Abstract
Our energy sources such as fossil fuels and coal are limited and cause air pollution. Hydrogen has been promoted as an alternative source of energy, which is renewable, cost-effective, and nature-friendly. Hydrogen evolution reaction (HER) can be used for the mass production of hydrogen at a very low cost. An active and efficient electrocatalyst is required to perform this reaction. To date, platinum (Pt) shows the highest efficiency; however, its high cost and low abundance hinder its large-scale uses. Molybdenum carbide has a similar electronic structure as that of platinum (Pt); hence, it shows high electrocatalytic activity towards HER. In this study, Mo2C/MoC/C composite has been synthesized using magnesium as a reducing agent. Carbon provides a highly conducting environment to Mo2C and MoC nanoparticles, and hence, the electrochemical performance is enhanced. The prepared sample shows a small Tafel slope of 125.5 mV/dec and long-term stability up to 5000 cyclic voltammetry cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing population of the world demands more and more energy. About 80% of the energy demand we require is fulfilled by traditional fossil fuels. However, a variety of air pollutants which are detrimental to both the atmosphere and public health are emitted from the combustion of fossil fuels. This encourages scientists to search for new sources of energy which are efficient and environment-friendly. Hydrogen can replace our traditional fossil fuels as it has a high energy density and zero emissions. At a large scale, hydrogen can be produced through electrochemical water splitting or hydrogen evolution reaction (HER). A high-performance, stable, and cost-effective electrocatalyst is a must for this reaction. Platinum (Pt) exhibits the highest HER activity to date; however, its expensiveness and low abundance hinder its large-scale uses. A variety of electrocatalysts has been investigated, which shows significant electrochemical performance towards HER [1,2,3,4,5,6].
Many transition metal–based electrocatalysts have been investigated for HER, including nitrides, oxides, sulfides, and carbides, due to their outstanding electrochemical performance [7, 8]. As a promising material for HER, transition metal carbides (TMCs) have received a lot of interest. Among TMCs, molybdenum carbide is one of the most studied compounds because of its platinum-like electronic structure and analogous electrochemical activity towards HER. Between the two phases of molybdenum carbide (Mo2C and MoC), Mo2C shows higher electrochemical activity than MoC [9]. They show outstanding stability and electrochemical activity in both acidic and basic mediums [10]. The main drawback of Mo2C is its low surface area due to high-temperature synthesis. Therefore, people tried to conjugate Mo2C with carbon, nickel, graphene, etc., to enhance the surface area, and hence, the electrochemical performance of Mo2C improves [11,12,13]. Others tried to prepare Mo2C based composites/heterostructures which show high electrocatalytic activity and high stability. However, the mass production of Mo2C at a large scale is still a challenge.
Recently, Mo2C-based composites have been synthesized by many groups, which show excellent electrochemical performance. Mir and Pandey demonstrated that the incorporation of Mo2C nanoparticles with carbon enhances the electrochemical activity and stability [14]. Pant et al. prepared Ni/MoC-decorated carbon fibers which showed high electrochemical activity with a specific capacitance of 312 F/g at 1 A/g [15]. Jingyu Chen and co-workers reported molybdenum carbide@ nickel foam electrodes synthesized via hydrothermal and calcination process. It showed a Tafel slope of 76 mV/dec and an overpotential of 42 mV to achieve a current density of 10 mA/cm2 [16]. Further, Wang et al. synthesized Mo2C-MoC heterostructures synthesized via hydrothermal followed by the annealing method. The prepared heterostructure showed a Tafel slope of only 54.3 mV/dec [17]. Therefore, it can be concluded that the conjugation of molybdenum carbide with other compounds is an effective strategy to improve the electrochemical performance of the material. In short, the reported methods for the synthesis of Mo2C-based composites are typically complex and expensive, which limit large-scale production. Also, to the best of our knowledge, Mo2C/MoC/C composite has not been synthesized yet.
In this work, we have synthesized Mo2C/MoC/C composite through a high-temperature carburization-reduction route in a single step. Magnesium (Mg) was used as a reducing agent during the reaction. The XRD analysis revealed the formation of Mo2C, MoC, and C phases within the sample. The synthesized sample shows high electrocatalytic activity towards HER with a Tafel slope of 125.5 mV/dec. It shows long cycle stability of up to 5000 cyclic voltammetry (CV) cycles.
