Abstract
Spawning salmon deliver nutrients (salmon-derived nutrients, SDN) to natal watersheds that can be incorporated into terrestrial and aquatic food webs, potentially increasing ecosystem productivity. Peterson Creek, a coastal watershed in southeast Alaska that supports several species of anadromous fish, was sampled over the course of a storm during September 2006 to test the hypothesis that stormflows re-introduce stored SDN into the stream. We used stable isotopes and PARAFAC modeling of fluorescence excitation–emission spectroscopy to detect flushing of DOM from salmon carcasses in the riparian zone back into a spawning stream. During the early storm hydrograph, streamwater concentrations of NH4–N and total dissolved phosphorus (TDP), the fluorescent protein tyrosine and the δ15N content of DOM peaked, followed by a rapid decrease during maximum stormflow. Although δ15N has previously been used to track SDN in riparian zones, the use of fluorescence spectroscopy provides an independent indicator that SDN are being returned from the riparian zone to the stream after a period of intermediate storage outside the stream channel. Our findings further demonstrate the utility of using both δ15N of streamwater DOM and fluorescence spectroscopy with PARAFAC modeling to monitor how the pool of streamwater DOM changes in spawning salmon streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrient transfer from marine to coastal ecosystems by anadromous salmon (Oncorhynchus spp.) has received much attention in recent years. Salmon-derived nutrients (SDN) may enter coastal stream food webs through bottom up and top down pathways (Gende and others 2002). Thus, identifying the pathways by which SDN are stored and released within streams is critical for developing an understanding of the ecological role salmon fill within coastal ecosystems.
SDN can be assimilated into freshwater food webs either by direct consumption of carcass material and eggs by invertebrates or fish (Bilby and others 1996; Wipfli and others 1998; Chaloner and others 2002) or through uptake of limiting dissolved nutrients by bacterial communities (Yoder and others 2006) and epilithon (Mitchell and Lamberti 2005). SDN can also be delivered to riparian ecosystems through hyporheic flowpaths (O’Keefe and Edwards 2003), as excreta by predators and scavengers (Hilderbrand and others 1999), through the deposition of partially consumed salmon carcasses by bears (Hilderbrand and others 1999; Gende and others 2001) and by floods, which can deposit carcasses in streamside vegetation, woody debris, or on the soil surface (Ben-David and others 1998). Moreover, the assimilation of SDN into riparian ecosystems via these pathways varies over time and among different sites (Hellfield and Naiman 2006). Once in the riparian zone, SDN are incorporated into a variety of pools including soil organic matter, vegetation, microbial biomass, and roots (Bilby and others 2003; Bartz and Naiman 2005; Wilkinson and others 2005; Drake and others 2006). Despite numerous studies confirming SDN storage in riparian zones, movement of SDN back into streams has not previously been documented.
Stable isotopes (primarily 14N and 15N) have been used successfully in ecological studies to quantify the contribution of SDN to freshwater ecosystems (Kline and others 1993; Bilby and others 1996), follow the flow of SDN into aquatic food webs (Bilby and others 1996; Chaloner and others 2002; Hicks and others 2005), and document the uptake and storage of SDN by riparian vegetation and soil organic matter pools (Bilby and others 2003; Drake and others 2006). 15N is a particularly useful tracer of salmon N because anadromous salmon acquire most of their biomass in marine ecosystems that are relatively enriched in 15N compared to riparian soil and biomass N pools.
In addition, fluorescence spectroscopy excitation–emission matrices (EEMs) have recently been used to identify streamwater dissolved organic matter (DOM) derived from spawning salmon (Hood and others 2007). During spawning, salmon contribute protein-rich DOM to streams that has a unique fluorescent fingerprint which can be used to detect DOM derived from salmon within the larger pool of stream DOM. Excitation–emission matrices can be analyzed with the multivariate modeling technique, parallel factor analysis (PARAFAC). PARAFAC is a three-way, decomposition method similar to principal component analysis that decomposes EEMs into trilinear components. PARAFAC has been shown to successfully decompose the fluorescent signature of aquatic DOM into individual components, thereby providing information about the composition and origin of DOM (Stedmon and others 2003).
