; palatable plants lost 6.4 species under the combined model and 9.9 species under the additive model. That is, for both medicinal and palatable plant species, approximately 35% more species were lost under the strictly additive effects of warming and grazing as compared to the combined effects of warming and grazing in 2001.
Additive (dashed line) and combined (solid line) warm and clip effects on (A) medicinal plant species numbers and (B) palatable plant species numbers from 1998 to 2001. The combined effect = (T WARM*CLIP − T CONTROL), whereas the additive effect = [(T WARM − T CONTROL) + (T CLIP − T CONTROL)]. Double asterisks represent a significant combined or additive effect at P ≤ 0.05 for that year. Single asterisk represents a marginally significant effect at 0.05 < P ≤ 0.1. ANOVA results are presented in Table 2.
Relative Vulnerability of Medicinal and Palatable Plant Groups to Warming
Because the initial number of species within each plant-use group differed, we examined warming effects on the proportion of species and the percent of warming-induced species loss in different plant-use groups. This allowed us to compare relative responses between the plant-use groups. Because clipping effects on species numbers were small in comparison to warming effects, we do not discuss clipping effects on the proportion of species and the percent of clipping-induced species changes. Warming had no effect on the proportion of medicinal plant species in 1998, but had significant effects in subsequent years (Table 3). Warming increased the proportion of medicinal plants by 0.08 in 1999 (P = 0.001) and by 0.05 in both 2000 and 2001 (P = 0.03 and 0.05, respectively), for an average increase of 0.06 over the 3-year period. Commensurate decreases in the proportion of non-medicinal plant species occurred. In contrast, the proportion of pala table and non-palatable plant species did not significantly change with warming in all years (Table 3).
As reported previously, significant warming-induced species losses occurred in both plant-use groups from 1999 to 2001. Medicinal plants lost a relatively smaller percent of species with warming (21% averaged from 1999 to 2001) as compared to the other plant-use groups and the overall plant community. In contrast, non-medicinal plants lost a relatively higher percent of species with warming (40% averaged from 1999 to 2001) as compared to the other plant-use groups and the overall plant community (Figure 5A). Palatable and non-palatable plants experienced an intermediate percent of species loss with warming (25 and 30%, respectively, averaged from 1999 to 2001); this decline was similar to the loss observed for the overall plant community (27% averaged from 1999 to 2001) (Figure 5B).
Percent warming-induced species loss (warmed species numbers relative to non-warmed species numbers) from 1998 to 2001 for (A) medicinal and non-medicinal plant species, (B) palatable and non-palatable plant species, and (C) deep and shallow-rooted plant species. We present the percent warming-induced species loss for all species (the ‘plant community’) in all three panels as a reference. We conducted the statistical tests on species numbers (see Table 1 and Figures 2 and 6); however, we converted these into percent warming-induced species losses for comparison purposes. There were warm × clip effects on shallow-rooted species numbers, as described in the text and presented in Figure 6. However, here we present warming effects averaged over clip and non-clip plots to compare the warming effects on shallow-rooted species to warming effects on (1) deep-rooted species and (2) medicinal and palatable plant-use groups (where warm × clip interactions were absent).
Plant Traits and Medicinal/Palatable Plant Groups
To evaluate whether the different plant group responses to warming, as described above, were related to plant traits, we examined χ2 associations between plant-use groups and plant traits (Table 4). We also examined χ2 associations between the medicinal and palatable plant-use groups. The majority of medicinal plants were non-palatable (61%) whereas less than half were palatable (39%). Of the palatable plants, approximately half were medicinal (48%) and half were non-medicinal (52%).