Experimental synthesis
Synthesis
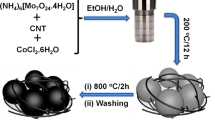
In the typical synthesis, 1.23 g of ammonium molybdate tetrahydrate ((NH4)6Mo7O24 · 4H2O), 1.40 g of hexamethylenetetramine C6H12N4, and 1 g of magnesium metal powder (Mg) have been used as molybdenum source, carbon source, and reducing agent, respectively. These materials were mixed in an agate mortar and transferred to a specially designed stainless steel autoclave. The autoclave was then sealed and placed in a pot furnace. The temperature was raised to 800 °C from room temperature at a heating rate of 5 °C/min for 12 h. The autoclave was allowed to cool to room temperature, and the black powder was collected. The obtained powder was then washed with dilute HCl and distilled water several times. The final product was obtained after drying it at 80 °C in a hot air oven.
Characterization
The X-ray diffraction measurements were done using PANalytical X-Pert-Pro diffractometer with CuKα radiation (λ = 1.5406 Å). The morphological and microstructural investigations were done through field emission scanning electron microscopy (FESEM; JEOL JSM 5600) and transmission electron microscopy (TEM; JEOL 2100F) at 15 kV and 200 kV.
The electrochemical measurements were obtained through a three-electrode cell assembly of Biologic EC lab instrument (SP-300). Reversible hydrogen electrode and platinum electrode were used as reference and counter electrodes. To prepare the working electrode, 1.5 mg of the prepared sample was dissolved in 250 µl of ethanol and sonicated for 30 min. Twenty microliters of the uniformly distributed particles of the sample was dropped on the top of the glassy carbon electrode having a surface area of 0.07 cm2. All three electrodes were dipped in a 0.5 M H2SO4 electrolyte solution. Before recoding any measurements, IR correction has been done using biologic EC lab software (ZIR program). The ZIR technique experimentally determines the solution resistance (Ru) of 38 Ω and compensates (80%) for it in real time. After initial stabilization of activities, linear sweep voltammetry (LSV) curves were measured at a scan rate of 5 mV/s. To check the stability of the sample, cyclic voltammetry (CV) measurements were done at a scan rate of 100 mV/s for 5000 cycles within a potential window of 0.1 to 0.4 V. LSV curves were recorded before and after 5000 CV cycles.
Result and discussion
XRD
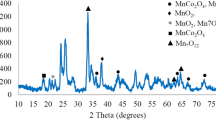
The XRD analysis has been done to investigate the structure and phase formation of the synthesized sample. As shown in Fig. 1, the XRD pattern of the sample confirms the formation of Mo2C (ICDD card no. 00–035–0787), MoC (ICDD card no. 03–065–6664, 01–089–4305), and C (ICDD card no. 01–089–8487) phases within the powder. The volume percent (vol%) of Mo2C, MoC, and C is about 73.34, 19.59, and 7.07, respectively. The composition of the composite has been estimated using Rietveld refinement of the XRD pattern, and the graphical representation is shown in Fig. S1 (Supplementary Information). It revealed the composition of the composite to be 76.3% of Mo2C, 15.1% of MoC, and 8.6% of C. It means the amount of Mo2C within the sample is higher than MoC and C. However, C has the least amount.
At high temperatures, AHM decomposes to MoO3, NH3, and H2O according to Eq. 2.
MoO3 reduces to Mo with the help of Mg, according to Eq. 2 [18]. Also, Mg reacts with oxygen present in the autoclave to form MgO and hence limits the transformation of Mo2C and MoC to their oxides.
Furthermore, Mo reacts with carbon (C) to form Mo2C and MoC. The synthesis mechanism and phase formation of molybdenum carbides using AHM and hexamethylenetetramine as precursors were explained by Upadhyay and Pandey [9]. The crystallite size of the sample has been calculated using the Debye–Scherrer equation [19]. The crystallite size of the composite has been calculated to be equal to 31.6 nm.
