Abstract
Gross rates of N mineralization and nitrification, and soil–atmosphere fluxes of N2O, NO and NO2 were measured at differently grazed and ungrazed steppe grassland sites in the Xilin river catchment, Inner Mongolia, P. R. China, during the 2004 and 2005 growing season. The experimental sites were a plot ungrazed since 1979 (UG79), a plot ungrazed since 1999 (UG99), a plot moderately grazed in winter (WG), and an overgrazed plot (OG), all in close vicinity to each other. Gross rates of N mineralization and nitrification determined at in situ soil moisture and soil temperature conditions were in a range of 0.5–4.1 mg N kg−1 soil dry weight day−1. In 2005, gross N turnover rates were significantly higher at the UG79 plot than at the UG99 plot, which in turn had significantly higher gross N turnover rates than the WG and OG plots. The WG and the OG plot were not significantly different in gross ammonification and in gross nitrification rates. Site differences in SOC content, bulk density and texture could explain only less than 15% of the observed site differences in gross N turnover rates. N2O and NO x flux rates were very low during both growing seasons. No significant differences in N trace gas fluxes were found between plots. Mean values of N2O fluxes varied between 0.39 and 1.60 μg N2O-N m−2 h−1, equivalent to 0.03–0.14 kg N2O-N ha−1 y−1, and were considerably lower than previously reported for the same region. NO x flux rates ranged between 0.16 and 0.48 μg NO x -N m−2 h−1, equivalent to 0.01–0.04 kg NO x -N ha−1 y−1, respectively. N2O fluxes were significantly correlated with soil temperature and soil moisture. The correlations, however, explained only less than 20% of the flux variance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China is covered by approximately 400 million hectare of grassland (42% of the total area), of which 87 million hectare belong to Inner Mongolia. The Inner Mongolian steppe is a part of approximately 12.5 million km2 of temperate grasslands, which is more than 8% of the Earth’s land surface. The temperate grasslands comprise the Eurasian steppe belt that ranges from Eastern Europe to Eastern Asia at mid-latitudes, with a remarkable branch extending into Inner Mongolia and further to the Southwest of China (Tibet). They play a substantial role in balancing the global greenhouse gas budget due to their ability to sequester carbon in the soil (Batjes 1998), storing some 300 Gt C – that is, approximately one-eighth of the global terrestrial carbon stock –, of which 97% is localized below ground (IPCC 2001). However, the role of grasslands in the global N cycle, including the nitrous oxide budget, is less clear.
In steppe areas of Asia, a nomadic land-use system prevailed for the past centuries but is only in practice today in some areas. The greatest recent changes in this practice were the sedentarization of the nomads, the collectivization of their livestock and, after re-privatization, the increase of livestock and the establishment of arable land which resulted in over-cropping and over-grazing of some areas. This has led to a severe decrease in soil organic matter, depletion of soil nutrients, facilitation of erosive processes and finally desertification (Chuluun 2001). It is estimated by the UNEP (United Nations Environment Programme) that 60–70% of the grasslands in China, Mongolia and the Asian parts of the former USSR are affected by deterioration and desertification mainly due to over-grazing, but in some areas also due to over-cropping (Graetz 1994).
Grazing can lead very quickly to changes in nutrient pools and fluxes (for example, Ross and others 1999; Augustine and Frank 2001), in vegetation cover (Paruelo and others 2001), plant community composition in grasslands (Li 2001; Oba and others 2001; Zhang and Skarpe 1996), and in the worst case even to desertification (Li and others 2000). Recent investigations of key microbial N turnover processes show that gross rates of N-mineralization and nitrification can be substantial in steppe ecosystems (Chen and Stark 2000; Corre and others 2002; Verchot and others 2002), comparable in the range to temperate forest ecosystems (for example, Davidson and others 1991; Stark and Hart 1997). Intensive studies in steppe ecosystems in North America have revealed that the N cycle and thus the involved consumptive and productive N cycling processes, such as mineralization, nitrification and immobilization, are in most cases tightly coupled in semi-arid temperate grasslands (Davidson and others 1990), and, thus, the N2O formation does not appear to be a significant pathway in the N cycle (Billings and others 2002) of such ecosystems. Reported mean emission rates are mostly below 6.5 μg N2O-N m−2 h−1 or smaller than 0.6 kg N ha−1 y−1 (for example, Mosier and others 1991, 1996, 2002; Epstein and others 1998; Mummey and others 1997, 2000). But due to the high spatial variability of N2O fluxes (for example, Billings and others 2002; Corre and others 1996) and a potential importance of wintertime fluxes in steppe ecosystems for total annual N2O losses, which are mostly associated with snow melting events (Mosier and others 1996), estimates are still uncertain.
In contrast to N2O fluxes, emission rates of NO-N from steppe ecosystems (0–200 μg N m−2 h−1, Epstein and others 1998; Smart and others 1999; Mosier and others 2002) have been reported to be significantly higher, in a range of 0.8–1.8 kg N ha−1 y−1 for a short-grass steppe in Colorado, USA (Martin and others 1998). NO fluxes have been shown to be strongly correlated with temperature (Martin and others 1998), but rainfall events can initiate short-term pulses of up to 400-fold increased NO fluxes (Martin and others 1998; Smart and others 1999).