There was a significant χ2 association between medicinal plant groups and growth form. Specifically, medicinal plants were primarily forbs (99%), whereas non-medicinal plants were both forbs (33%) and graminoids (67%). There was also a significant χ2 association between medicinal plant groups and rooting depth. In particular, 66% of medicinal plants were deep-rooted, whereas 70% of non-medicinal plants were shallow-rooted. There were no significant χ2 associations between medicinal plant groups and life history and between medicinal plant groups and phenology. Seventy-seven percent of both medicinal and non-medicinal plants were perennial. Moreover, the majority of medicinal and non-medicinal plants bloomed during July and August (65% for each group). Of medicinal plants, only 10% were early blooming species, whereas 25% were late blooming species; of non-medicinal plants, 13% were early blooming, whereas 23% were late blooming.
There were significant χ2 associations between palatable plant groups and growth form as well as between palatable plant groups and life history. Palatable plants consisted of both forbs (52%) and graminoids (45%), whereas all non-palatable plants were forbs (100%). Palatable plants were almost all perennial (95%), whereas non-palatable plants were a mixture of perennials (57%) and annuals (43%). There was no significant χ2 association between palatable plant groups and rooting depth. Of the palatable plants, 48% were shallow-rooted and 52% were deep-rooted. Of non-palatable plants, 47% were shallow-rooted and 53% were deep-rooted. There was also no significant χ2 association between palatable plant groups and phenology. The majority of palatable plants (67%) and non-palatable plants (60%) bloomed during July and August. Of palatable plants, 14% were early blooming species, whereas 19% were late blooming species; of non-palatable plants, 8% were early blooming, whereas 32% were late blooming.
Warming Effects on the Rooting Depth Plant Trait
The associations between plant traits and plant groups reported above suggest that rooting depth could be responsible for the different plant group responses to warming (as expressed by the percent warming-induced species losses and the proportional changes in plant groups). To examine the link between plant group responses to warming and rooting depth, we describe how shallow and deep-rooted species responded to warming. Because it is unlikely that the other plant traits were responsible for the different plant group responses to warming (we elaborate on this point in the “Discussion” section), we do not present results regarding how the other plant traits responded to warming.
For both deep and shallow-rooted plant species, there was a warm × year interaction with no effect in 1998 and significant effects thereafter (Table 1). These effects were independent of habitat. For deep-rooted plants, the warming effect was also independent of clipping. Warming decreased deep-rooted plants by 4.0 species in 1999 (P = 0.01), 3.9 species in 2000 (P = 0.01), and 4.5 species in 2001 (P = 0.002) for an average loss of 4.1 species from 1999 to 2001. For shallow-rooted species, warming also interacted with clipping such that the warming-induced species loss was greater in the absence than in the presence of clipping. Warming in the absence of clipping resulted in shallow-rooted species losing 4.4 species in 1999 (P = 0.005), 6.7 species in 2000 (P < 0.001), and 7.6 species in 2001 (P < 0.001) for an average loss of 6.2 species from 1999 to 2001. Warming in the presence of clipping resulted in shallow-rooted species losing 4.9 species in 1999 (P = 0.002), 4.2 species in 2000 (P = 0.007), and 4.5 species in 2001 (P = 0.004) for an average loss of 4.5 species from 1999 to 2001 (Figure 6).
Warming effects on shallow-rooted plant species numbers in the absence of clipping (black triangles, solid line) and in the presence of clipping (open triangles, dashed line) from 1998 to 2001. “Warm (no clip)” = (T WARM − T CONTROL) and “warm (+clip)” = (T WARM*CLIP − T CLIP). Asterisks represent a significant warm (no clip) or warm (+clip) effect at P ≤ 0.05 for that year. ANOVA results are presented in Table 1.
From 1998 to 2001, warming increased the proportion of deep-rooted species by 0.05, with a commensurate decrease in the proportion of shallow-rooted species over the same timeframe; the warming effect was independent of both habitat and year (Table 3). The proportional changes in deep and shallow-rooted plant species were similar to the proportional changes in medicinal and non-medicinal plant species, respectively. In response to warming, deep-rooted species lost an average of 20% species from 1999 to 2001. Shallow-rooted species lost an average of 39% species from 1999 to 2001. The percent warming-induced species losses for deep and shallow-rooted species reflected the losses experienced by the medicinal and non-medicinal plant groups, respectively (Figure 5C).