FESEM and TEM
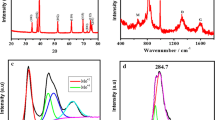
The FESEM images of the prepared Mo2C/MoC/C composites are shown in Fig. 2a, b at different magnifications. These micrographs indicate the formation of highly agglomerated carbide structures. Some of the smaller particles show spheroidal structures which are below 1 µm in size. The porous structure of the samples can be observed in Fig. 2b. Further, the TEM micrograph of the sample suggests that the Mo2C and MoC particles are covered by highly conductive carbon layers (Fig. 2c). These layers promote structural stability and facilitate the fast transfer of electrons and ions. The average particle size calculated from TEM is 33.4 nm. The selected area electron diffraction (SAED) pattern of the sample is shown in Fig. 2d. It shows many diffraction rings consisting of distinct spots that are indexed to ((200), (101)) Mo2C, and (311) MoC. These results are consistent with XRD analysis.
Electrochemical studies
HER activity
To investigate the HER activity of the as-synthesized sample, linear sweep voltammetry (LSV) measurements were done at a scan rate of 5 mV/s, as shown in Fig. 3a. At a potential of −400 mV, the sample provides a current density of about −20.16 mA cm−2. The prepared Mo2C/MoC/C composite exhibited an onset overpotential of ∼102 mV. Also, it showed an overpotential of −336 mV to reach a current density of 10 mA cm−2. For an efficient electrocatalyst, stability is a crucial factor that has been tested using CV measurements for 5000 cycles at a scan rate of 100 mV/s. The LSV curve after 5000 CV cycles is shown in Fig. 3a. Here, it can be observed that there is a negligible difference between LSV curves before and after 5000 cycles which indicates the long-term stability of the sample in an acidic medium. Such high stability can be attributed to the structure and crystal phase stability of β-Mo2C along with the protective action of carbon [14]. The Tafel plots are fitted with the Tafel equation (η = a + b log |j|) to further analyze the HER reaction mechanism, where j is the current density and b is the Tafel slope. As shown in Fig. 3b, the prepared Mo2C/MoC/C electrocatalyst shows a Tafel slope of 125.5 mV/dec, suggesting that the prepared electrocatalyst might be based on the Volmer − Heyrovsky mechanism [20]. In our previous work, we have synthesized Mo2C and MoC nanoparticles using the same synthesis procedure as adopted in the present work. Mo2C and MoC showed a Tafel slope of 129.7 and 266 mV dec−1, respectively, which are higher than Mo2C/MoC/C composite (125.5 mV dec−1) [9]. Therefore, these results indicate that the prepared Mo2C/MoC/C composite showed improved electrochemical activity. Table 1 shows the comparison of water splitting performances of Mo2C-based electrocatalysts.
Conclusion
In summary, we have synthesized Mo2C/MoC/C composite through a high-temperature carburization-reduction route. Magnesium was used as a reducing agent during the synthesis process. The XRD results show the presence of Mo2C, MoC, and C phases within the prepared powder. The Mo2C/MoC/C composite shows a Tafel slope of 125.5 mV/dec with long-term cyclic stability up to 5000 CV cycles. Such high electrochemical performance can be attributed to the synergistic effects between Mo2C, MoC, and C and the high conductivity environment provided by carbon. The presence of carbon within the composite is responsible for such high cyclic stability. The above process for synthesizing a noble metal-free catalyst can be an efficient and environmentally safe way to synthesize Mo2C/MoC/C composite.