Our knowledge about rates of microbial N cycling and biosphere–atmosphere exchange of N trace gases in East Asian steppe ecosystems, which are significantly different from steppe ecosystems in North America with respect to floristic composition and rangeland management, is still rudimentary. To contribute to constraining the uncertainties associated with N cycling and N trace gas exchange in East Asian steppe ecosystems, a study was conducted in a typical steppe area in Inner Mongolia, P. R. China, by means of chamber measurements of soil–atmosphere N2O, NO and NO2 exchange with high time resolution in the field, accompanied by laboratory incubation studies for the quantification of gross N turnover rates.
Materials and Methods
Site Characteristics
The study was performed at four differently managed Leymus chinensis (Trin.) Tzvel. steppe sites in a typical steppe ecosystem of the Xilin river catchment, Inner Mongolia, China, approximately 450 km north of Beijing. Three of the sites, that is, a site fenced and ungrazed since 1979 (“UG79”, 25 hectare), a site fenced and ungrazed since 1999 (“UG99”, 35 hectare), and a site fenced and occasionally grazed in the winter period from November to April by 3–4 sheep units per hectare (“WG”, 40 hectare), are permanent. These three sites belong to the Inner Mongolia Grassland Ecosystem Research Station (IMGERS, 116°42′ E, 43°38′ N), a station within the Chinese Ecological Research Network (CERN) managed by the Institute of Botany, Chinese Academy of Sciences. To our knowledge, these sites are the only sites in this region with a well-documented management history. Floristic composition and seasonal variation of aboveground biomass at the UG79 site were described previously (Chen and others 2003; Bai and others 2004). It is noteworthy to mention that the ungrazed sites were moderately grazed before fencing.
The fourth site, which is approximately 2 km away from the other sites, is an unfenced and overgrazed site, located in the vicinity of a hamlet and used by a local farmer for grazing (“OG”, approximately 5–6 sheep units per hectare during the vegetation period). At this site grazing has already led to changes in vegetation composition. For example, the abundances of Potentilla acaulis L. and Artemisia frigida Willd. were found to be significantly increased, and also the abundances of C4 grasses [for example, Cleistogenes squarrosa (Trin.) Keng] increased at the cost of C3 grasses [for example, L. chinensis (Trin.) Tzvel., Stipa grandis P. Smirn.]. These vegetation changes are typical indicators of overgrazing (Wang and Ripley 1997; Wang 2002; Tong and others 2004). High evaporation and transpiration rates during summer reduce plant available water, which is, besides temperature, the most limiting factor for plant growth in this semi-arid grassland ecosystem. A short growing season of approximately 150 days, a frost-free period of less than 100 days, and low mean monthly temperatures October–March further limit plant growth. Precipitation, mainly of convective nature, is restricted mostly to the summer months. For the period 1982–2003, the mean annual temperature of this area was 0.7°C, and mean annual precipitation was 343 mm. The soil type at all sites is a calcic chernozem soil on a loess parent material, with a soil organic carbon (SOC) content of 1.8–2.2% in the uppermost 10 cm, and of 1.7–3.1% in the uppermost 4 cm. The C to N ratio of all sites was approximately 10 and, hence, rather narrow. The soil texture was a sandy loam, with pH values of 6.6–6.8. For further site details see Table 1.
Experimental Design
Gross ammonification and nitrification rates, and soil inorganic N concentrations were determined in the 2004 and 2005 growing season at the UG99 and WG sites, and additionally at the UG79 and WG sites in the 2005 growing season in at least monthly intervals.
N2O fluxes were measured during the 2004 and 2005 growing season at the adjacent WG and UG99 sites with a fully automated measuring system for every 3 h, using three chambers for each site. At the UG79 and OG sites manual N2O measurements were performed every 3–4 days during the 2005 growing season with four chambers for each site. At the latter two sites no automated measurements were possible because only one automated measuring system was available, and the UG79 and OG sites were too far away from the location of the automated system. Therefore, automated and manual N2O flux measurements were considered as two separate sets of two treatments each.
NO x flux measurements were exclusively conducted at the UG99 and WG sites because, like for the N2O measurements, only one automated measuring system was available, and the other two sites were too far away with respect to maximum tube length. As manual NO x measurements with static chambers do not yield results comparable with dynamic chamber measurements, they were not performed at the UG79 and OG sites. Technical problems with the chemoluminescence detector restricted our measurements of NO x fluxes and ambient air concentrations of NO and NO2 to a few weeks in August and September 2004.
Determination of Gross N Turnover Rates
Soil samples of 0.5 kg from the top 0.05 m at three randomly chosen positions were taken from each treatment and immediately processed at IMGERS. Soil samples were homogenized by sieving carefully with a mesh width of 3.15 mm. The moisture content of the soil samples was determined gravimetrically by drying an aliquot of each unlabeled and labeled soil sample at 105°C for 24 h. Gross N mineralization and gross nitrification rates were determined according to Rosenkranz and others (2006a) with the 15N pool dilution technique at in situ soil temperature and moisture conditions. 15/14N isotope ratios were determined at the mass spectrometer laboratory at IMK-IFU, Garmisch-Partenkirchen, Germany, using an elemental analyzer (Flash EA 1112, Thermo Electron, Milan, Italy) coupled to a DeltaPlusXP mass spectrometer (Thermo Electron, Bremen, Germany). Ammonium and nitrate contents of the soil extracts were directly analyzed at the IMGERS using a Foss FIA Star 5000 Analyzer (Foss Inc., Hillerød, Denmark). Gross N mineralization and gross nitrification rates were calculated from the isotope ratios and NH +4 soil contents as described by Kirkham and Bartholomew (1954).