In this study, we used stable isotopes and fluorescence spectroscopy to investigate the hydrologic flushing of SDN stored in the riparian zone back into a spawning stream. This represents a previously undocumented pathway for SDN transfer to aquatic ecosystems and elucidates a potential mechanism for the storage and delayed release of DOM and inorganic forms of N and P, which are typically limiting to primary production in aquatic ecosystems.
Methods
Site Description
The study was conducted in the Peterson Creek watershed in southeast Alaska, approximately 40 km north of Juneau, Alaska. Southeast Alaska has a moderate maritime climate with mean monthly temperatures ranging from −2 to 14°C. Mean annual precipitation is 1400 mm, much of which falls during large frontal storms in the fall. The Peterson Creek watershed is approximately 25 km2 and 53% of the watershed area is covered with wetlands, making Peterson Creek a brownwater stream where DOC concentrations typically range from 5 to 13 mg C l−1 (Hood and others 2007). Annual discharge in the creek ranges from 0.1 m3 s−1 to more than 8.0 m3 s−1. The Peterson Creek watershed consists of a landscape mosaic of water-logged peatlands mixed with coniferous forest (predominantly Picea sitchensis and Tsuga heterophylla). The creek supports anadromous pink (Oncorhynchus gorbuscha), chum (O. keta), and coho salmon (O. kisutch), with peak spawner densities occurring from mid-July through mid-August. Spawner densities can be as high as 0.53 fish m−2 in the active stream channel (Mitchell and Lamberti 2005).
Field and Laboratory Methods
Surface water was sampled with an ISCO (model 3700) automated sampler over the course of a storm lasting from September 6–8, 2006. Spawning in Peterson Creek began in mid-July, peaked in early August, and the last live spawners were observed in mid-August. One-liter streamwater samples were collected every 2 h on the ascending limb of the hydrograph and every 4–8 h on the descending limb. Water samples were removed from the ISCO daily, filtered through pre-combusted, Gelman A/E, glass fiber filters (0.7 μm), and refrigerated until analysis. Dissolved nutrients were analyzed within 48 h of collection and fluorescence measurements were completed within one week of collection. To estimate salmon carcass densities in the riparian zone before the storm, the number of carcasses was visually counted along three, 10 m transects that extended 2 m from the active stream channel. Our approach provided a rough estimate of salmon carcass densities before the storm. Observed salmon carcass density in the riparian zone ranged from 0 to 5 fish m−2 with an average of 0.45 fish m−2.
DOC concentrations were determined by high-temperature combustion using a Shimadzu TOC-V Organic Carbon Analyzer and the standard deviation of standards during analysis was ±0.3 mg C l−1. Total dissolved phosphorus (TDP) was measured using a persulfate digestion (Valderrama 1981) in conjunction with the ascorbic acid method (Murphy and Riley 1962). A 10-cm, quartz flow through cell was used for TDP analysis to enable the detection of low P concentrations (0.5–1.0 μg P l−1). Ammonium (NH4–N) was measured on a Dionex Ion Chromatograph (ICS-1500) with a detection limit of 5 μg N l−1. The standard deviation of standards re-analyzed during sample analyses was ±0.59 μg N l−1 for NH4–N and ±0.14 μg P l−1 for TDP.
