Abstract
Climate change may affect ecosystem functioning through increased temperatures or changes in precipitation patterns. Temperature and water availability are important drivers for ecosystem processes such as photosynthesis, carbon translocation, and organic matter decomposition. These climate changes may affect the supply of carbon and energy to the soil microbial population and subsequently alter decomposition and mineralization, important ecosystem processes in carbon and nutrient cycling. In this study, carried out within the cross-European research project CLIMOOR, the effect of climate change, resulting from imposed manipulations, on carbon dynamics in shrubland ecosystems was examined. We performed a 14C-labeling experiment to probe changes in net carbon uptake and allocation to the roots and soil compartments as affected by a higher temperature during the year and a drought period in the growing season. Differences in climate, soil, and plant characteristics resulted in a gradient in the severity of the drought effects on net carbon uptake by plants with the impact being most severe in Spain, followed by Denmark, with the UK showing few negative effects at significance levels of p ≤ 0.10. Drought clearly reduced carbon flow from the roots to the soil compartments. The fraction of the 14C fixed by the plants and allocated into the soluble carbon fraction in the soil and to soil microbial biomass in Denmark and the UK decreased by more than 60%. The effects of warming were not significant, but, as with the drought treatment, a negative effect on carbon allocation to soil microbial biomass was found. The changes in carbon allocation to soil microbial biomass at the northern sites in this study indicate that soil microbial biomass is a sensitive, early indicator of drought- or temperature-initiated changes in these shrubland ecosystems. The reduced supply of substrate to the soil and the response of the soil microbial biomass may help to explain the observed acclimation of CO2 exchange in other ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shrubland ecosystems are often natural or seminatural by nature and constitute large areas of Europe. Nutrient-poor shrubland ecosystems are vulnerable to multiple environmental pressures such as climate change, eutrophication by nitrogen deposition, changes in the water table, as well as ongoing fragmentation by changes in land use and because of the limited lifespan of the shrub vegetation. Its size, both aboveground and belowground, makes it susceptible to shading and water and nutrient shortages. Thus, although a shrubland appears rather stable and often accommodates a large biodiversity, it can quickly change to other ecosystems or states.
The increased anthropogenic emission of CO2 has led to a continually rising atmospheric CO2 concentration (Keeling and others 1995), which poses an additional threat through a predicted increase in temperature and altered rainfall patterns.
Temperature and water availability are important drivers for ecosystem processes such as photosynthesis, carbon translocation, and organic matter decomposition; therefore, climatic changes are likely to affect ecosystem functioning (Meharg and Killham 1989, Meharg and Killham 1990; Bardgett and others 1999; Gordon and others 1999; Oechel and others 2000; Shaver and others 2000; Luo and others 2001).
The carbon flow through the terrestrial system (that is, photosynthesis → carbon input into soil → decomposition) will be directly and indirectly affected by increased temperature and changes in water availability. A small increase in temperature will be expected to result in few changes in rates of photosynthesis and carbon fixation, especially when temperatures are close to optimum (Llorens and others 2004); however, water deficiency may strongly affect photosynthesis and carbon translocation (Deng and others 1990). Decomposition and mineralization play a key role in ecosystem functioning and are mainly conducted by soil microorganisms (Grayston and others 1996). It is expected that these processes will increase with increasing temperature (Kirschbaum 1995), although feedback mechanisms may cause acclimation of these processes (Oechel and others 2000; Luo and others 2001). The impact of changes in soil moisture will be dependent on the existing moisture conditions in the soil and could either stimulate or depress microbial processes. The activity of the soil microbial population also strongly depends on the input of easily decomposable substrates (for example, exudates and lysates) released from roots (Røsberg and others 1981; Helal and Sauerbeck 1989; Van Ginkel and others 1997, Van Ginkel and others 2000). It has been shown that rhizodeposition has a major effect on decomposition, mineralization, and nutrient cycling (Högberg and others 2001; Whipps and Lynch 1985, Whipps and Lynch 1986), yet, despite this, it is often an overlooked aspect of terrestrial ecosystems.
