Abstract
A novel conjugated polymer PProDOT(MeEO)2 based on dioxythiophene with symmetric double ethoxymethyl pendant groups was successfully synthesized through electrochemical polymerization. The polymer film exhibits reversible electrochromism switching between broadly absorbing bluish black neutral state (− 0.1 V) and transmissive sky blue oxidized state (0.7 V). The symmetric double short-chain ethoxymethyl substitution pattern was utilized to realize the broadly absorbing bluish black neutral state of the polymer film. The electrochemical, spectroelectrochemical, and electrochromic switching properties of the polymer film were studied in detail.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrochromic materials relate to a class of brilliant materials that respond to externally applied potentials by exhibiting color or optical changes reversibly and persistently [1,2,3]. Conjugated polymers are attaining popularity in the research field of electrochromism because of their high optical contrast, fast switching speed, and superior coloration efficiency, which make them promising candidates for various electrochromic applications such as smart windows, nonemissive displays, textiles, and camouflage devices [1,2,3]. The most advantageous property of conjugated polymers to serve as outstanding electrochromes is that their electrochromic properties can be fine-tuned through structural modification of conjugated backbones [4] or side chains [5].
Most of the electrochromic applications require materials to switch between an absorptive state and a transmissive state in the visible and/or near infrared region. Therefore, when utilized in the visible region, colored-to-transmissive electrochromic polymers are of great interests and long lived aspirations in electrochromic field [6]. Among the full color palette, extensive efforts have been devoted to achieve electrochromic polymers exhibiting broadly absorbing behavior in the visible region such as black-to-transmissive one, which would find real-world applications such as information displays and e-papers [7]. In this subject, Reynolds group reported the pioneering works. They prepared broadly absorbing black-to-transmissive electrochromic polymers through donor-acceptor structures [8, 9] in which propylenedioxythiophene (ProDOT) was utilized as the donor unit, and 2,1,3-benzothiadiazole (BTD) was employed as the acceptor unit. Meanwhile, bluish black dyes are one of the most often utilized dyes in textile industries [10, 11] which are the potential application field of electrochromic materials. However, to the best of our knowledge, bluish black-to-transmissive electrochromic polymer has not been reported up to date.
The development of novel electrochromic polymers by functionalizing precursor molecules is an attractive method to adjust the optical and electronic characteristics of these materials. The optoelectronic properties of electrochromic polymer can be fine-tuned by change the structure of the monomer and/or by introduction of various pendant groups such as alkyl or alkoxy substituents [12]. Polypropylenedioxythiophene (PProDOT) is a famous star platform in colored-to-transmissive electrochromic polymers because it can be symmetrically di-substituted on the central carbon atom of the propylene bridge for realizing improved electrochromic properties [13, 14]. The most successfully developed PProDOT derivative is PProDOT-Me2 which shows a rapid switching time less than 1 s and a promising optical contrast of 78% at 578 nm, higher than 62% of PProDOT or 45% of PEDOT [13]. Based on this discovery, as in the case of long-chain alkoxy substituted polypropylenedioxythiophene (PProDOT) such as PProDOT-(CH2OEtHx)2 and PProDOT-(CH2OC18)2, the polymer film often shows a magenta color because they often exhibits the maximum absorption at about 550–600 nm [15]. That means both a part of red light and a part of blue light will transmit and be combined together to give a magenta color. In our opinion, this could be due to the steric repulsion of long-chain alkoxy substituents, resulting in the decrease of the conjugation length of polymer chains and the shift of absorption to the shorter wavelength [16].
In this contribution, we report the electrochemical synthesis of a homopolymer based on ProDOT derivative with symmetric double short-chain ethoxymethyl pendant groups (PProDOT(MeEO)2). We hope that introducing short-chain alkoxy substituents to the polymer chain will reduce the steric repulsion of side chains and the blue shift of the absorption compared with long-chain alkoxy substituted polymer, therefore realizing a broadly absorbing electrochromic polymer. The results indicate that PProDOT(MeEO)2 exhibits broadly absorbing behavior at about 480–660 nm in the visible region; therefore, it shows a bluish black color in its neutral form. The polymer film has a reversible electrochromism switching between bluish black and transmissive sky blue under very low driving potentials (< 1 V). The electrochemical, spectroelectrochemical, and electrochromic properties have been investigated in detail as presented in the following.