Fluorescence of DOM was measured on a Fluoromax-3 (Jobin Yvon Horiba) fluorometer with a xenon lamp, following the procedures of Hood and others (2007). Excitation–emission matrices (EEMs) were created by measuring fluorescence intensity across excitation wavelengths ranging from 240 to 450 nm and emission wavelengths ranging from 300 to 600 nm. Water samples were diluted according to Green and Blough (1994) to avoid inner filter effects. EEMs were corrected for instrument bias and subsequently Raman normalized using the area under the water Raman peak at excitation wavelength 350 nm. PARAFAC modeling of fluorescence EEMs was conducted with MATLAB using the PLS_toolbox version 3.7 following the procedures described in Stedmon and others (2003). A non-negativity constraint was applied to the PARAFAC model and the model was successfully validated using a split-plot analysis and core consistency diagnostics (Stedmon and others 2003). If the correct number of fluorescent components is selected using the PARAFAC model, the components can be compared by determining the relative proportion of each component to the total DOM fluorescence. The relative proportion of each fluorescent component was determined for the individual streamwater samples during the storm by quantifying the relative abundance of each component in comparison to the other components identified by the PARAFAC model.
Streamwater DOM from filtered water samples was lyophilized using a Labconco freeze drier (FreezeZone 2.5) at −50°C and was stored in a desiccator until isotope analysis. Analyses for δ15N of DOM were performed at the UC Davis Stable Isotope Facility by continuous-flow Isotope Ratio Mass Spectrometry (IRMS). Instrument precision as measured by the standard deviation of standards during the run was ±0.12‰ for δ15N.
Results
Inorganic Nutrient Concentrations and δ15N of Streamwater DOM
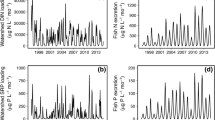
During September 6–8, 4.8 cm of precipitation fell in the Peterson Creek watershed. Stream discharge in Peterson Creek increased from 0.6 to 4.8 m3 s−1 in 22 h and receded to pre-storm levels approximately 72 h after the beginning of the storm. During the early portion of the storm hydrograph, TDP concentrations increased approximately 40% to a maximum of 31 μg P l−1 and NH4–N concentrations increased 30% to a maximum of 51 μg N l−1 (Figure 1A). As streamflow increased, both TDP and NH4–N concentrations decreased to below 10 μg l−1 and stayed below 15 μg l−1 for the remainder of the storm.
Relationship between stream discharge and (A) inorganic nutrient concentrations, (B) DOC concentrations, (C) δ15N values and relative percent contribution of the fluorescent protein tyrosine for Peterson Creek during the September 6–8 storm. Capital numbers 1 and 2 on Figure 1C indicate the samples for which EEM plots are shown in Figure 2.
Concentrations of streamwater DOC increased sharply on the ascending limb of the hydrograph, peaked at approximately 15 mg C l−1, and decreased back toward pre-storm levels on the descending limb of the hydrograph (Figure 1B). The δ15N values for streamwater DOM ranged from 0.45 to 9.9‰ and peaked coincident with the peaks in inorganic N and P on the early portion of the storm hydrograph (Figure 1C). This peak was followed by a rapid decrease in δ15N of DOM as stream discharge increased.
Fluorescence Spectroscopy
We employed a PARAFAC model to evaluate the fluorescence properties of streamwater DOM. Using this model, we identified seven unique components within the fluorescence EEMs. Of the seven components identified by the PARAFAC model, we focus our analysis on the fluorescent component linked to the protein tyrosine, which has an excitation maximum of 275 nm and an emission maximum of 306–308 nm. We refer to tyrosine fluorescence in this study as “tyrosine-like” because this fluorophore is likely a mixture of proteinaceous materials rather than free tyrosine (Maie and others 2007). Tyrosine-like fluorescence has been observed in other studies (Hood and others 2007; Maie and others 2007) and was validated in our study using a pure tyrosine standard. We use tyrosine-like fluorescence for our analysis because salmon-derived DOM has been shown to be rich in tyrosine-like fluorescence while lacking the characteristic humic-like fluorescence typical of DOM leached from peatland soils (Hood and others 2007).