Potentially, increased nitrogen availability due to temperature-stimulated mineralization, supplemented by atmospheric nitrogen deposition and changes in water availability, may subsequently affect plant growth. It also may be expected that changes will be seen in carbon uptake, carbon allocation patterns (for example, shoot-to-root ratios), and quantity and quality (for example, C:N ratio) of leaf litter and root deposits (Simmons and others 1996). Such quantitative and qualitative changes in litter quality and rhizodeposition could ultimately be reflected in plant species composition (Berendse 1994; Grime and Hunt 1975; Wedin and Tilman 1996; Van der Krift and others 2001).
From this perspective, we performed a 14C-labeling experiment carried out within the cross-European research project CLIMOOR (climate-driven changes in the functioning of heathland and moorland ecosystems). CLIMOOR was initiated in 1998 and involved experimental field-scale manipulation of the temperature and water input at four shrubland ecosystems in Spain, UK, Denmark, and The Netherlands (Beier and others 2004). The aim of the 14C-labeling experiment was to probe changes in net carbon uptake and allocation to the roots and soil compartment under field conditions as affected by a higher temperature during the whole year and a drought period in the growing season. The hypotheses tested were (1) summer drought will decrease net carbon uptake from photosynthesis and will decrease the amount of carbon allocated to roots and carbon supply to the soil microbial biomass, and (2) a higher temperature will increase net carbon uptake from photosynthesis as a result of stimulated nutrient mineralization and will increase carbon allocation to roots and soil, thus further stimulating microbial activity.
Materials and Methods
Sites and Manipulations
Manipulations were carried out in shrubland ecosystems at four sites (UK, Denmark, The Netherlands, Spain) spanning a gradient in temperature and precipitation (Table 1). The four sites were seminatural ecosystems dominated by Ericacea plants with no management activities conducted for at least the last ten years prior to the experiment. At each site, field-scale warming and drought treatments were conducted in three replicated 20-m2 plots per treatment and treatment effects were compared with three untreated control plots.
Warming Treatment
Warming was applied as nighttime warming. This was chosen because the temperature increase of 0.6°C experienced so far over land has mainly been due to an increase in the daily minimum temperatures (T min), which have increased twice as much as maximum temperatures (T max), primarily because of increased cloudiness (IPCC 2001). Solar energy accumulates in the ecosystem during the day and a fraction of the energy is radiated back to the atmosphere at night as long-wave infrared (IR) radiation. Nighttime warming was done by covering the vegetation at night by reflective aluminum curtains to reduce the loss of IR radiation. The plot cover system was activated automatically according to preset light (less than 200 lux) conditions. To avoid influencing the hydrological cycle, the covers were automatically removed during rain events, triggered by rain sensors. However, registration of these rain events caused a small delay from the start of the rain until the curtains were opened. Overall, the warming treatment reduced water input to the heathlands by 10%–22% (Beier and others 2004). The warming treatment began in March 1999 with an average temperature increase to the soil and plants on the order of 0.5–2°C during the day and night (Beier and others 2004).
Drought Treatment
The drought treatment was performed for a 2-month period in the spring/summer growing season of 2000. The time of the growing season differs across Europe, peaking in the dry, warm midsummer in the northern UK, Danish, and Dutch sites. The peak growing season in Spain, however, is in the spring and autumn when most of the precipitation occurs. Covering the vegetation with waterproof, transparent covers served as the drought treatment. The drought plots were constructed similar to the warming plots except that the curtain material consisted of transparent plastic and that curtain movement was governed by rain. During the drought period, the rain sensors activated the curtain to cover the plots whenever it rained and removed the curtains after the rain stopped. For the part of the year without drought treatment, the drought plots were run parallel to the control plots.
Untreated Control
The treatment effects were assessed by comparison with three untreated control plots with similar scaffolding as for the warming and drought treatments but without a curtain. More details on the experimental setup and the treatments are given by Beier and others (2004).