References
Huang Y, Miao YE, Fu J et al (2015) Perpendicularly oriented few-layer MoSe2 on SnO2 nanotubes for efficient hydrogen evolution reaction. J Mater Chem A 3:16263–16271. https://doi.org/10.1039/c5ta03704b
Li W, Liu D, Yang N et al (2019) Molybdenum diselenide – black phosphorus heterostructures for electrocatalytic hydrogen evolution. Appl Surf Sci 467–468:328–334. https://doi.org/10.1016/j.apsusc.2018.10.127
Yang L, Yu J, Wei Z et al (2017) Co-N-doped MoO2 nanowires as efficient electrocatalysts for the oxygen reduction reaction and hydrogen evolution reaction. Nano Energy 41:772–779. https://doi.org/10.1016/j.nanoen.2017.03.032
Hua W, Sun H-H, Xu F, Wang J-G (2020) A review and perspective on molybdenum-based electrocatalysts for hydrogen evolution reaction. Rare Met 394(39):335–351. https://doi.org/10.1007/S12598-020-01384-7
Xia L, Zhang X, Song H et al (2020) Structural engineering of hierarchically hetestructured Mo2C/Co conformally embedded in carbon for efficient water splitting. Int J Hydrogen Energy 45:22629–22637. https://doi.org/10.1016/J.IJHYDENE.2020.06.049
Wang Y-Z, Ding Y-M, Zhang C-H et al (2021) Formation of hierarchical Co-decorated Mo2C hollow spheres for enhanced hydrogen evolution. Rare Met 4010(40):2785–2792. https://doi.org/10.1007/S12598-021-01765-6
Pi C, Zhao Z, Zhang X et al (2021) In situ construction of γ-MoC/VN heterostructured electrocatalysts with strong electron coupling for highly efficient hydrogen evolution reaction. Chem Eng J 416:129130. https://doi.org/10.1016/J.CEJ.2021.129130
Zhou F, Zhou Y, Liu G-G et al (2021) Recent advances in nanostructured electrocatalysts for hydrogen evolution reaction. Rare Met 4012(40):3375–3405. https://doi.org/10.1007/S12598-021-01735-Y
Upadhyay S, Pandey OP (2020) One-pot synthesis of pure phase molybdenum carbide (Mo2C and MoC) nanoparticles for hydrogen evolution reaction. Int J Hydrogen Energy 45:27114–27128. https://doi.org/10.1016/j.ijhydene.2020.07.069
Xiao P, Yan Y, Ge X et al (2014) Investigation of molybdenum carbide nano-rod as an efficient and durable electrocatalyst for hydrogen evolution in acidic and alkaline media. Appl Catal B Environ 154–155:232–237. https://doi.org/10.1016/j.apcatb.2014.02.020
Ren JT, Song YJ, Yuan ZY (2018) Facile synthesis of molybdenum carbide nanoparticles in situ decorated on nitrogen-doped porous carbons for hydrogen evolution reaction. J Energy Chem. https://doi.org/10.1016/j.jechem.2018.07.006
Das D, Santra S, Nanda KK (2018) In situ fabrication of a nickel/molybdenum carbide-anchored N-doped graphene/CNT hybrid: an efficient (pre)catalyst for OER and HER. ACS Appl Mater Interfaces 10:35025–35038. https://doi.org/10.1021/acsami.8b09941
Chaitoglou S, Giannakopoulou T, Speliotis T, Vavouliotis A, Trapalis C, Dimoulas A (2018) Mo2C/graphene heterostructures: low temperature chemical vapor deposition on liquid bimetallic Sn-Cu and hydrogen evolution reaction electrocatalytic properties. Nanotechnology 30:125401. https://doi.org/10.1088/1361-6528/aaf9e8
Mir RA, Pandey OP (2020) An ecofriendly route to synthesize C-Mo2C and C/N-Mo2C utilizing waste polyethene for efficient hydrogen evolution reaction (HER) activity and high performance capacitors. Sustain Energy Fuels 4:655–669. https://doi.org/10.1039/c9se00516a
Pant B, Ojha GP, Acharya J, Park M (2021) Eggshell membrane templated synthesis of Ni/MoC decorated carbon fibers with good electrochemical behavior. Int J Hydrogen Energy 46:2774–2782. https://doi.org/10.1016/j.ijhydene.2020.10.139
He T, He Y, Li H et al (2021) Mo2C nanospheres anchored on nickel foam as self-supported electrode for high-performance hydrogen production. J Solid State Chem 294:121825. https://doi.org/10.1016/j.jssc.2020.121825
Wang Q, Mi F, Li J et al (2021) Tungsten doping generated Mo2C-MoC heterostructure to improve HER performance in alkaline solution. Electrochim Acta 370:137796. https://doi.org/10.1016/j.electacta.2021.137796