The relative contribution of tyrosine-like fluorescence increased rapidly from 1.1 to 6.6% early in the storm hydrograph followed by a rapid decrease during peak stormflow (Figure 1C). This protein-like fluorescence is evident in the fluorescence EEMs. Before the onset of the storm, the primary fluorescence peak, which has been attributed to humic-like material of terrestrial origin (Stedmon and others 2003), occurs at approximately 240 nm excitation and 452 nm emission (Figure 2). However, in the early storm hydrograph, there is an increase in protein fluorescence with the appearance of the tyrosine-like peak corresponding to an excitation of 275 nm and an emission maximum of 306–308 nm (Figure 2). The large initial increase in tyrosine-like fluorescence was similar in timing to the increases in δ15N of DOM and inorganic nutrient concentrations. There was a second, smaller peak in tyrosine-like fluorescence on the descending limb of the hydrograph with a maximum percent contribution of 2.8%.
EEMs for DOM from Peterson Creek streamwater (1) before the September 6–8 storm and (2) during the pulse of SDN when the tyrosine-like fluorescence was elevated. Tyrosine-like fluorescence has an excitation maximum of 275 nm and an emission maximum of 306–308 nm, whereas the terrestrial-derived, humic-like material has an excitation maximum of 240 nm and an emission maximum of 452 nm. Fluorescence intensities are in Raman units.
Discussion
Increases in streamwater concentrations of inorganic N and P (primarily NH +4 and SRP) during salmon spawning have been reported in numerous studies (Minakawa and Gara 1999; Mitchell and Lamberti 2005; Hood and others 2007). These aquatic nutrient pulses are associated with the presence of both spawning salmon and salmon carcasses within the stream channel. At the end of the spawning period, carcasses are typically flushed from the stream channel during high flow events and streamwater nutrient concentrations decrease to pre-spawning levels. Our results suggest that salmon-derived inorganic nutrients flushed from carcasses that are close to but out of the stream channel can elevate streamwater concentrations of inorganic N and P.
Several studies have documented increases in streamwater concentrations of DOM during salmon spawning (Sarica and others 2004; Hood and others 2007); however, few studies have characterized DOM derived from salmon. Adult salmon tissue and eggs have δ15N values of 12–15‰ (Kline and others 1993; Hicks and others 2005). The enriched DOM δ15N values reported here are consistent with these values and indicate that salmon carcasses contribute to the streamwater DOM pool during the storm. Fluorescence characterization of streamwater DOM supports this hypothesis, because the tyrosine-like protein peaks observed early during the storm are identical to the tyrosine-like protein peaks identified previously during the spawning period (Hood and others 2007). Moreover, the relative contribution of the tyrosine (6.6%) and humic-like fluorescent (24.1%) components observed during the SDN pulse early in the storm was in between spawning (tyrosine: 15–35%; humic: 5–15%) and non-spawning levels (tyrosine: 1–3%; humic: 30–35%) typically observed in Peterson Creek (Hood and others 2007). As a result of the distinct chemical differences between salmon and terrestrially-derived DOM (which dominates the aquatic DOM load in Peterson Creek throughout most of the year), salmon-derived DOM can be easily fingerprinted using fluorescence spectroscopy. Taken together, these independent characterization techniques suggest that organic SDN stored outside the active stream channel returned to the stream early in the storm. Our findings further demonstrate the utility of both δ15N of streamwater DOM and fluorescence spectroscopy with PARAFAC modeling to monitor how the pool of streamwater DOM changes in spawning salmon streams.
In forested watersheds without spawning salmon, most streamwater DOM is derived from allochthonous sources within the watershed, particularly DOM mobilized from soil organic matter pools during high streamflow events, such as snowmelt (Boyer and others 1997) and storms (Hinton and others 1998; McGlynn and McDonnell 2003). In many forested watersheds, DOC concentrations peak on the ascending limb of the storm hydrograph because of early, DOC-rich contributions from riparian areas that are later diluted by low DOC hillslope water (Hinton and others 1998; McGlynn and McDonnell 2003). Our results depict an early increase in DOC with a high protein and 15N content, consistent with salmon DOM being flushed from a near-stream reservoir early on the storm hydrograph. The pulses of TDP and NH4–N that occur coincident with the delivery of salmon DOM further corroborate this finding. The continued increase in DOM during the storm has fluorescent properties and 15N values which are consistent with inputs from the extensive wetlands within the Peterson Creek watershed.