14C-Labeling Experiment
A 14C-pulse-labeling experiment was performed with one of the dominant species on each site. In the UK (UK), Denmark (DK), and The Netherlands (NL) this was Calluna vulgaris (L.) and in Spain (ES) it was Erica multiflora (L.). The Dutch site had to be discarded from the experiment before the 14C-labeling was performed because the plants selected for labeling were severely damaged by rabbits during the winter period. Nine individual plants of the same size were selected outside the plots for transplantation into the nine plots (3 control, 3 drought, and 3 warming plots). The plants were about 5–7 years old. To avoid damage to the root systems as much as possible, the plants were carefully dug out using a PVC column with an open bottom (internal diameter 24 cm; height 20 cm). The columns were placed around the selected plants and the column was pushed downwards while the soil was removed around the outside of the columns. Subsequently, the plants were transferred to similar PVC columns, which were closed at the bottom with a small-mesh polyamide netting (31.5 Μm; Stokvis & Smits BV, IJmuiden). This netting was used to prevent outgrowth of roots without restricting water movement in and out the column. The height of the columns at the Spanish site had to be reduced to 15 cm because of the rocky subsoil. The upper part of the column included a closed-cell foam band on the outside to enable an airtight fastening of a plastic bag during the 14C-labeling.
Inside the nine experimental plots, a hole was carefully prepared in which the columns fitted. The nine columns with plants were placed in the plots during autumn 1999 (DK: September 28–29; UK, October 5–6, and ES: October 19–20).
The plants in the control (C) treatment were exposed to ambient temperature and precipitation. The plants in the warming (W) treatment were continually exposed to this treatment from installation in autumn 1999 until labeling and harvesting in summer 2000. The plants in the drought (D) treatment were exposed to a severe drought before pulse-labeling in summer 2000.
A heather beetle attack (Lochmaea suturalis Thompson) occurred in the DK site during 1999/2000, which to some extent damaged the plants. Only two of the three replicates per treatment in this site survived and were analyzed.
Because of a severe natural drought in winter 2000, all plants at the Spanish site were irrigated on February 9 (4 mm), February 11 (4 mm), February 16 (20 mm), and February 25 (20 mm).
The drought period in 2000 at the different sites started in ES on April 18, in DK on May 23, and in UK on July 11. The 14C-pulse-labeling was carried out when plants had reached the same phenological growing stage in all treatments, that is, 4–6 weeks after starting the imposed drought treatment (ES: May 24, DK: July 12, UK: August 16).
14C-Labeling
At labeling, a glass vial with a solution containing 4.9 MBq of 14C-labeled Na2CO3 (2.0 GBq mmol−1) was placed on top of the column. The shoot was subsequently enclosed in a polythene cover and fixed airtight around the foam band at the top of the column. 14C–CO2 was released by injecting 20 mL of 5 N HCl into the glass vial through a septum in the plastic cover. The CO2 concentration in the chamber was increased by about 30 ΜL L−1. After 200 min, the plastic cover was flushed and the air was forced through 75 mL of 0.5 M NaOH solution to trap the remaining 14CO2 in the atmosphere. Labeling took place between 8 and 11 a.m. to prevent high temperatures in the plastic bag. Temperatures during labeling were measured using a TinytagPlus thermometer (Intab Benelux, Cuijk, The Netherlands). Temperature increased from 17 to 37°C at the Spanish site, from 17 to 31°C at the Danish site, and from 12 to 21°C at the UK site. The plastic bags (chambers) were removed and the plants were left in the plots for 72 h to allow the 14CO2 taken up by the plants to be allocated to the different compartments of the plant/soil system. After 72 h, the columns were lifted from the soil, the shoots were clipped at the base, and the soil columns and the plants were transported to the laboratory where they were kept at 2°C until further processing.
Measurements
Soil in the columns was divided into an upper layer of 10 cm and a lower layer of 5 cm (ES) or 10 cm (DK, UK). Stones and roots were removed by sieving (5 mm) and handpicking, and part of the soil was oven-dried at 70°C until constant weight for 14C determination and soil moisture measurement. Soil microbial biomass was determined in fresh soil using the fumigation–extraction method (Vance and others 1987). Duplicate soil samples were chloroform-fumigated for 24 h. Twenty-five grams of unfumigated and fumigated soil were shaken with 100 mL of 0.5 M K2SO4 for 1 h. One-half milliliter of the extracts was mixed with 3 mL of UltimaGold scintillation liquid (Packard) and counted (Tri-Carb 2100, Packard). Solutions of the unfumigated soil samples were used to determine the soluble 14C fraction. The difference (14C flush) between the fumigated and unfumigated soil samples was used to calculate soil microbial biomass 14C (see below).