Gas Flux Measurements
The automated system for N2O measurements was installed in a bus, which was placed approximately 10 m from the border between the WG and UG99 sites. All chambers were installed at a distance of 20–40 m from the bus. The chambers (side length 0.7 × 0.7 m, height 0.3 m at the UG99 site; side length 0.5 × 0.5 m, height 0.15 m at the WG site) were fixed on stainless steel frames, which were driven approximately 0.1 m deep into the soil. The static chambers were pneumatically closed gas-tight for 90 min, followed by a period of 90 min with open lids to allow for the aeration of the chambers. Thus, one measurement cycle lasted 180 min resulting in eight flux measurements per day. Alternately, five air samples were taken by the automated sampling system from each closed chamber during the closure period (flow rate 100 ml min−1, sampling time 3 min), followed by five reference gas injections (400 ppbv N2O in synthetic air, Air Liquide, Munich, Germany). Samples were automatically injected into a gas chromatograph (SRI 8610C, Texas Instruments, Torrance, CA, USA), equipped with an electron capture detector (ECD) for N2O detection. A pre-column filled with Ascarite (Sigma Aldrich, Munich, Germany), which was renewed in weekly intervals, was installed upstream of the ECD to remove CO2. The carrier gas for N2O analysis was N2 (99.999% purity). The ECD was heated to 350°C, while the column temperature was kept at 60°C. The detection limit of the automated system was approximately 2 ppbv N2O concentration difference to ambient air when using five sample injections for one flux calculation. This is equivalent to an N2O flux of 0.4 μg N m−2 h−1. For further details of the automated measuring system and the calculation of fluxes, see Butterbach-Bahl and others (1998 and 2004), Papen and Butterbach-Bahl (1999), Breuer and others (2000), and Rosenkranz and others (2006b).
For the manual measurements, we installed four stainless steel frames (size 0.4 × 0.4 m) at each of the two sites, driven approximately 0.15 m into the soil. In intervals of 3–4 days, we mounted temperature-isolated chambers (chamber height 0.4 m) gas-tight onto the frames. Over a period of 80 min five gas samples were taken in 20 min intervals from the chambers using plastic syringes with a stopcock. Directly after sampling, the gas samples were brought to a laboratory at the IMGERS, where they were immediately analyzed for N2O. Analytical conditions were the same as described above. The system used for manual N2O analyses was described in detail by Wang and Wang (2003) and Wang and others (2005). However, to avoid interference with CO2 during N2O analysis we installed a pre-column filled with Ascarite upstream of the analytical column. The detection limit for manual N2O flux measurements using five data points for flux calculation was significantly higher than that for the automated system due to uncertainties associated with manual sampling and injection into the gas chromatograph. Using multiple injections of calibration standard, we calculated the precision of sampling and gas-chromatography analysis to be 5 ppbv concentration change of N2O, equal to a flux of approximately 1 μg N2O-N m−2 h−1. A comparison between measurements with the automated system and manual measurements at the same spot indicated no differences in N2O fluxes determined independently with the two methods (data not shown).
For the determination of NO x fluxes six dynamic measuring chambers and one reference chamber (side lengths 0.5 × 0.5 m; height 0.3 m) with a gas-tight perspex bottom were used. Three chambers were placed at the UG99 site, whereas the other three were located at the adjacent WG site. Each of the measuring chambers and the reference chamber were sampled alternately for 6 min, thus, within 72 min all six chambers had been sampled once. Besides the chambers, the measuring system consisted of a sampling device, an air pump and flow controller to achieve a controlled sample air flow of approximately 50 l min−1, a chemoluminescence detector for the determination of NO (CLD 770 AL ppt, Ecophysics AG, Dürnten, Switzerland), a photolysis converter for the determination of NO2 (PLC 760, Ecophysics AG, Dürnten, Switzerland) and an ozone analyzer (TE 49 C, Thermo Environmental Instruments Inc., Franklin, MA, USA). The chemoluminescence detector was calibrated weekly using 10 ppbv NO in synthetic air made by dilution of a reference gas (0.998 ppmv NO in N2, Air Liquide, Munich, Germany) with synthetic air (79% N2, 21% O2), using a computerized multi-gas calibrator (Environics 100, Environics Inc., West-Wellington, USA). The efficiency of photolytic conversion of NO2 into NO was determined weekly as described by Butterbach-Bahl and others (1997). NO x fluxes were calculated as the difference between measured NO and NO2 mixing ratios of sample and reference chamber air, multiplied by the flow rate, and were corrected for the reaction of NO with O3 to NO2 in the chambers and the sample lines. Details of flux calculation procedures are described by Butterbach-Bahl and others (1997), and Rosenkranz and others (2006b). The detection limit of the NO x measuring equipment was equivalent to fluxes of 0.2 and 0.4 μg N m−2 h−1 for NO and NO2, respectively (Gasche and Papen 1999).