Discussion
Our results demonstrate that both medicinal and palatable plant groups were vulnerable to species losses with warming. These important plant-use groups are likely to be vulnerable to future warming in this region of the Tibetan Plateau, potentially leading to the reduced well-being of the pastoralists. However, for some groups of species, grazing can mitigate some of the negative warming effects on species numbers. In previous work, we demonstrated that experimental warming decreased palatable plant ANPP (Klein and others 2007). The warming-induced loss of palatable plant species numbers and reduction in palatable plant ANPP indicate a decrease in the robustness of that plant-use group with warming.
Plant Group Responses to Warming Versus Grazing
The different plant group responses to simulated warming and grazing suggest that knowledge of the mechanisms through which a driver operates, as well as the evolutionary interaction of plants with that driver, is critical for predicting which plant group will be more resistant to which environmental and land-use drivers. Warming and grazing differ in their primary effects on plants. The primary warming effect is to directly alter microclimate (Klein and others 2005), with maximum air temperatures occasionally exceeding the mean heat tolerance temperature of plants (Klein and others 2004). Thus, the species in our experimental plots might lack resistance to the novel microclimate conditions presented by experimental warming. In contrast, the primary grazing effect is the direct removal of plant biomass and litter. Because plants on the Tibetan Plateau evolved with grazing, these plants have developed mechanisms for persistence under grazing. Our results suggest that the current policy of removing grazing from the system in some regions of the Tibetan Plateau (Yeh 2005) could be detrimental to some species. Moreover, our finding that the combined warm and clip treatment effect differed from the additive warm and clip treatment effect suggests we cannot predict the combined effects of environmental and land-use drivers from single factor studies.
Plant Traits Mediate the Plant Group Response to Warming
Knowledge of plant traits, their distribution within a plant-use group, and their response to specific drivers, can also enhance predictions of how that plant group will respond to environmental and land-use drivers. In our system, rooting depth could explain the different plant group responses to warming. Non-medicinal plants (the group relatively more vulnerable to warming) were primarily shallow-rooted species, whereas medicinal plants (the group relatively less vulnerable to warming) were primarily deep-rooted. Palatable and non-palatable plants, which were both moderately vulnerable to warming, had a relatively even mixture of both deep and shallow-rooted plant species. Warming can favor deep-rooted plant species by two different mechanisms. First, warming could directly damage plants through occasional heat stress or enhanced susceptibility to freezing events (Klein and others 2004; Loik and others 2004; Lambrecht and others 2007). Plants with larger belowground reserves can both tolerate and recover from these stressful physiological events better than plants with smaller belowground reserves. For example, higher levels of photosynthates could provide for greater cryoprotection or other acclimation mechanisms (Loik and others 2004). Second, warming can affect plants by altering the availability of soil resources (Chapin and others 1995; Harte and others 1995). Because warming increased nitrogen availability in this study (Klein and others 2004), it is unlikely that this resource explains differential responses to warming between deep and shallow-rooted plants. Warming at our site led to no change in soil moisture (measured at 12-cm depth) averaged over the growing season across all sites; however, at certain sites, and at certain times during the growing season, warming did decrease soil moisture (Klein and others 2005). Moreover, the depth of our soil moisture probes was generally beyond the rooting depth of shallow-rooted species. The warming-induced decreased decomposition rates of litterbags placed at the soil surface (Klein and others 2004) suggest that some amount of near surface soil drying may have occurred at certain periods of the study. If this was the case, the deep-rooted species would be at an advantage over shallow-rooted species with respect to greater access to soil moisture pools. Another high elevation warming experiment found that warming decreased the annual production and flowering success of shallow-rooted forbs, but either increased or had no effect on the production and flowering success of deep-rooted forbs (De Valpine and Harte 2001; Saavedra and others 2003).