Roots were carefully washed and dried at 70°C until constant weight. Shoots were divided into leaves and stems and also dried at 70°C. Total carbon and total 14C content in dried shoots, roots, and soil were determined after wet digestion (Dalal 1979). Plant material (30 mg) and soil (1 g) were digested with 5 mL of a 10% (w/v) K2Cr2O7 solution in H2SO4 and H3PO4 (3:2 v/v) at 160°C for 2 h. Released CO2 was trapped in 10 mL of a 0.5 N NaOH solution. 14C was determined in 0.5 mL of NaOH as described above. A carbon budget was calculated including total net 14C uptake and distribution of 14C among shoots, roots, and soil.
Calculations
A conversion factor (K c factor) of 0.45 was used to calculate soil microbial 14C from the 14C flush (Vance and others 1987). The measured total 14C content in soil consists of soluble 14C, microbial biomass 14C, and residual 14C. This soil residue was calculated by subtracting soluble 14C in the unfumigated soil extracts and microbial biomass 14C from total soil 14C. This residue fraction will mainly consist of insoluble secretions and lysates, including root hairs and small roots.
Statistics
The experiment consisted of three treatments (control, warming, and drought) and three sites (ES, UK, and DK). Each treatment consisted of three replicates, except for Denmark with two replicates per treatment. The effects of drought and warming within sites were separately analyzed against the control treatment by ANOVA (Genstat 5, version 4.2). Differences are considered significant for p ≤ 0.10.
Results
Soil Water and Plant Biomass
Nighttime warming increased the mean daily soil temperature during the period just before labeling by 1.3°C (UK), 0.2°C (DK), and 0.8°C (ES) compared with the controls (Figure 1). Table 2 shows the accumulated rainfall before the 14C-labeling inside the plots. The results show that the roofs of the drought plots reduced the cumulative rain input during about eight weeks before labeling by 52%, 97%, and 41% in UK, DK, and ES, respectively, compared with the control. At all sites a large decrease in rainfall was created in the drought plots. In Spain and Denmark, the reduced rain input resulted in a relative decrease of over 65% in soil water content (in both soil layers; Table 3). No difference was found in the soil moisture content in the columns at harvest time at the UK site (Table 3), a measurement that we can not explain. However, four days before labeling, probes measured a 50% reduction in soil moisture in the drought treatment compared with the control, confirming the reduced cumulative rainfall in the drought plots. More detailed information about temperatures and rainfall is given by Beier and others (2004).
Soil temperature in the warming treatment compared with the control at the different sites during the last 50 days before 14C-labeling. More details in Beier and others (2004).
Dry weights of the plant fractions were not significantly affected by any of the treatments (Table 4). The dry weight of the leaves at the Danish site was low as a result of the heather beetle (Lochmaea suturalis Thompson) attack at the site in 1999.
At each of the sites most of the root biomass (85%–95%) was found in the upper 10 cm of the soil profile. Root mass was not significantly affected by the treatments and was comparable for all sites (Table 4).
Net 14C Uptake and Absolute 14C Allocation
Total net 14C uptake was lowest in Spain and highest in Denmark and the UK (Table 4). The low net uptake at the Spanish site resulted in 14C amounts allocated to the soil compartments that were too low for accurate measurements (Tables 4 and 5). An interaction was found between drought and site (D × S) for the 14C uptake. In Spain, total net 14C uptake by the plants was significantly reduced by 96% in the drought treatment compared with 39% in Denmark. The reduction in net 14C uptake in the warming treatment in Spain and Denmark was not significant. In contrast, at the UK site the drought resulted in a slight (13%) but not significant increase in net 14C uptake, whereas the warming treatment resulted in no change. This was also observed in 14C uptake per unit leaf (Figure 2).