Auxiliary Measurements
Daily rainfall was measured at the climate station at the IMGERS, approximately 10 km from the experimental sites. During the growing season, air and soil temperatures were automatically recorded every 1 min at UG99 and WG sites using PT100 thermocouples. In addition, soil moisture was measured daily at all experimental sites using a portable TDR probe (ThetaKit, Delta-T Devices, Cambridge, UK). At each site the TDR probe was inserted vertically at least five times. From the individual readings a mean value was calculated representing the soil moisture in 0-6 cm soil depth.
Statistical Analysis
Statistical analysis was performed as a blocked analysis with the different plots serving as the blocks, using SPSS 8.0 (SPSS Inc., Chicago, IL, USA) and Origin 7.0 (OriginLab Corp., USA) software packages. Tests of significant differences (P < 0.05) between the experimental sites were either performed with the multiple range test (LSD) of the ANOVA, with a parametric t-test or a non-parametric U-test (Mann–Whitney). Because no treatment replications were available, a separate analysis of the effect of grazing on soil N turnover and N trace gas exchange was not possible.
Results
Meteorology
The year 2004 was a “normal” year with a total sum of rainfall of 265 mm within the period June 1 to September 30 (average sum for the years 1982–2003: 262.3 mm). In contrast, rainfall in 2005 during the same period was only 119 mm, and, thus, far below average. The mean air temperature during the period June to September was 17.7 ± 0.7°C in 2004 and 19.3 ± 0.8°C in 2005, but the difference was not significant. However, it should be noted that daily air temperatures varied widely within a range from 2.5°C up to 35°C.
Inorganic Soil N Concentrations and Microbial N Turnover Rates
Ammonium concentrations in the uppermost 5 cm of the soil ranged from 0.1 to 5.0 mg N kg−1 soil dry weight (SDW) during the 2005 growing season, with mean values (± s.e.) of 0.51 ± 0.05 mg N kg−1 SDW for the UG99 site, 0.67 ± 0.09 mg N kg−1 SDW for the WG site, 0.73 ± 0.16 mg N kg−1 SDW for the OG site, and 1.71 ± 1.06 mg N kg−1 SDW for the UG79 site. At all sites soil nitrate concentrations were significantly higher than soil ammonium concentrations and ranged from 0.4 to 14.5 mg N kg−1 SDW. The highest mean nitrate concentration (6.96 ± 2.64 mg N kg−1 SDW) was found at the UG79 site, and was significantly higher than at all other three sites. The lowest soil nitrate concentrations were found at the WG site (1.59 ± 0.15 mg N kg−1 SDW). Mean nitrate concentrations at the UG99 and OG sites were 2.77 ± 0.17 mg N kg−1 SDW and 1.75 ± 0.29 mg N kg−1 SDW, respectively.
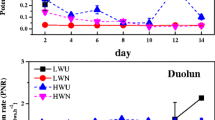
Monthly mean ammonification rates at the different steppe sites varied within a range of 0.1–7.5 mg N kg−1 SDW day−1 (Figure 1). Mean ammonification rates were significantly higher at the ungrazed sites than at the grazed sites (Table 2). The highest growing season mean ammonification rate was found at the UG79 site (Table 2). A comparable difference between ungrazed and grazed sites and between the sites UG79 and UG99 was also found for gross nitrification (Table 2, Figure 1).
N2O Fluxes
The N2O fluxes observed at the UG99 and WG sites were close to zero in both years, showing both net emission and net uptake (Figures 2, 3). Net emission of N2O was mainly observed in periods with volumetric soil water contents higher than 15%, whereas uptake of atmospheric N2O was especially observed when volumetric soil water content was low (Figures 2, 3). N2O fluxes were significantly correlated with soil moisture and soil temperature. However, the correlations explained only less than 20% of the variance of the measured fluxes. The linear regression of all data from the automated measurements in 2004 and 2005 at WG and UG99 plotted against volumetric soil water content resulted in an r 2 value of 0.166 (f(x) = 0.098 + 0.05x, P < 0.01), and the linear regression with soil temperature yielded an r 2 value of 0.19 (f(x) = 2.24 − 0.094x, P < 0.01). The mean N2O flux at the UG99 site was slightly, but not significantly lower than at the WG site in 2004 (Table 3). N2O fluxes during the much drier 2005 growing season tended to be lower than that in the 2004 growing season at both sites (Figure 3, Table 3). However, these differences were not statistically significant. The manual N2O flux measurements performed in 2005 at the UG79 and OG sites revealed somewhat higher mean N2O fluxes (Figure 4, Table 3). Nevertheless, N2O fluxes at both sites were not significantly different from N2O fluxes at the UG99 and WG sites, neither in 2004 nor in 2005.
Temporal course of soil (5 cm soil depth) and air temperatures, soil moisture (0–6 cm) and daily mean N2O fluxes during the 2004 growing season at the sites UG99 (filled circle) and WG (open circle). N2O fluxes are daily mean values (± s.e.), representing measurements with three chambers and eight flux measurements chamber−1 day−1.
Temporal course of mean daily N2O fluxes, soil moisture (0–6 cm) and soil and air temperature (5 cm soil depth) during the 2005 growing season at the sites UG99 (filled circle) and WG (open circle). N2O fluxes are daily mean values (± s.e.), representing measurements with three chambers and eight flux measurements chamber−1 day−1.