Experimental
Materials
3,3-Bis(bromomethyl)-3,4-dihydro-2H-thieno[3,4-b] [1, 4]-dioxepine (ProDOT(CH2Br)2) was synthesized according to the procedure reported by Reeves et al. [15]. Sodium ethoxide (C2H5ONa) and lithium perchlorate (LiClO4, 99%, anhydrous) were purchased from Aladdin Industrial Inc. Dimethylformamide (DMF), acetonitrile (ACN, HPLC grade), and propylene carbonate (PC, AR) were bought from Sinopharm Chemical Reagent Co., Ltd. All reagents were used as received.
Instruments
NMR spectra were collected on a 400 MHz Bruker Avance 400 spectrometer in deuterochloroform (CDCl3). IR spectra were determined using NICOLET 6700 FTIR spectrometer (Thermo Scientific). SEM images were collected on a JSM-7500F field emission scanning electron microscope (JEOL). The sheet resistance of the polymer film was measured using RTS-9 model 4-point probes resistivity measurement system (4 Probes Tech., China, Guangzhou). Cyclic voltammetry, spectroelectrochemistry, and colorimetry studies were performed using a CHI600E electrochemical analyzer (CH Instruments Ins.) combined with T6 New Happy UV-Vis-NIR spectrometer (Beijing Persee General).
Synthesis of diethyl 3,3-bis(ethoxymethyl)-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine (ProDOT(MeEO) 2)
ProDOT(CH2Br)2 (10 mmol), C2H5ONa (30 mmol), and 100 mL DMF were combined in a 250-mL round bottom flask. The reaction mixture was heated at 110 °C for 24 h, and then cooled and deionized water was added. The collected water phase was extracted with diethyl ether for three times. The organic phase was collected and dried with MgSO4; filtered and diethyl ether was removed by rotary evaporation under vacuum. The residue was subjected to column chromatography (silica, petroleum ether/ethyl acetate = 10:1, v/v) to give the final product as a colorless oil. 1H NMR (400 MHz, CDCl3, δ): 6.47 (s, 2H), 4.04 (s, 4H), 3.52 (s, 4H), 3.50 (d, 4H), 1.19 (t, 6H). 13C NMR (100 MHz, CDCl3, δ): 149.7, 105.1, 73.8, 69.4, 67.0, 47.5, 15.0. The synthetic route of ProDOT(MeEO)2 is shown in Scheme 1.
Synthesis of PProDOT(MeEO) 2
The electrochemical polymerization was carried out in an electrochemical cell (an indium tin oxide (ITO)-coated glass slide as the working electrode, Pt wire as the counter electrode, Ag wire as the pseudo-reference electrode). The reaction solution consists of 0.01 M ProDOT(MeEO)2 and 0.1 M LiClO4/ACN electrolyte-solvent couple. Five millimolars of solution of ferrocene (Fc/Fc+) in the same electrolyte was employed to calibrate Ag wire pseudo-reference electrode (E1/2(Fc/Fc+) = 0.36 V versus Ag wire). The potential data in this study are reported versus Ag wire. The reaction solution was deaerated by dry N2 gas and maintained under a minor overpressure throughout the polymerization process to avoid the effect of O2. The polymer was directly deposited on ITO-coated glass potential dynamically through successive cyclic voltammetry scan between 0 and 1.7 V for total 5 cycles. After the polymerization, the polymer film was electrochemically reduced to its neutral form in monomer-free electrolytic solution and repeatedly washed with ACN to remove the electrolyte, monomers, and oligomers. The synthetic route is depicted in Scheme 2.
Electrochemical and spectroelectrochemical experiments
The electrochemical and spectroelectrochemical characterizations were performed in an electrochemical cell consisting of a polymer-coated ITO/glass (4 cm × 0.7 cm) as the working electrode, a Pt wire as the counter electrode and an Ag wire as the pseudo-reference electrode. All measurements were performed in monomer-free lithium perchlorate (LiClO4)—propylene carbonate (PC) electrolytic solution. Following the suggestion of Silva and Ribeiro et al. [17], CIE (Commission Internationale de l’Eclairage) 1976 color coordinates (a*, b*) and relative luminance were calculated from UV-Vis-NIR spectroscopy using a Spectra Lux Software [18] and a Microsoft Excel spreadsheet developed by Mortimer and Varley [19].