By far the most significant result from the 14C labeling of the plant and soil cores was the reduced 14C allocation to the soil compartment as a result of the drought treatment. For the warming treatment, the same tendencies (p values between 0.10 and 0.20) were observed in all soil fractions. At the Danish site total 14C translocated belowground was reduced by 80% for the drought treatment and 74% as a result of the warming treatment (Table 4). This decrease was also observed at the UK site (39% by drought and 27% by warming). Within the soil compartments at both these sites the drought treatment also significantly reduced 14C allocation to soluble soil 14C and soil microbial biomass (Table 4). Reductions observed in the warming treatment were not significant. 14C allocation to the soil compartment at the Spanish site was undetectable in any of the treatments (including the control).
Relative 14C Allocation
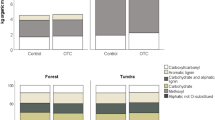
When expressed as a percentage of total net 14C uptake, relative 14C allocation showed that overall more than 97% of the 14C was found in the plants and less than 3% in the soil compartments, in Spain even less than 1% (Table 5). Drought and warming did not significantly effect relative 14C allocation within the plant compartments at any of the sites (Table 5).
Within the soil compartments, drought significantly reduced the relative 14C allocation to the soil microbial biomass in Denmark and the UK. As with the absolute 14C allocation, drought decreased the relative 14C allocation to the soil soluble fraction and the soil microbial biomass at the Danish and UK sites (Figure 3) Reductions observed in the warming treatment were significant only for the soluble fraction in Denmark.
Discussion
Climate change, caused by increasing atmospheric levels of CO2 and other greenhouse gasses, poses an additional threat to already vulnerable European shrublands. Warming of the atmosphere and the earth’s surface may invoke changes in precipitation patterns, evapotranspiration, and water availability in soils. Shrubland-like ecosystems may be sensitive to these changes because, in contrast to forest ecosystems growing on comparable soils, shrubs are less capable of exploring deeper soil layers for water. In many areas this will exacerbate existing problems caused by altered water tables. This is directly applicable for the CLIMOOR sites with the majority of root biomass (85%–95%) found in the upper 10 cm of the soil profile. In the longer term, root biomass may be a good indicator of changes in carbon allocation to the soil compartment. In the short term, small changes are difficult to observe and the apparent differences in this study were probably already present at the start of the experiment.
This study shows that drought reduces total net carbon uptake of these shrubland ecosystems and the subsequent allocation of carbon to soil compartments. The release of carbon compounds by roots decreased not only because of reduced net carbon uptake by plants, but also as a result of a change in the carbon allocation to root exudates; the percentage of 14C found in the soluble fraction and in microbial biomass decreased by more than 60%, whereas there was no observed change in the relative allocation pattern within the plant. Generally, the relative amount of carbon allocated to the soil through the release by roots is usually small, but a prolonged reduction in the supply of easily decomposable carbon compounds and energy as seen in this study may alter soil microbial activity.
Carbon Uptake
General net carbon uptake was lower in Spain than in the UK and Denmark, indicating a low photosynthetic activity probably caused by the dry conditions at this site. This low net uptake at the Spanish site resulted in 14C amounts allocated to the soil compartments that were very low and not reliable (Tables 4 and 5).
Drought.
In the experiment, not all sites reacted equally to the drought treatment. Beforehand, one would expect the strongest effect of drought on carbon dynamics at the warmest and drier site with the highest evapotranspiration, Spain, and the weakest effect in the colder and wetter site, the UK. The drought treatment at the Spanish site was severe and caused a strong reduction in the already low net carbon uptake. This lower CO2 uptake resulted from a reduced photosynthetic area (as indicated by the lower dry leaf mass) and a reduced uptake per unit leaf. Dry and warm conditions generally force plants to close their stomata to control transpiration, causing an accompanying reduction in photosynthesis (Schulze and others 1987). The lower plant activity in Erica multiflora at the Spanish site compared with Calluna vulgaris at the Danish and the UK sites was reflected in measurements on the photosynthetic rates that were less than 25% of the rates measured in the UK and Denmark (Llorens and others 2004).