NO x Fluxes
Ambient air concentrations of NO and NO2 were mostly well below 1 ppbv during the measurement period in August and September 2004. Only during some days when wind was blowing predominantly from the direction of a nearby country road (distance approximately 1 km) did we observe slightly higher mean daily NO2 concentrations of up to 2.1 ppbv (Figure 5). Ambient air O3 concentrations varied in a range of 15–50 ppbv. Soil–atmosphere NO x fluxes at the UG99 site and the WG site were always close to the detection limit of the system (Figure 5). The magnitude of mean NO x fluxes was as low as observed for N2O fluxes (Table 3). However, it should be noted that NO x fluxes, like N2O fluxes, tended to be higher at the WG site than at the UG99 site.
Temporal course of mean daily NO x fluxes at the sites UG99 (filled circle) and WG (open circle), and ambient air concentrations of NO, NO2 and O3 in late August and September 2004. NO x fluxes are daily mean values (± s.e.), representing measurements with three chambers and 20 flux measurements chamber−1 day−1.
Discussion
Meteorology
Although the 2005 growing season was much drier and warmer than in 2004 – such a year-to-year variability of rainfall and temperature is not unusual in Inner Mongolia and the Xilin river basin (Liang and others 2003) – no significant effect on gross ammonification or on gross nitrification rates could be found. Also the growing season means of N2O fluxes were not significantly different in the two years. However, given the very low level of N2O emission rates, there was obviously not a sufficiently high potential of N gas formation to be stimulated.
Inorganic Soil N Concentrations and Microbial N Turnover Rates
In the present study, total inorganic N (nitrate + ammonium) concentrations tended to be higher at the ungrazed sites as compared to the grazed sites. At all sites nitrate concentrations in the top soil were on average significantly higher than ammonium concentrations with concentrations of up to 14.5 mg N kg−1 soil at the ungrazed sites. Our results are in agreement with a previous study carried out at the ungrazed and grazed sites (Wang and others 2006), but are in contrast to findings by Frank and others (2000) who found no significant effect of fencing on soil inorganic N concentrations in Yellowstone Park grasslands. Nitrate concentrations exceeding ammonium concentrations as found in this study were also reported for autumn measurements in Bromus-dominated shrub-steppe ecosystems in northern Utah (Booth and others 2003). The authors hypothesized that increased inorganic N concentrations in the topsoil of Bromus-dominated steppe sites as compared to Artemisia and Elymus steppe sites are most likely associated with the dense thatch at Bromus sites resulting in a more homogenous litter input as compared to litter inputs under perennials. Also the ungrazed sites of the present study had a well-developed thatch, whereas the thatch of the grazed sites was sparse and inhomogeneously distributed.
Changes in soil inorganic N concentrations are a result of differences in production, consumption and N loss processes, that is, ammonification, nitrification, denitrification, plant and microbial N uptake, gaseous N losses, and nitrate and DON leaching. Gross mineralization and gross nitrification rates, and, thus, N availability were significantly higher in the topsoil of the ungrazed plots of this study than that in the grazed plots. This was especially pronounced at the site, which had been protected from grazing for 26 years (UG79), but was still significant at the UG99 site (protected from grazing for 6 years) in the year 2005, that is, the year with strongly reduced rainfall during the growing season. In contrast, there was no significant difference between moderately (winter-)grazed and heavily (over-) grazed plots.
The significant differences in gross N turnover rates between the grazed and ungrazed plots of our study could have potentially been a result of differences in soil physical and chemical site properties, concealed by sufficient rainfall during the 2004 growing season, but unveiled by the very dry conditions in 2005. The sites exhibited small but measurable differences in soil texture, soil organic C (SOC) content and bulk density (Table 1). Although it is likely the differences in soil properties had been caused by grazing and sheep trampling (for example, Golodets and Boeken 2006), this could not be proved in the present work due to the lack of replication.
Our findings are in agreement with a livestock exclusion experiment in the Columbia Basin, USA, in which 32-fold and 149-fold higher potential net mineralization and net-nitrification rates, respectively, were found for ungrazed riparian meadow sites as compared to grazed sites (Kauffman and others 2004). In contrast, in a study including the determination of net mineralization and net nitrification rates under grazing disturbance at the sites of the present study in Inner Mongolia, Wang and others (2006) could not observe differences between grazed and ungrazed plots, supporting our finding that microbial inorganic N immobilization rates were in the same range as gross ammonification and gross nitrification rates and therefore masking differences in gross N turnover rates during the determination of net N turnover rates. Yet our results are in contradiction to most measurement and modeling studies in grasslands of North America, where grazing was mostly found to stimulate mineralization, nitrification and denitrification (Groffman and others 1993; Frank and Groffman 1998; Frank and others 2000; Le Roux and others 2003) which may be due to the stimulation of rhizosphere microbial mineralization activity as a result of increased root exudation after defoliation (Holland and others 1996; Hamilton and Frank 2001). However, a statistically sound testing of an effect of grazing on soil N turnover and N trace gas exchange in steppe ecosystems of Inner Mongolia was not possible in this study due to the lack of experimental plot replication.