Results and discussion
Electrochemical polymerization
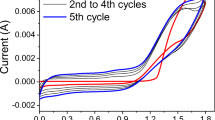
ProDOT(MeEO)2 (0.01 M) in 0.1 M LiClO4/ACN solution was subjected to successive cyclic voltammetry (CV) between 0 and 1.7 V at a potential scan rate of 100 mV/s for 5 cycles as shown in Fig. 1. As can be seen in Fig. 1, in the first CV cycle, the current exhibits a sharp increase when the externally applied potential is beyond 1.4 V, indicating the onset of oxidation of the monomer occurs. Upon repetitive CV scan, a new reversible redox wave between 0 and 0.7 V can be found which is corresponding to the oxidation and reduction of the polymer film. Concomitantly as the cycle number of CV scan increases, the current of each CV cycle increases, indicating that a layer of redox-active and conductive polymer was deposited on the working electrode.
Polymer characterization
The FTIR spectra of the monomer and the polymer are shown in Fig. 2a, b respectively in order to elucidate the structure of the polymer and interpret the polymerization mechanism. As can be seen in Fig. 2a, peaks located at 2925, 2855, and 1453 cm−1 correspond to the CH2 vibrations; 2974 cm−1 corresponds to the CH3 vibration; 3115 cm−1 corresponds to the vibration of CH group in the thiophene ring; 1476, 1445, and 1370 cm−1 are attributed to the vibration of C=C and C-C in the thiophene ring; and 763 cm−1 corresponds to the stretching of C-S respectively [20,21,22]. Upon polymerization, the absorption bands of the polymer were obviously broadened in comparison with the monomer as can be seen in Fig. 2b. This is mainly due to the wide chain dispersity of the polymer [22]. The band at 3115 cm−1 disappeared in the spectra of the polymer indicating that the electropolymerization occurred at 2,5-positions of the thiophene ring [20,21,22].
Surface morphology and cross-section view of the polymer film deposited on the ITO-coated glass slide were investigated by SEM. Morphologies of formed conducting polymer films which are represented in SEM images are closely related to their electrical and optical properties [23]. As can be seen in Fig. 3 in this study, the ProDOT(MeEO)2 film exhibits an uneven surface with many grains and some larger particles and the thickness of the film is found to be about 4 μm.
The sheet resistance of the polymer film was measured using four-point probes resistivity measurement system. PProDOT(MeEO)2 film has a sheet resistance R = 22.6 Ω/sq. The thickness of the film xj = 4 μm which was obtained through SEM image of the film from the cross-section view. Therefore, the resistivity ρ = R·xj = 9 × 10−5 Ω m, and the conductivity of the film is κ = 1/ρ = 110 S/cm. This value is relatively lower than that of poly(ethylenedioxythiophene) (PEDOT) which has a high conductivity of ca. 300 S/cm in the oxidized state [24]. The high conductivity of PProDOT(MeEO)2 film makes it a potential candidate for optoelectronic applications.
Electrochemistry
According to the experimental results of CV scan during the electropolymerization in Fig. 1, cyclic voltammetry studies of the as-prepared PProDOT(MeEO)2 film were performed in the potential range from − 0.1 V to 0.7 V with a Pt wire counter and Ag wire pseudo-reference electrode in monomer-free 0.1 M LiClO4/PC solution to investigate the electrochemical response of the polymer film as can be seen in Fig. 4. The results indicate clearly that PProDOT(MeEO)2 shows an oxidation peak at 0.6 V and a reduction peak at 0.05 V when the potential scan rate is 50 mV/s in CV experiments.
Spectroelectrochemistry
The combination of electrochemistry and spectroscopy into spectroelectrochemistry (SEC) provides a detailed interpretation of chemically driven electron transfer processes and redox events for different kinds of molecules and nanoparticles [25]. Therefore, spectroelectrochemistry technique could be very informative in electrochromic research because the optical and electronic properties of the polymer upon redox reaction can be explored simultaneously.