In contrast, the peaty soil in the UK had a much higher water-holding capacity, and, although soil moisture was affected by the drought treatment, the soil still contained much more water compared with the sandy soils at the Spanish site and at the Danish site. The differences in soil, climate, and plant characteristics caused a gradient in the drought effect on net carbon uptake by the plants with the effect in Spain greater than in Denmark which is greater than in the UK, a gradient also found for photosynthetic rates (Llorens and others 2004). Carbon uptake at the UK site was not clearly affected; if anything, a trend for higher net 14C fixation was observed in the plants in the drought plots. This contradicts previous findings by Gordon and others (1999), who found a clear decrease in potential photosynthetic activity in Calluna vulgaris during a summer drought in the UK. However, their drought treatment was apparently more severe than in the CLIMOOR experiment as heather shoots were wilting, a symptom that was not observed in our experiment.
Warming.
The warming treatment did not increase net 14C uptake as had been hypothesized. The expected stimulating effect of increased mineralization on plant growth was also not observed in leaf dry weights.
Carbon Allocation
Drought.
The amount of 14C allocated belowground in Spain was so small that subsequent transfer of the 14C to the soil compartments could not be detected. At the Danish site and the UK site, drought reduced absolute carbon allocation to the soluble fraction and the residue fraction. The amounts recovered in solubles (probably mainly consisting of exudates) and in the residue (three days after labeling probably mainly containing insoluble secretions and lysates, including root hairs and small roots) serve as substrate for the microbial biomass and a differential response of these soil fractions (soluble − insoluble) to drought treatment could affect the soil microbial community differently. In this study, the decrease in 14C transfer into both fractions resulted in an overall reduction of the 14C microbial biomass. Also, the relative amount of carbon (that is, the fraction of the total 14C fixed) that was allocated to soil microbial biomass was strongly depressed by the drought treatment, suggesting that microbial biomass may be a sensitive, early indicator of drought-initiated changes in shrubland ecosystems.
Warming.
The warming treatment also affected 14C allocation to the soil. Given the drier conditions in the warming treatment at the Danish site, this reduction is to some extent comparable with the drought treatment. Certainly at this site carbon uptake and distribution seemed to be related to soil moisture conditions. The warming treatment did not significantly reduce 14C allocation to the soil fractions at either the Danish or the UK site. In theory, a reduction in carbon allocation may follow an increased availability of soil nutrients as a result of increased mineralization (Emmett and others 2004) that enables plants to invest relatively less carbon and energy in the root system.
In conclusion, this field study shows that soil microbial biomass seems to be an early indicator of changes in soil carbon dynamics induced by drought in the shrubland ecosystems studied in CLIMOOR. The results of the warming treatment showed similar, but not significant, effects. Repeated analyses and a time-integrated assessment are certainly warranted in future research of these shrublands and would give us a better judgment of the changes in 14C fixation and 14C allocation caused by warming and especially drought. We have confidence that such an assessment would reinforce the 14C results because results on biomass accumulation and net primary productivity after the whole growing period point in the same direction as the 14C fixation response (Peñuelas and others 2004). The climate manipulations, especially drought and to a lesser extent warming, within the CLIMOOR project are significantly reducing carbon allocation to the soil. These observations on a reduced supply of substrate may play a significant role in explaining the observed acclimation of CO2 exchange in tundra (Oechel and others 2000) and tall grass prairie (Luo and others 2001) ecosystems. At present we cannot extrapolate how the reduced carbon allocation to the microbial population, and subsequent effects on carbon and nutrient cycles, will accumulate over the years. However, as microbial decomposition processes play a central role in soil nutrient cycles (Van Veen and others 1989), it could be predicted that altering microbial activity may affect ecosystem functioning in the longer term.
References
RD Bardgett E Kandeler D Tscherko PJ Hobbs TM Bezemer TH Jones LJ Thompson (1999) ArticleTitleBelow-ground microbial community development in a high temperate world Oikos 85 193–203
C Beier B Emmett P Gundersen A Tietema J Peñuelas M Estiarte C Gordon A Gorissen L Llorens F Roda D Williams (2004) ArticleTitleNovel approaches to study climate change effects on terrestrial ecosystems in the field: Drought and passive nighttime warming Ecosystems 7 .