N2O and NO Fluxes
Emission rates of N2O and NO x were in the range of 0.03–0.14 kg N2O-N ha−1 y−1 and 0.01-0.04 kg NO-N ha−1 y−1, respectively, and, thus, at the lower detection limit of the equipment used in the field experiments. N2O fluxes showed a highly significant correlation with soil moisture and soil temperature. The correlations, however, explained only less than 20% of the variance of the fluxes. However, other factors contributing to N2O flux variance could not be identified in this work.
The average growing season N2O emission rate found in this study was approximately 30–50% lower than was previously reported by Wang and others (2005), who calculated a range of 0.06–0.21 kg N2O-N ha−1 y−1 as the average annual N2O emission for the same region. Wang and others (2005) found differences in N2O emission rates between grazed and ungrazed plots in the same region at certain times during the growing season, especially during flowering of the grass. Whenever N2O fluxes were different, they were higher at the ungrazed plot. Xu and others (2003) found an annual average value for N2O emissions of 0.76 g N2O-N ha−1 day−1 for the typical steppe of Inner Mongolia, equivalent to 3.2 μg N2O-N m−2 h−1 and to 0.28 kg N2O-N ha−1 y−1, respectively, and Du and others (2006) even reported a mean total annual N2O flux of 0.73 ± 0.52 kg N2O-N ha−1 y−1. The higher values in these studies could be a result of not using sufficient means to exclude cross-sensitivity of electron capture detectors for CO2 if the gas-chromatographic separation of N2O and CO2 is insufficient. This can especially become a problem in studies with static soil chambers, leading to high CO2 accumulation during the measurements and, hence, to a higher bias in the N2O measurements. In our study ascarite, that is, sodium hydroxide on a silica carrier, was used as a CO2 absorbent. In the studies cited above the use of a CO2 absorbent was not mentioned in the respective “Materials and methods”, and by discussions with the involved colleagues we could confirm that a complete removal of CO2 could not be assured. Tests using both methods, that is, no or an ascarite pre-column for CO2 absorption revealed that at low N2O fluxes (<10 μg N m−2 h−1) not using an ascarite pre-column and N2 as carrier and purge gas will result in an overestimation of N2O fluxes up to a factor of 10 due to the cross-sensitivity with CO2. Therefore, we hypothesize that those previous methods used to measure N2O fluxes in Inner Mongolia grasslands tended to overestimate N2O fluxes. This is especially supported by the high number of single chamber flux rates determined in this study (approximately 10,000). N2O emission rates previously reported for temperate semi-arid grassland of North America were also low, albeit not as low as the ones found in this study, with emission rates below 6.5 μg N m−2 h−1 or 0.6 kg N ha−1 y−1 (for example, Mosier and others 1991, 1996, 2002; Epstein and others 1998; Mummey and others 1997). Mummey and others (2000) estimated an annual mean N2O emission of 0.24 kg N2O-N ha−1 y−1 from the Great Plain States of the US, that is, almost twice as high as the upper limit of the range of emission rates in this study. The higher rates found in North America could be a result of differences in vegetation composition between the two different steppe types and, hence, differences in soil nitrogen cycling. This is also supported by the very low average NO emission rates found in the present study, which are approximately two orders of magnitude lower than those found in a shortgrass steppe in northeastern Colorado (Epstein and others 1998), with the highest rates found for C4-dominated plots, whereas the Inner Mongolian typical steppe and likewise the experimental plots of the present study were dominated by C3 plants.
Conclusion
N2O and NO x fluxes were at a very low level at all four experimental sites in a typical steppe ecosystem of Inner Mongolia. No significant differences in N trace gas fluxes between the experimental plots, both grazed and ungrazed, were found. In contrast, at the grazed study sites microbial gross N turnover was significantly lower than at the ungrazed plots. Although the N2O fluxes were at least 30% lower than previously reported for the same region, the contribution of Eurasian steppe ecosystems to regional and global emission budgets could nonetheless be significant due to the vast extension of this ecosystem. For a statistical confirmation of our preliminary results on potential grazing effects on soil N cycling and N trace gas exchange further work on replicated sites and in other regions of Central and East Asian steppe is required.
REFERENCES
Augustine DJ, Frank DA. 2001. Effects of migratory grazers on spatial heterogeneity of soil N properties in a grassland ecosystem. Ecology 82:3149–62.
Bai Y, Han X, Wu J, Chen Z, Li L. 2004. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431:181–4.
Batjes NH. 1998. Mitigation of atmospheric CO2 concentrations by increased carbon sequestration in the soil. Biol Fertil Soils 27:230–5.
Billings SA, Schaeffer SM, Evans RD. 2002. Trace N gas losses and N mineralization in Mojave desert soils exposed to elevated CO 2 . Soil Biol Biochem 34:1777–84.
Booth MS, Stark JM, Caldwell MM. 2003. Inorganic N turnover and availability in annual- and perennial-dominated soils in a northern Utah shrub-steppe ecosystems. Biogeochemistry 66:311–30.
Breuer L, Papen H, Butterbach-Bahl K. 2000. N2O emission from tropical forest soils of Australia. J Geophys Res 105:26353–67.
Butterbach-Bahl K, Gasche R, Breuer L, Papen H. 1997. Fluxes of NO and N2O from temperate forest soils: impact of forest type, N deposition and of liming on the NO and N2O emissions. Nutr Cycl Agroecosys 48:79–90.