None of the other traits we identified could explain the differential responses to warming between medicinal and palatable plant groups. Although most medicinal plants were forbs, most non-palatable plants were also forbs. Therefore, if forbs were susceptible to warming, then the non-palatable plants would have experienced similar % species loss and proportional changes as the medicinal plants. Because this was not the case, growth form could not explain our results. Because both medicinal and non-medicinal plants were primarily perennial plants and also plants that bloomed in July/August, the different responses of medicinal versus non-medicinal plants can be explained neither by life history nor by phenology.
The shared deep-rooted plant trait among most medicinal plant species can explain this group’s lowered vulnerability to warming as compared to non-medicinal plants. Here, the vulnerability of medicinal plants to a driver depends on whether that driver favors deep or shallow-rooted plants: for drivers that favor the former, medicinal plants will do better; for drivers that favor the latter, medicinal plants species will do worse. In contrast to the situation for medicinal and non-medicinal plants, the distribution of plant rooting depths across palatable and non-palatable plant groups was relatively equitable. This relatively even distribution of plant rooting depths across palatable and non-palatable plant groups can explain these two groups’ similar responses to warming and their intermediate sensitivity to warming as compared to the medicinal and non-medicinal plant groups. These results suggest that palatable and non-palatable plant species are likely to be similarly and moderately responsive to a driver (or a suite of drivers) regardless of which rooting depth that driver favors. That is, some degree of resistance is built into the response of the palatable plant group due to the presence of both deep and shallow-rooted plant species within this group. Thus, in our particular case, deep-rooted plants (and, therefore, medicinal plants) were most resistant to warming. However, in a more general case, (1) where there is uncertainty regarding how a given driver will affect plant traits; or (2) where there are a suite of drivers that might differentially affect plant traits, a plant-use group comprised of species which do not share similar plant traits is more likely to be resistant to that driver, or suite of drivers (as demonstrated by palatable and non-palatable plants). These findings are consistent with the literature (Chapin and others 1997; Walker and others 1999; Yachi and Loreau 1999; Diaz and Cabido 2001; Lavorel and Garnier 2002; Hooper and others 2005); however our work extends this discourse beyond the maintenance of ecosystem function to the preservation of plant groups that provide a common and direct use to humans.
Implications for Ecosystem Services
Medicinal and palatable forage plant species can be characterized as plant species that deliver provisioning ecosystem services. The Millennium Ecosystem Assessment (MEA) describes provisioning ecosystem services as the products obtained from ecosystems (MEA 2003). We have already described above how medicinal and palatable plants provide important products to pastoralists and their livestock on the Tibetan Plateau. Additionally, Tibetan medicine occupies a central social and cultural role in modern Tibetan society (Janes 1999). Janes (1999) argues that the success of Tibetan medicine can be central to a process of cultural revitalization in Tibet. Moreover, because Tibetan culture, history, and identity are inextricably linked to the Tibetan pastoral production system (Ekvall 1968; Miller 1999), plants palatable to domestic livestock are also essential to Tibetan culture and identity. Our work, therefore, suggests that important provisioning ecosystem services that are of central importance to Tibetan culture and identity may be vulnerable to future warming on the Tibetan Plateau. Quantifying the impact of this decline on local communities will require a socio-economic analysis that is beyond the scope of this work.
Conclusion
This study is an example of how to examine and predict how global changes and human activities may affect locally important and utilized plant species in the near future. We conclude that the nature of the drivers, the evolutionary interaction of plants with those drivers, the way in which the drivers affect plant traits, and the distribution of plant traits within the plant-use groups, are all important components for predicting the vulnerability of these plant groups to environmental and land-use drivers. We found that plant groups that deliver important provisioning ecosystem services and that are of central importance to Tibetan culture on the Tibetan Plateau are vulnerable to species losses with warming. However, grazing management can mitigate some of the negative warming effects. The generality of our findings regarding which plant groups are vulnerable to which human activities should be tested in other systems. Moreover, these findings illustrate the relatively short-term response to warming and grazing; understanding the long-term implications of changing climate and land use will require an analysis of a distinct set of response variables associated with reproduction and recruitment. Future steps to protect important plant groups that furnish ecosystem services, particularly for people who rely on subsistence-based livelihoods, will be required under a warmer climate.