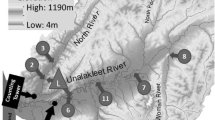
The SDN pulse early in the storm suggests the riparian zone is most likely the near-stream area for SDN storage. In our study stream, previous storm deposition of salmon carcasses in the riparian zone resulted in localized carcass densities as high as 5 fish m−2. The Peterson Creek watershed is similar to many other anadromous salmon streams in southeast Alaska where the highest carcass densities are typically found on large woody debris (LWD) in the riparian zone and along the high-water mark from previous storms (Figure 3). Salmon carcasses stored in the riparian zone could leach DOM and inorganic nutrients back into the stream channel through several pathways. Precipitation could fall on salmon carcasses lodged in LWD and contribute SDN directly to the stream. SDN could be leached from carcasses lying on the soil and transported to the stream through overland flow, shallow-soil flowpaths, or movement into underlying hyporheic flowpaths. As salmon carcasses decompose, SDN could be incorporated into soil organic matter pools (Reimchen and others 2002; Drake and others 2006) where they could be leached by rainwater percolating through the soil and rapidly transported to the stream.
A portion of the observed pulse of SDN could be from return flow from hyporheic zones within the active channel and beneath the floodplain. O’Keefe and Edwards (2003) found elevated concentrations of NH +4 and SRP in the hyporheic zone when spawning salmon were present and postulated that if SDN enters the biologically active, upstream edge of the hyporheic zone, there is the potential for biological uptake and storage of SDN. This form of SDN spiraling through the hyporheic zone could allow for the delayed release of nutrients through mineralization and subsequent release to the active stream channel as return flow.
Our findings have important implications for watersheds containing anadromous fish streams. First, salmon carcass storage in the riparian zone is a potential source of nutrients for aquatic ecosystems. For example, Gende and others (2007) found maximum soil inorganic N loadings of 6–7 g N m−2 from salmon carcasses deposited in the riparian zone. In our study, using the inorganic N loading rate by Gende and others (2007), average riparian salmon carcass density in Peterson Creek (0.45 fish m−2), and a time period of 2 weeks (estimated length of time salmon carcasses were in the riparian zone before the storm), we estimate that approximately 2 g N m−2 (6% of total N in riparian salmon carcasses) was available to flush from salmon carcasses in the riparian zone to Peterson Creek during the storm. Second, evidence continues to accumulate that SDN inputs are assimilated into aquatic food webs and can have important effects on natal streams, comprising as much as 50% of juvenile coho salmon diets in Alaskan streams (Hicks and others 2005). However, for SDN to have a substantial impact on annual stream production, nutrients must influence annual production and not simply stimulate short-term processes. Most large salmon runs occur near or at the end of the growth season for aquatic producers and riparian vegetation in southeast Alaska. These salmon runs often occur during or just prior to scouring late summer floods that remove most salmon carcasses from the stream channel. To impact annual stream production, SDN must be retained within the ecosystem and returned to the stream after a period of storage that can last weeks to potentially as long as the following spring. Riparian and/or hyporheic zones are likely areas for this to occur, and even though our proposed mechanism for storage and return of SDN to streams may not significantly increase annual stream production, our study is the first to document the return of SDN to the stream after a period of intermediate storage outside the stream channel.