Butterbach-Bahl K, Gasche R, Huber C, Kreutzer K, Papen H. 1998. Impact of nitrogen input by wet deposition on N-trace gas fluxes and CH4-oxidation in spruce forest ecosystems of the temperate zone in Europe. Atmos Environ 32:559–64.
Butterbach-Bahl K, Kock M, Willibald G, Hewett B, Buhagiar S, Papen H, Kiese R. 2004. Temporal variations of fluxes of NO, NO2, N2O, CO2, and CH4 in a tropical rain forest ecosystem. Glob Biogeochem Cycles 18:GB3012.
Chen J, Stark JM. 2000. Plant species effects and carbon and nitrogen cycling in a sagebrush-crested wheatgrass soil. Soil Biol Biochem 32:47–57.
Chen SP, Bai YF, Han XG. 2003, Variations in composition and water use efficiency of plant functional groups based on their water ecological groups in the Xilin river basin. Acta Bot Sin 45:1251–60.
Chuluun T. 2001. Land use change and carbon cycle in arid and semi-arid lands of east and central asia. In: Land use change and the terrestrial carbon cycle in Asia. http://www.gcte.org/APN%20Kobe%20Report.pdf
Corre MD, van Kessel C, Pennock DJ. 1996. Landscape and seasonal patterns of nitrous oxide emissions in a semiarid region. Soil Sci Soc Am J 60:1806–15.
Corre MD, Schnabel RR, Stout WL. 2002. Spatial and seasonal variation gross nitrogen transformations and microbial biomass in a Northeastern US grassland. Soil Biol Biochem 34:445–57.
Davidson EA, Hart SC, Firestone MK. 1990. Microbial production and consumption of nitrate in an annual grassland. Ecology 71:1968–75.
Davidson EA, Hart SC, Shanks CA, Firestone MK. 1991. Measuring gross nitrogen mineralization, immobilization, and nitrification by 15N isotopic pool dilution in intact soil cores. J Soil Sci 42:335–49.
Du R, Lu D, Wang G. 2006. Diurnal, seasonal, and inter-annual variations of N2O fluxes from native semi-arid grassland soils of Inner Mongolia. Soil Biol Biochem 38:3474–3482.
Epstein HE, Burke IC, Mosier AR, Hutchinson GL. 1998. Plant functional type effects on trace gas fluxes in the shortgrass steppe. Biogeochemistry 42:145–68.
Frank DA, Groffman PM. 1998. Ungulate vs. landscape control of soil C and N processes in grasslands of Yellowstone National Park. Ecology 79:2229–41.
Frank DA, Groffman PM, Evans RD, Tracy BF. 2000. Ungulate stimulation of nitrogen cycling and retention in Yellowstone Park grasslands. Oecologia 123:116–21.
Gasche R, Papen H. 1999. A 3-year continuous record of nitrogen trace gas fluxes from untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany –2. NO and NO2 fluxes. J Geophys Res 104:18505–20.
Golodets C, Boeken B. 2006. Moderate sheep grazing in semiarid shrubland alters small-scale soil surface structure and patch properties. Catena 65:285–91.
Graetz D. 1994. Grasslands. In: Meyer W, Turner II B, Eds. Change in land use and land cover, a global perspective. Cambridge: Cambridge University Press. pp 287–328.
Groffman PM, Rice CW, Tiedje JM. 1993. Denitrification in a tallgrass prairie landscape. Ecology 74:855–62.
Hamilton EW, Frank DA. 2001. Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82:2397–402.
Holland JN, Cheng W, Crossley DA Jr. 1996. Herbivore-induced changes in plant carbon allocation: assessment of below-ground C fluxes using carbon−14. Oecologia 107:87–94.
IPCC. 2001. Climate Change 2001: the scientific basis. In: Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA, Eds. Contribution of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press. 881 pp.
Kauffman JB, Thorpe AS, Brookshire ENJ. 2004. Livestock exclusion and belowground ecosystem responses in riparian meadows of Eastern Oregon. Ecol Appl 14:1671–79.
Kirkham D, Bartholomew WV. 1954. Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci Soc Am Proc 18:33–4.
Le Roux X, Bardy M, Loiseau P, Louault F. 2003. Stimulation of soil nitrification and denitrification by grazing in grasslands: do changes in plant species composition matter? Oecologia 137:417–25.
Li XR. 2001. Study on shrub community diversity of Ordos Plateau, Inner Mongolia, Northern China. J Arid Environ 47:271–9.
Li SG, Harazono Y, Oikawa T, Zhao HL, He ZY, Chang XL. 2000. Grassland desertification by grazing and the resulting micrometeorological changes in Inner Mongolia. Agr For Meteorol 102:125–37.
Liang EY, Vennetier M, Lin JX, Shao XM. 2003. Relationships between tree increment, climate and above-ground biomass of grass: A case study in the typical steppe, north China. Acta Oecol 24:87–94.
Martin RE, Scholes MC, Mosier AR, Ojima DS, Holland EA, Parton WJ. 1998. Controls on annual emissions of nitric oxide from soils of the Colorado shortgrass steppe. Glob Biogeochem Cycles 12:81–91.
Mosier AR, Schimel DS, Valentine DW, Bronson KF, Parton WJ. 1991. Methane and nitrous oxide fluxes in native, fertilized, and cultivated grasslands. Nature 350:330–2.