F Berendse (1994) ArticleTitleCompetition between plant populations at low and high nutrient supplies Oikos 71 253–60
RC Dalal (1979) ArticleTitleSimple procedure for the determination of total carbon and its radioactivity in soils and plant materials Analyst 104 151–4 Occurrence Handle1:CAS:528:DyaE1MXkslems7o%3D
X Deng RJ Joly T Hahn (1990) ArticleTitleThe influence of water deficit on photosynthesis and translocation of 14C-labeled assimilates in cacao seedlings Physiol Plant 78 623–7 Occurrence Handle10.1034/j.1399-3054.1990.780419.x
BA Emmett C Beier M Estiarte A Tietema HL Kristensen D Williams J Peñuelas IK Schmidt A Sowerby (2004) ArticleTitleThe response of soil processes to climate change: Results from manipulation studies of shrublands across an environmental gradient Ecosystems 7 .
C Gordon SJ Woodin IJ Alexander CE Mullins (1999) ArticleTitleEffects of increased temperature, drought and nitrogen supply on two upland perennials of contrasting functional type: Calluna vulgaris and Pteridium aquilinum. New Phytol 142 243–58 Occurrence Handle10.1046/j.1469-8137.1999.00399.x
SJ Grayston D Vaughan D Jones (1996) ArticleTitleRhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability Appl Soil Ecol 5 29–56 Occurrence Handle10.1016/S0929-1393(96)00126-6
JP Grime R Hunt (1975) ArticleTitleRelative growth rate: its range and adaptive significance in a local flora J Ecol 63 393–422
HM Helal D Sauerbeck (1989) ArticleTitleCarbon turnover in the rhizosphere Z Pfanzenernährung Bodenk 152 211–6 Occurrence Handle1:CAS:528:DyaL1MXkvFKju7s%3D
P Högberg A Nordgren N Buchmann AFS Taylor A Ekblad MN Högberg G Nyberg M Ottosson–Lofvenius DJ Read (2001) ArticleTitleLarge scale forest girdling shows that current photosynthesis drives soil respiration Nature 411 789–92 Occurrence Handle10.1038/35081058 Occurrence Handle11459055
InstitutionalAuthorNameIPCC JT Houghton Y Ding DJ Griggs M Noguer PJ Van der Linden X Dai K Maskell CA Johnson (2001) Climate Change 2001: The Scientific Basis Cambridge University Press Cambridge
CD Keeling TP Whorf M Wahlen J Van der Plicht (1995) ArticleTitleInterannual extremes in the rate of rise of atmospheric dioxide since 1980 Nature 375 666–70 Occurrence Handle10.1038/375666a0 Occurrence Handle1:CAS:528:DyaK2MXmsFKrtrY%3D
MUF Kirschbaum (1995) ArticleTitleThe temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage Soil Biol Biochem 27 753–60 Occurrence Handle10.1016/0038-0717(94)00242-S Occurrence Handle1:CAS:528:DyaK2MXlsF2msrk%3D
L Llorens J Peñuelas C Beier B Emmett M Estiarte A Tietema (2004) ArticleTitleEffects of an experimental increase of temperature and drought on the photosynthetic performance of two ericaceous shrub species along a north–south European gradient Ecosystems 7 .
Y Luo S Wan D Hui LL Wallace (2001) ArticleTitleAcclimation of soil respiration to warming in a tall grass prairie Nature 413 622–5 Occurrence Handle10.1038/35098065 Occurrence Handle1:CAS:528:DC%2BD3MXnslegtL4%3D Occurrence Handle11675783
AA Meharg K Killham (1989) ArticleTitleDistribution of assimilated carbon within the plant and rhizosphere of Lolium perenne: influence of temperature Soil Biol Biochem 21 487–9 Occurrence Handle10.1016/0038-0717(89)90119-3
AA Meharg K Killham (1990) ArticleTitleCarbon distribution within the plant and rhizosphere in laboratory and field-grown Lolium perenne at different stages of development Soil Biol Biochem 22 471–7 Occurrence Handle10.1016/0038-0717(90)90180-8 Occurrence Handle1:CAS:528:DyaK3MXjsFGg
WC Oechel GL Vourlitis SJ Hastings RC Zulueta L Hinzman D Kane (2000) ArticleTitleAcclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming Nature 406 978–81 Occurrence Handle10.1038/35023137 Occurrence Handle1:CAS:528:DC%2BD3cXmtlKqsbg%3D Occurrence Handle10984048
J Peñuelas C Gordon L Llorens T Nielsen A Tietema C Beier P Bruna B Emmett M Estiarte A Gorissen (2004) ArticleTitleNon-intrusive field experiments show different plant responses to warming and drought among sites, seasons and species in a north–south European gradient Ecosystems 7 .