Riparian zones have long been recognized for influencing channel morphology and increasing stream habitat complexity by contributing LWD to streams (Bilby and others 1991). These direct effects have been shown to improve juvenile coho salmon survival (Quinn and Peterson 1996), create habitat for over-wintering juvenile coho salmon (Bustard and Narver 1975), and reduce stream velocity which in turn attracts spawning salmon (Shirvell 1990). Our findings suggest that riparian zones may provide storage for salmon carcasses and facilitate the delayed release of organic and inorganic SDN back into the active stream channel. As a result, we postulate that habitat complexity modulates the feedbacks between salmon inputs and coastal ecosystems and thus, watershed restoration strategies should consider the intricacies of this linkage.
References
Bartz KK, Naiman RJ. 2005. Effects of salmon-borne nutrients on riparian soils and vegetation in southwest Alaska. Ecosystems 8:529–45
Ben-David M, Hanley TA, Schell DM. 1998. Fertilization of terrestrial vegetation by spawning Pacific salmon: the role of flooding and predator activity. OIKOS 83:47–55
Bilby RE, Ward JW. 1991. Characteristics and function of large woody debris in streams draining old-growth, clear-cut, and second-growth forests in southcentral Washington. Can J Fish Aquat Sci 48:2499–508
Bilby RE, Frassen BR, Bisson PA. 1996. Incorporation of nitrogen and carbon from spawning coho salmon into the trophic system of small streams: evidence from stable isotopes. Can J Fish Aquat Sci 53:164–73
Bilby RE, Beach EW, Fransen BR, Walker JK, Bisson PA. 2003. Transfer of nutrients from spawning salmon to riparian vegetation in western Washington. Trans Am Fish Soc 132:733–45
Boyer EW, Hornberger GM, Bencala KE, McKnight DM. 1997. Response characteristics of DOC flushing in an alpine catchment. Hydrol Process 11:1635–47
Bustard DR, Narver DW. 1975. Aspects of the winter ecology of juvenile coho salmon and steelhead trout. J Fish Res Board Can 32:667–80
Chaloner DT, Martin KM, Wipfli MS, Ostrom PH, Lamberti GA. 2002. Marine carbon and nitrogen in south-eastern Alaskan stream food webs. Evidence from artificial and natural streams. Can J Fish Aquat Sci 59:1257–65
Drake DC, Naiman RJ, Bechtold JS. 2006. Fate of nitrogen in riparian forest soils and trees: an 15N tracer study simulating salmon decay. Ecology 87(5):1256–66
Gende SM, Quinn TP, Wilson MF. 2001. Consumption choice by bears feeding on salmon. Oecologia 127:372–82
Gende SM, Edwards RT, Wilson MF, Wipfli MS. 2002. Pacific salmon in aquatic ecosystems. BioScience 52(10):917–28
Gende SM, Miller AE, Hood E. 2007. The effects of salmon carcasses on soil nitrogen pools in a riparian forest of southeastern Alaska. Can J For Res 37:1194–202
Green SA, Blough NV. 1994. Optical absorption and fluorescence properties of chromophoric dissolved organic matter in natural waters. Limnol Oceanogr 39:1903–16
Hellfield JM, Naiman RJ. 2006. Keystone interactions: Salmon and bear in riparian forests of Alaska. Ecosystems 9:167–80
Hicks BJ, Wipfli MS, Lang DW, Lang ME. 2005. Marine-derived nitrogen and carbon in freshwater-riparian food webs of the Copper River Delta, southcentral Alaska. Oecologia 144:558–69
Hilderbrand GV, Hanley TA, Robbins CT, Schwartz CC. 1999. Role of brown bears (Ursus arctos) in the flow of marine nitrogen into a terrestrial ecosystem. Oecologia 121:546–50
Hinton MJ, Schiff SL, English MC. 1998. Sources and flowpaths of dissolved organic carbon during storms in two forested watersheds of the Precambrian Shield. Biogeochemistry 41:175–97
Hood E, Fellman JB, Edwards RT. 2007. Salmon influences on dissolved organic matter in a coastal temperate brownwater stream: an application of fluorescence spectroscopy. Limnol Oceanogr 52(4):1580–7