Mosier AR, Parton WJ, Valentine DW, Ojima DS, Schimel DS, Delgado JA. 1996. CH4 and N2O fluxes in the Colorado shortgrass steppe: 1. Impact of landscape and nitrogen addition. Glob Biogeochem Cycles 10:387–99.
Mosier AR, Morgan JA, King JY, Milchunas DG. 2002. Soil–atmosphere exchange of CH4, CO2, NO x , and N2O in the Colorado shortgrass steppe under elevated CO2. Plant Soil 240:201–11.
Mummey DL, Smith JL, Bolton H. 1997. Small-scale spatial and temporal variability of N2O flux from a shrub-steppe ecosystem. Soil Biol Biochem 29:1699–706.
Mummey DL, Smith JL, Bluhm G. 2000. Estimation of nitrous oxide emissions from US grasslands. Environ Manage 25:169–75.
Oba G, Vetaas OR, Stenseth NC. 2001. Relationships between biomass and plant species richness in arid-zone grazing lands. J Appl Ecol 38:836–45.
Papen H, Butterbach-Bahl K. 1999. A 3-year continuous record of nitrogen trace gas fluxes from untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany. 1. N2O emissions. J Geophys Res 104:18487–503.
Paruelo JM, Burke IC, Lauenroth WK. 2001. Land-use impact on ecosystem functioning in eastern Colorado, USA. Glob Change Biol 7:631–9.
Rosenkranz P, Brüggemann N, Papen H, Xu Z, Horváth L, Butterbach-Bahl B. 2006a. Soil N and C trace gas fluxes and microbial soil N turnover in a sessile oak (Quercus petraea (Matt.) Liebl.) forest in Hungary. Plant Soil 286:301–22.
Rosenkranz P, Brüggemann N, Papen H, Xu Z, Seufert G, Butterbach-Bahl K. 2006b. N2O, NO and CH4 exchange, and microbial N turnover over a Mediterranean pine forest soil. Biogeosciences 3:121–33.
Ross DJ, Tate KR, Scott NA, Teltham CW. 1999. Land-use change: effects on soil carbon, nitrogen and phosphorus pools and fluxes in three adjacent ecosystems. Soil Biol Biochem 31: 803–13.
Smart DR, Stark JM, Diego V. 1999. Resource limitations of nitric oxide emissions from a sagebrush-steppe ecosystem. Biogeochemistry 47:63–86.
Stark JM, Hart SC. 1997. High rates of nitrification and nitrate turnover in undisturbed coniferous forests. Nature 385:61–4.
Steffens M, Kölbl A, Totsche KW, Kögel-Knabner I. 2007. Heavy grazing significantly deteriorates topsoils in a shortgrass steppe in Inner Mongolia (P. R. China). Geoderma (to appear)
Tong C, Wu J, Yong S, Yang J, Yong W. 2004. A landscape-scale assessment of steppe degradation in the Xilin river basin, Inner Mongolia, China. J Arid Environ 59:133–49.
Verchot LV, Groffman PM, Frank DA. 2002. Landscape versus ungulate control of gross mineralization and gross nitrification in semi-arid grasslands of Yellowstone National Park. Soil Biol Biochem 34:1691–9.
Wang RZ. 2002. Photosynthetic pathways, life forms, and reproductive types for forage species along the desertification gradient on Hunshandake desert, North China. Photosynthetica 40:321–9.
Wang R, Ripley EA. 1997. Effects of grazing on a Leymus chinensis grassland on the Songnen plain of north-eastern China. J Arid Environ 36:307–18.
Wang YS, Wang YH. 2003. Quick measurement of CH4, CO2 and N2O emissions from a short-plant ecosystem. Adv Atmos Sci 20:842–4.
Wang Y, Xue M, Zheng X, Ji B, Du R, Wang Y. 2005. Effects of environmental factors on N2O emission from and CH4 uptake by the typical grasslands in the Inner Mongolia. Chemosphere 58:205–15.
Wang C, Wan S, Xing X, Zhang L, Han X. 2006. Temperature and moisture interactively affected soil net N mineralization in temperate grassland in Northern China. Soil Biol Biochem 38:1101–10.
Xu R, Wang Y, Zheng X, Ji B, Wang M. 2003. A comparison between measured and modeled N2O emissions from Inner Mongolian semi-arid grassland. Plant Soil 255:513–28.
Zhang W, Skarpe C. 1996. Small-scale vegetation dynamics in semi-arid steppe in Inner Mongolia. J Arid Environ 34:421–39.
ACKNOWLEDGEMENTS
The work has been supported by the German Research Foundation (DFG, Research Unit No. 536, “Matter fluxes in grasslands of Inner Mongolia as influenced by stocking rate”, MAGIM) and by the National Natural Science Foundation of China (NSFC, project no. 40331014). We thank Ralf Kiese, Georg Willibald and Ursula Berger for their technical assistance in the field and laboratory work. Our thanks also go to Zhihong Yu and Shubin Yu for their valuable assistance in manual gas sampling and GC analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holst, J., Liu, C., Brüggemann, N. et al. Microbial N Turnover and N-Oxide (N2O/NO/NO2) Fluxes in Semi-arid Grassland of Inner Mongolia. Ecosystems 10, 623–634 (2007). https://doi.org/10.1007/s10021-007-9043-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-007-9043-x