References
Adams V 2001. The sacred in the scientific: ambiguous practices of science in Tibetan medicine. Cult Anthropol 16:542–75
Balvanera P, Pfisterer AB, Buchmann N, He J-S, Nakashizuka T, Raffaelli D, Schmid B 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett 9:1146–56
Chapin FS, Bret-Harte MS, Hobbie SE, Zhong HL 1996. Plant functional types as predictors of transient responses of arctic vegetation to global change. J Veg Sci 7:347–58
Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76:694–711
Chapin FS, Walker BH, Hobbs RJ, Hooper DU, Lawton JH, Sala OE, Tilman D 1997. Biotic control over the functioning of ecosystems. Science 277:500–4
De Valpine P, Harte J 2001. Plant responses to experimental warming in a montane meadow. Ecology 82:637–48
Diaz S, Cabido M 2001. Vive la difference: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–55
Dobson A, Lodge D, Alder J, Cumming GS, Keymer J, McGlade J, Mooney H, Rusak JA, Sala O, Wolters V, Wall D, Winfree R, Xenopoulos MA 2006. Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 87:1915–24
Eckvall RB 1968. Fields on the hoof: nexus of Tibetan nomadic pastoralism. New York: Holt, Rinehart and Winston
Giorgi F, Hewitson B, Christensen J, Hulme M, Von Storch H, Whetton P, Jones R, Mearns L, Fu C 2001. Climate change 2001: regional climate information—evaluation and projections. In: Houghton JT, Ding Y, Griggs DJ, Noguer M, Linden PJV, Dai X, Maskell K, Johnson CA, Eds. Climate change 2001: the scientific basis. Contribution of working group i to the third assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press. pp 583–636
Golluscio RA, Sala OE 1993. Plant functional types and ecological strategies in Patagonian forbs. J Veg Sci 4:839–46
Grime JP 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–94
Harte J, Torn M, Chang F, Feifarek B, Kinzig A, Shaw R, Shen K 1995. Global warming and soil microclimate: results from a meadow-warming experiment. Ecol Appl 5:132–50
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setala H, Symstad AJ, Vandermeer J, Wardle DA 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Janes CR 1999. The health transition, global modernity and the crisis of traditional medicine: the Tibetan case. Soc Sci Med 48:1803–20
Klein JA, Harte J, Zhao XQ 2004. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecol Lett 7:1170–9
Klein JA, Harte J, Zhao XQ 2005. Dynamic and complex microclimate responses to warming and grazing manipulations. Glob Change Biol 11:1440–51
Klein JA, Harte J, Zhao XQ 2007. Experimental warming, not grazing, decreases rangeland quality on the Tibetan Plateau. Ecol Appl 17:541–57
Kremen C 2005. Managing ecosystem services: what do we need to know about their ecology? Ecol Lett 8:468–79
Kremen C, Ostfeld RS 2005. A call to ecologists: measuring, analyzing, and managing ecosystem services. Front Ecol Environ 3:540–8
Kremen C, Raymond I, Lance K 1998. An interdisciplinary tool for monitoring conservation impacts in Madagascar. Conserv Biol 12:549–63
Lambrecht SC, Loik ME, Inouye DW, Harte J 2007. Reproductive and physiological responses to simulated climate warming for four subalpine species. New Phytol 173:121–34
Lavorel S, McIntyre S, Landsberg J, Forbes TDA 1997. Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends Ecol Evol 12:474–8
Lavorel S, Garnier E 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–56
Law W, Salick J 2005. Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae). Proc Natl Acad Sci USA 102:10218–20
Leishman MR, Westoby M 1992. Classifying plants into groups on the basis of associations of individual traits: evidence from Australian semiarid woodlands. J Ecol 80:417–24