Kline TC Jr, Goering JJ, Mathisen OA, Poe PH, Parker PL, Scalan RS. 1993. Recycling of elements transported upstream by runs of Pacific Salmon: II. δ15N and δ13C evidence in the Kvichak River watershed, Bristol Bay, southwestern Alaska. Can J Fish Aquat Sci 50:2350–65
Maie N, Scully NM, Pisani O, Jaffe R. 2007. Composition of protein-like fluorophore of dissolved organic matter in coastal wetland and estuarine ecosystems. Water Res 41:563–70
McGlynn BL, McDonnell JJ. 2003. Role of discrete landscape units in controlling catchment dissolved organic carbon dynamics. Water Resour Res 39(4). DOI 10.1029/2002WR001525
Minakawa N, Gara RI. 1999. Ecological effects of a chum salmon spawning run in a small stream of the Pacific Northwest. J Fresh Ecol 14(3):327–35
Mitchell NL, Lamberti GA. 2005. Responses in dissolved nutrients and epilithon abundance to spawning salmon in southeast Alaska streams. Limnol Oceanogr 50:217–27
Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–6
O’Keefe TC, Edwards RT. 2003. Evidence for hyporheic transfer and removal of marine-derived nutrients in sockeye streams in southeast Alaska. In: Stockner JG, Ed. Nutrients in salmonid ecosystems: sustaining production and biodiversity. Am Fish Soc Sympos 34:99–107
Quinn TP, Peterson NP. 1996. The influence of habitat complexity and fish size on over-winter survival and growth of individually marked juvenile coho salmon in Big Beef Creek, Washington. Can J Fish Aquat Sci 53:1555–64
Reimchen TE, Mathewson D, Hocking MD, Moran J, Harris D. 2002. Isotopic evidence for enrichment of salmon-derived nutrients in vegetation, soil and insects in riparian zones in coastal British Columbia. Am Fish Soc Sympos 34:59–69
Sarica J, Amyot M, Hare L, Doyon MR, Stanfield LW. 2004. Salmon-derived mercury and nutrients in a Lake Ontario spawning stream. Limnol Oceanogr 49:891–9
Shirvell CS. 1990. Role of instream rootwads as juvenile coho salmon cover habitat under varying streamflows. Can J Fish Aquat Sci 47:852–61
Stedmon CA, Markager S, Bro R. 2003. Tracing DOM in aquatic environments using a new approach to fluorescence spectroscopy Mar Chem 82:239–54
Valderrama JC. 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar Chem 10:109–22
Wilkinson CE, Hocking MD, Reimchen TE. 2005. Uptake of salmon-derived nitrogen by mosses and liverworts in coastal British Columbia. OIKOS 108:85–98
Wipfli MS, Hudson J, Caouette J. 1998. Influence of salmon carcass densities on stream productivity: response of biofilm and benthic macroinvertebrates in southeastern Alaska. Can J Fish Aquat Sci 55:1503–11
Yoder DM, Viramontes A, Kirk LL, Hanne LF. 2006. Impact of salmon spawning on microbial communities in a northern California river. J Fresh Ecol 21:147–55
Acknowledgments
The authors wish to thank Karen Michaels for her assistance with field and laboratory analyses and Jacob Berkowitz, Nick Bonzi, and Erik Norberg for their comments on an earlier version of this manuscript. This study was funded by the U.S. Department of Agriculture National Research Initiative, grant number 2005-35102-16289, the USDA Forest Service, Aquatic and Land Interactions Program, and the Resource Management and Productivity Program at the Pacific Northwest Research Station in Juneau, AK. The use of trade or firm names in this publication is for reader information and does not imply endorsement by the U.S. Department of Agriculture of any product or service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fellman, J.B., Hood, E., Edwards, R.T. et al. Return of Salmon-Derived Nutrients from the Riparian Zone to the Stream during a Storm in Southeastern Alaska. Ecosystems 11, 537–544 (2008). https://doi.org/10.1007/s10021-008-9139-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-008-9139-y