Firstly, PProDOT(MeEO)2 film was reduced to the neutral state under a negative applied potential − 0.1 V before the spectroelectrochemical experiments to remove the doping ions and the residual charges away. Subsequently, the Vis-NIR absorption spectra of the polymer film were recorded under applied potentials increasing incrementally from − 0.1 V to 0.7 V as shown in Fig. 5. The digital photos of the neutral state and the oxidized state of the polymer film are also included in Fig. 5 to indicate the hue of bluish black perceived and the degree of transmissivity obtained upon the complete oxidation of the polymer film. Meanwhile, Figs. 6 and 7 depict the changes of CIE1976 color coordinates (a*, b*) and the relative luminance of the PProDOT(MeEO)2 film during the oxidation process.
It can be seen from Fig. 5 that PProDOT(MeEO)2 film displays a broad absorption band at 480–660 nm with the absorbance beyond 1.0 corresponding to π-π* transition, and the maximum absorption wavelength is located at about 575 nm. A bluish black color of the polymer neutral state is realized with color coordinates a* = 18.58, b* = − 36.03 as can be seen in the digital photo in Fig. 5 and the color coordinates in Fig. 6. In our point, introducing double short-chain alkoxy substituents to the polymer chain will reduce the steric repulsion of side chains and the blue shift of the absorption compared with long-chain alkoxy substituted polymer, therefore realizing a broadly absorbing neutral state of the electrochromic polymer PProDOT(MeEO)2.
While the applied potential is increased from − 0.1 V to 0.7 V, the polymer film is successively oxidized. The absorption bands at 480–660 nm decrease and the polymer absorbs at longer wavelength in the near-IR region (about 950 nm and > 1000 nm) corresponding to the polaronic transition and the bipolaron absorption respectively.
Upon fully oxidation (a* = 1.58, b* = − 20.42), the absorption at 480–660 nm decreases notably and the polymer film displays a transmissive sky blue oxidized state as can be seen in the digital photo in Fig. 5 and the color coordinates in Fig. 6.
The bluish black-to-transmissive transition during the oxidation of the polymer film can also be proved by the increase of the relative luminance from 7.39% in the neutral state (− 0.1 V) to 43.51% in the oxidized state (0.7 V) of the polymer film as shown in Fig. 7.
Electrochromic switching
Square wave potential step chronoabsorptometry and chronocoulometry method were employed to perform the electrochromic switching experiments of PProDOT(MeEO)2 film (2 cm × 0.7 cm) at 575 nm. The results are shown in Fig. 8a, b. The potential square waves were set as − 0.1 and 0.7 V with various potential residence time.
It can be seen from Fig. 8 that the transmittance of the neutral state and the fully oxidized state are 8.9 and 44.1% at 575 nm respectively. The polymer film reveals a reasonable optical contrast ΔTmax = 35.2% at 575 nm. At 95% full contrast during bleaching process, PProDOT(MeEO)2 shows an appropriate coloration efficiency of 106.59 cm2/C (CE = ΔOD/Qd, ΔOD is the change of optical density at the monitoring wavelength, Qd is the inserted charge per area of the electrode).
However, the response time of the polymer during the bleaching process is relatively longer (t95 = 9.5 s) compared to PProDOT-Me2. The relatively lower switching speed of PProDOT(MeEO)2 may be due to the interaction between ethoxy groups and counterions which make the doping and dedoping of counterions be slower.
In a long-term electrochromic switching experiment, the PProDOT(MeEO)2 film was subjected to repeated CV scans between − 0.1 V and 0.7 V for total 200 cycles as shown in Fig. 9. It can be seen in Fig. 9 that after 200 cycles, the anodic and the cathodic current density do not show any loss, suggesting that PProDOT(MeEO)2 has a reasonable switching stability and can be employed in electrochromic applications.
Conclusions
In summary, in this contribution, a novel regiosymmetric electrochromic polydioxythiophene PProDOT(MeEO)2 with double ethoxymethyl pendant groups was electrochemically synthesized and characterized. The polymer reveals a broadly absorbing bluish black neutral state and a transmissive sky blue oxidized state. It exhibits a reasonable optical contrast of 35.2% at 575 nm, an appropriate coloration efficiency of 106.59 cm2/C, and a relative longer switching time t95 = 9.5 s during the bleaching process.