Aydinyan SV, Gumruyan Z, Manukyan KV, Kharatyan SL (2010) Self-sustaining reduction of MoO3 by the Mg-C mixture. Mater Sci Eng B Solid-State Mater Adv Technol 172:267–271. https://doi.org/10.1016/j.mseb.2010.05.028
Upadhyay S, Pandey OP (2020) Synthesis of layered 2H–MoSe2 nanosheets for the high-performance supercapacitor electrode material. J Alloys Compd 157522. https://doi.org/10.1016/j.jallcom.2020.157522
Mir RA, Pandey OP (2018) Influence of graphitic/amorphous coated carbon on HER activity of low temperature synthesized Β-Mo2C@C nanocomposites. Chem Eng J 348:1037–1048. https://doi.org/10.1016/j.cej.2018.05.041
Li J, Zhou C, Mu J et al (2018) In situ synthesis of molybdenum carbide/N-doped carbon hybrids as an efficient hydrogen-evolution electrocatalyst. RSC Adv 8:17202–17208. https://doi.org/10.1039/c8ra02020e
Kang Q, Li M, Wang Z et al (2020) Agaric-derived N-doped carbon nanorod arrays@nanosheet networks coupled with molybdenum carbide nanoparticles as highly efficient pH-universal hydrogen evolution electrocatalysts. Nanoscale 12:5159–5169. https://doi.org/10.1039/C9NR10236A
Chaitoglou S, Giannakopoulou T, Tsoutsou D et al (2019) Direct versus reverse vertical two-dimensional Mo2C/graphene heterostructures for enhanced hydrogen evolution reaction electrocatalysis. Nanotechnology 30:415404. https://doi.org/10.1088/1361-6528/AB3155
Chen X, Qi J, Wang P et al (2018) Polyvinyl alcohol protected Mo2C/Mo2N multicomponent electrocatalysts with controlled morphology for hydrogen evolution reaction in acid and alkaline medium. Electrochim Acta 273:239–247. https://doi.org/10.1016/j.electacta.2018.04.033
Lv C, Wang J, Huang Q et al (2016) Facile synthesis of hollow carbon microspheres embedded with molybdenum carbide nanoparticles as an efficient electrocatalyst for hydrogen generation. RSC Adv 6:75870–75874. https://doi.org/10.1039/c6ra16490k
Tang Y, Li W, Jiao L et al (2017) Mo2C-Ni-modified nitrogen-doped carbon nanofiber toward efficient hydrogen evolution reaction. New J Chem 41:12956–12961. https://doi.org/10.1039/c7nj02611k
Cao Q, Zhao L, Wang A et al (2019) Tailored synthesis of Zn-N co-doped porous MoC nanosheets towards efficient hydrogen evolution. Nanoscale 11:1700–1709. https://doi.org/10.1039/c8nr07463a
Sun Y, Wang B, Yang N et al (2019) Synthesis of RGO-supported molybdenum carbide (Mo2C-RGO) for hydrogen evolution reaction under the function of poly(ionic liquid). Ind Eng Chem Res 58:8996–9005. https://doi.org/10.1021/ACS.IECR.9B00209
Šljukić B, Vujković M, Amaral L et al (2015) Carbon-supported Mo2C electrocatalysts for hydrogen evolution reaction. J Mater Chem A 3:15505–15512. https://doi.org/10.1039/c5ta02346g
Acknowledgements
The authors are highly thankful to SAI lab, Thapar Institute of Engineering and Technology, Patiala, for XRD analysis. The authors are grateful to Dr. R. Venkatesh (Scientist-E) for FESEM measurements. The authors offer special gratitude to Mr. Manu Vashistha, AIRF-JNU, New Delhi, for TEM analysis.
Funding
This work was supported by UGC-DAE Consortium for Scientific Research (project no.- CSR-IC-239/2017–18/1320) Indore, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•Mo2C/MoC/C nanocomposite: an efficient electrocatalyst for HER
•Carbon provides a high conductivity environment and guards active sites of the electrocatalyst
•Cost-effective method to prepare Mo2C/MoC/C in one step
•Mo2C/MoC/C has good HER activity in an acidic medium
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Upadhyay, S., Pandey, O.P. Synthesis of Mo2C/MoC/C nanocomposite for hydrogen evolution reaction. J Solid State Electrochem 26, 559–564 (2022). https://doi.org/10.1007/s10008-021-05096-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-05096-5