Loik ME, Still CJ, Huxman TE, Harte J 2004. In situ photosynthetic freezing tolerance for plants exposed to a global warming manipulation in the Rocky Mountains, Colorado, USA. New Phytol 162:331–41
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–8
MEA 2003. Ecosystems and human well-being: a framework for assessment. Washington, DC: Island Press
Miller DJ 1998. Nomads of the Tibetan Plateau rangelands in Western China part one: pastoral history. Rangelands 20:24–9
Miller DJ 1999. Nomads of the Tibetan Plateau rangelands in Western China part three: pastoral development and future challenges. Rangelands 21:17–20
Olsen CS 2005. Valuation of commercial central Himalayan medicinal plants. Ambio 34:607–10
Price MV, Waser NM 1998. Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 79:1261–71
Saavedra F, Inouye DW, Price MV, Harte J 2003. Changes in flowering and abundance of Delphinium nuttallianum (Ranunculaceae), in response to a subalpine climate warming experiment. Glob Change Biol 9:885–94
Salick J, Yongping Y, Amend A 2005. Tibetan land use and change near Khawa Karpo, Eastern Himalayas. Econ Bot 59:312–25
Salick J, Byg A, Amend A, Gunn B, Law W, Schmidt H 2006. Tibetan medicine plurality. Econ Bot 60:227–53
Schwartz MW, Brigham CA, Hoeksema JD, Lyons KG, Mills MH, van Mantgem PJ 2000. Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia 122:297–305
Thompson LG, Mosley-Thompson E, Davis M, Lin PN, Yao T, Dyurgerov M, Dai J 1993. Recent warming: ice core evidence from tropical ice cores with emphasis on Central Asia. Global Planet Change 7:45–156
Thompson LG, Yao T, Mosley-Thompson E, Davis ME, Henderson KA, Lin PN 2000. A high-resolution millennial record of the South Asian monsoon from Himalayan ice cores. Science 289:1916–9
Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C 2001. Diversity and productivity in a long-term grassland experiment. Science 294:843–5
Walker B, Kinzig A, Langridge J 1999. Plant attribute diversity, resilience, and ecosystem function: the nature and significance of dominant and minor species. Ecosystems 2:95–113
Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, Alatalo JM, Bret-Harte D, Calef MP, Callaghan TV, Carroll AB, Epstein HE, Jónsdóttir IS, Klein JA, Magnússon Bό, Molau U, Oberbauer SF, Rewa SP, Robinson CH, Shaver GR, Suding KN, Thompson CC, Tolvanen A, Totland O, Turner PL, Tweedie CE, Webber PJ, Wookey PA 2006. Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci 103:1342–6
Xu JC, Wilkes A 2004. Biodiversity impact analysis in northwest Yunnan, southwest China. Biodivers Conserv 13:959–83
Yachi S, Loreau M 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci USA 96:1463–8
Yeh ET 2005. Green governmentality and pastoralism in Western China: converting pastures to grasslands. Nomad People 9:9–29
Zavaleta ES, Hulvey KB 2004. Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science 306:1175–7
Acknowledgments
We thank the Haibei Research Station, E. Betts, J. Hu, S. Kloss, S. Lippert, Y. Li, S. McCarthy, and T. Thundup for field assistance. We thank J. Liu for assistance with plant identification and S. Chen for the plant-group and plant-trait categorizations. We also acknowledge A. Knapp, M. Sankaran, and two anonymous reviewers for their comments. This work was supported by the National Science Foundation (INT-9907375), the NOAA Postdoctoral Program in Climate and Global Change, and the American Alpine Club.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Klein, J.A., Harte, J. & Zhao, XQ. Decline in Medicinal and Forage Species with Warming is Mediated by Plant Traits on the Tibetan Plateau. Ecosystems 11, 775–789 (2008). https://doi.org/10.1007/s10021-008-9160-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-008-9160-1