References
Beaujuge PM, Reynolds JR (2010) Chem Rev 110(1):268–320
Gunbas G, Toppare L (2012) Chem Commun 48(8):1083–1101
Neo WT, Ye Q, Chua S, Xu J (2016) J Mater Chem C 4(31):7364–7376
Lv X, Li W, Ouyang M, Zhang Y, Wright DS, Zhang C (2017) J Mater Chem C 5(1):12–28
Beverina L, Pagani GA, Sassi M (2014) Chem Commun 50(41):5413–5430
Amb CM, Dyer AL, Reynolds JR (2011) Chem Mater 23(3):397–415
Dyer AL, Thompson EJ, Reynolds JR (2011) ACS Appl Mater Interfaces 3(6):1787–1795
Beaujuge PM, Ellinger S, Reynolds JR (2008) Nat Mater 7(10):795–799
Shi P, Amb CM, Knott EP, Thompson EJ, Liu DY, Mei J, Dyer AL, Reynolds JR (2010) Adv Mater 22(44):4949–4953
Krishnakumar B, Balakrishna A, Nawabjan SA, Pandiyan V, Aguiar A, Sobral AJFN (2017) J Phys Chem Solids 111:364–371
Ferkous H, Merouani S, Hamdaoui O, Petrier C (2017) Ultrason Sonochem 34:580–587
Tarkuc S, Sahmetlioglu E, Tanyeli C, Akhmedov IM, Toppare L (2006) Electrochim Acta 51(25):5412–5419
Welsh DM, Kumar A, Meijer EW, Reynolds JR (1999) Adv Mater 11(16):1379–1382
Gaupp CL, Welsh DM, Reynolds JR (2002) Macromol Rapid Commun 23(15):885–889
Reeves BD, Grenier CRG, Argun AA, Cirpan A, McCarley TD, Reynolds JR (2004) Macromolecules 37(20):7559–7569
Dyer AL, Craig MR, Babiarz JE, Kiyak K, Reynolds JR (2010) Macromolecules 43(10):4460–4467
Silva AJC, Nogueira VC, Santos TEA, Buck CJT, Worrall DR, Tonholo J, Mortimer RJ, Ribeiro AS (2015) Sol Energ Mater Sol Cells 134:122–132
Santa-Cruz PA, Teles FS (2003) Spectra Lux Software v2.0, Ponto Quântico Nanodispositivos, Recife-PE, Brazil, RENAMI
Mortimer RJ, Varley TS (2011) Displays 32(1):35–44
Soganci T, Gumusay O, Soyleyici HC, Ak M (2018) Polymer 134:187–195
Carbas BB, Kivrak A, Onal AM (2011) Electrochim Acta 58:223–230
Zhang S, Xu J, Lu B, Qin L, Zhang L, Zhen S, Mo D (2014) J Polym Sci Polym Chem 52(14):1989–1999
Hu B, Zhang X, Liu J, Chen X, Zhao J, Jin L (2017) Synth Met 228:70–78
Groenendaal LB, Jonas F, Freitag D, Pielartzik H, Reynolds JR (2000) Adv Mater 12(7):481–494
Zhai Y, Zhu Z, Zhou S, Zhu C, Dong S (2018) Recent advances in spectroelectrochemistry. Nanoscale 10(7):3089–3111
Funding
This work received financial support from the Support Plan Project of Excellent Young Talents in Universities in Anhui Province (gxyq2017023); Anhui Natural Science Foundation (1808085 MB29); Anhui Province College Natural Science Foundation (KJ2017A482); the Open Foundation of Anhui Province Key Laboratory of Advanced Building Materials of Anhui Jianzhu University (JZCL201602ZZ); College Students Innovation and Entrepreneurship Training Plan Project (201810878000); and College Quality Engineering Project of Anhui Province: Polymer Chemistry—Resources Sharing Course (2016gxk028).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Yang, M., Xu, W. et al. Broadly absorbing bluish black-to-transmissive sky blue electrochromic polymer based on 3,4-dioxythiophene. J Solid State Electrochem 23, 19–25 (2019). https://doi.org/10.1007/s10008-018-4106-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-4106-9