I Røsberg DO Øvstedal R Seljelid Ø Schreiner J Goksøyr (1981) ArticleTitleEstimation of carbon flow in a Calluna heath system Oikos 37 295–305
ED Schulze NC Turner T Gollan KA Shackel (1987) Stomatal responses to air humidity and to soil drought E Zeiger GD Farquhar IR Cowan (Eds) Stomatal function Stanford University Press Stanford, CA 311–21
JA Simmons IJ Fernadezm RD Briggs MT Delaney (1996) ArticleTitleForest floor carbon pools and fluxes along a regional climate gradient in Maine, USA For Ecol Manage 84 81–95 Occurrence Handle10.1016/0378-1127(96)03739-5
JH Van Ginkel A Gorissen JA Van Veen (1997) ArticleTitleCarbon and nitrogen allocation in Lolium perenne in response to elevated atmospheric CO2 with emphasis on soil carbon dynamics Plant Soil 188 299–308 Occurrence Handle10.1023/A:1004233920896 Occurrence Handle1:CAS:528:DyaK2sXktVSkurw%3D
JH Van Ginkel A Gorissen D Polci (2000) ArticleTitleElevated atmospheric carbon dioxide concentration: effects of increased carbon input in a Lolium perenne soil on microorganisms and decomposition Soil Biol Biochem 32 449–56 Occurrence Handle10.1016/S0038-0717(99)00097-8 Occurrence Handle1:CAS:528:DC%2BD3cXitlGjtL8%3D
AJ Van der Krift PJ Kuikman F Möller F Berendse (2001) ArticleTitlePlant species and nutritional-mediated control over rhizodeposition and root decomposition Plant Soil 228 191–200 Occurrence Handle10.1023/A:1004834128220
JA Van Veen R Merckx SC Van de Geijn (1989) Plant- and soil-related controls of the flow of carbon from roots through the soil microbial biomass M Clarholm L Bergström (Eds) Ecology of Arable Land Kluwer Academic Publishers Dordrecht 43–52
ED Vance PC Brookes DS Jenkinson (1987) ArticleTitleAn extraction method for measuring soil microbial biomass C Soil Biol Biochem 19 703–7 Occurrence Handle10.1016/0038-0717(87)90052-6 Occurrence Handle1:CAS:528:DyaL1cXjs1KqsA%3D%3D
B Wallén (1983) ArticleTitleTranslocation of 14C in adventitiously rooting Calluna vulgaris on peat Oikos 40 241–8
DA Wedin D Tilman (1996) ArticleTitleInfluence of nitrogen loading and species composition on the carbon balance in grasslands Science 274 1720–3 Occurrence Handle10.1126/science.274.5293.1720 Occurrence Handle1:CAS:528:DyaK28XntlyrtLc%3D Occurrence Handle8939865
JM Whipps JM Lynch (1985) ArticleTitleEnergy losses by the plant in rhizodeposition Annu Proc Phytochem Soc Europe 26 59–71
JM Whipps JM Lynch (1986) ArticleTitleThe influence of the rhizosphere on crop productivity Adv Microb Ecol 9 187–244
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorissen, A., Tietema, A., Joosten, N. et al. Climate Change Affects Carbon Allocation to the Soil in Shrublands. Ecosystems 7, 650–661 (2004). https://doi.org/10.1007/s10021-004-0218-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-004-0218-4