Abstract
Thin films of pure, Mn-substituted ZnO were synthesized by dip coating technique and irradiated thin films by Li3+ swift heavy ions (SHIs) with fluence 5 × 1012 ions/cm2. The XRD peaks revealed that all the samples exhibit hexagonal (wurtzite phase, P63 mc group) structures. The extra peaks were observed in XRD patterns, but after SHI irradiation, peaks were disappeared due to inelastic scattering. Pure ZnO manifests large spherical grain-shaped morphology after Li3+ SHI irradiation with fluence 5 × 1012 ions/cm2 as compared to pristine sample. The Mn-substituted ZnO thin films showed the rod-like shape and nanowire-like configuration for 5% after SHI irradiation. Raman spectra of the SHI-irradiated samples show oxygen vacancies, and also, phonon mode shifted to the blue shift as compared to pristine sample. Electrical properties were investigated by I–V measurements, which show that enhancement in conductivity as Mn content as well as SHI irradiation increased. Optical properties have been elaborated by UV-Vis, which confirm red shifting of the absorption edge after SHI irradiation.

Mn-doped ZnO thin films irradiated with 50 MeV Li3+ SHI of fluence 5 × 1012 ions/cm2. Electronic and nuclear loss is 0.010 keV/μm, and range is 206.1 μm. Electronic and nuclear losses are shown in the figure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A study of ZnO nanomaterials has attracted a lot of attention of scientists, technocrats, and materials scientists over the last two decades. Zinc oxide can act as basic building block materials for many advanced device technologies [1, 2]. It is a cheap semiconductor having a direct band gap 3.34 eV which has hexagonal (wurtzite) structure, and it has the large exciton binding energy of 60 meV [3,4,5]. ZnO thin films are stable under ambient conditions towards lights, water, and air [6]. Due to this large exciton binding energy, huge experimental work on Mn-doped ZnO materials has been initiated by researchers and scientists [7, 8].
Thin films of these materials were grown using different methods, and various properties of these materials were studied [9, 10]. ZnO has a significant insight into spin filters and transport media. It has a wide range of applications in solar cells, [11], piezoelectric transducers [12], transparent conductive films [13], gas sensors [14], light-emitting devices [15], and biological (antibacterial and drug delivery) and chemical sensors [16]. ZnO with doping transition metal ions outlines the oxygen deficiencies and area in heterogeneous photocatalytic activities. Sharma et al. [17] reported the presence of ferromagnetism at room temperature that creates interest for further spintronic study. Some researchers have prepared samples at relatively low temperatures and observed weak ferromagnetism at T C = 420 °C with an average magnetic moment of 0.16 B per ions.
SHI is a unique technique which was used to modify structural, optical, magnetic, electronic, optoelectronic, and transport properties of the materials. SHI-irradiated samples have many potential applications such as in space devices, astrophysics, and mass spectrometry of large molecules [18]. SHI ion beam interacts with materials that may result in the creation of defect or amorpholization and phase formation on nanometer scale [19,20,21,22]. The effect of SHI on the materials depends on the ion energy, current, fluencies, and ion species [23,24,25]. Dolia et al. [26] reported that due to SHI irradiation, surface area-to-volume ratio of Mn-doped ZnO nanoparticles was modified by the formation of columnar tracks. The electrical resistivity results for irradiated Mn-doped ZnO samples that show the shifting of metal–semiconductor transition temperature towards room temperature by enhancing irradiation fluences. The SHI ion beam interacts with thin films by two techniques namely (i) nuclear energy loss S n(de/dx)n, i.e., transfer of energy to target atoms through an elastic collision, and (ii) electronic energy loss S e(de/dx)e, i.e., inelastic collision is due to electronic ionization and excitation of target atoms [27,28,29]. The large value of the energy losses is due to the Coulombic interactions between the target atoms [30,31,32]. It was explicitly understood that defects could be created by electronic energy loss and nuclear energy loss, which play a significant role. The degree of damaged structures was found to be dependent on the mass of the incident ions and the thickness of the samples. SHI irradiation transfers its energy to the electrons of target materials as kinetic energy via inelastic collision. Electron–phonon interaction acquires kinetic energy which results in the escalation of lattice temperature above the melting point of the material which was monitored by rapid quenching. This states the formation of amorphous track along the path of the projectile ion when the melt solidifies [33,34,35,36,37,38,39]. In this article, we have studied the energy loss at 50 MeV Li3+ SHI irradiation on pure and Mn-substituted ZnO thin films thoroughly. It was observed by SRIM software calculation that the nuclear energy loss is insignificant as compared to the electronic energy loss. High electronic energy loss is due to the inelastic collision, and high energetic ions interact with a direct electron in the samples. We have used Li3+ ion due to its lighter element and transition metal ions like Li3+; it has incomplete electronic d-shell, which affects the ion–solid interaction to a greater extent than non-transition metal ions. They have promising effects of doping when these are added in the host solid as compared to Au and Ag ions. Structural, morphological, and electrical property modifications of Mn-substituted ZnO thin films were investigated by SHI irradiation systematically in this paper.
Experimental procedure
Synthesis of the materials
The pure ZnO thin film was synthesized by using 0.1 M zinc chloride solution dissolved in 40 ml distilled water with constant stirring for 1 h at room temperature. Sodium hydroxide (NaOH) 0.1 M solution was dissolved in 40 ml distilled water with constant stirring for 1 h at temperature 70 °C in a separate beaker. Glass substrate was immersed in hydrochloric acid for 24 h. It was ultrasonically cleaned with acetone, distilled water subsequently, and dried on the hot plate at temperature 75 °C for 1 h. Zincket bath was prepared by adding two to three drops of triethanolamine (TEA) and five to six drops of ammonia in zinc chloride solution for maintaining the pH 11. Sodium hydroxide solution was added in zincket bath and stirred at temperature 80 °C for 1 h using a magnetic stirrer. The cleaned substrate was dipped in the solution for 30 min. The film on the substrate was dried in air for 60 min at 250 °C. Similarly, for the synthesis of 3 and 5% Mn-doped ZnO thin films, similar procedure was used.
Irradiation experiment
The synthesized pure and Mn-substituted ZnO thin films were irradiated using 50 MeV Li3+ ion beam with fluence 5 × 1012 ions/cm2. The 15 UD Pelletron Accelerators used with allowed beam current of one particle nano ampere (PNA) are available at Materials Science, IUAC, New Delhi, India. The range of Li3+ ions for 50 MeV was estimated using SRIM program. It was corroborated that the electronic energy loss was found to be 1.466 × 102 keV/μm, nuclear energy loss was 8.190 × 102 keV/μm, and the projectile range was 206.1 μm for Mn-doped ZnO thin films (Fig. 1). The detailed information of Li3+ ion irradiation effect on pure and Mn-doped ZnO thin films is due to ion collision and ion recoil distribution; the energy loss of target phonon and energy loss of vacancy production are plotted and given in Supplementary material (SM).
Material characterizations
The XRD patterns were recorded using advanced D-8 X-ray diffractometer (Bruker AXS, Germany) with monochromatic CuKα radiation with λ = 1.5406 Å conventional X-ray source, Göbble mirror, LiF monochromator, and scintillator detector. The surface morphology was intervened by scanning electron microscope (SEM): MIRA II LMH from TESCAN, with a resolution of 1.5 nm at 30 kV. Raman spectra of pure and Mn-substituted ZnO samples were recorded by using Ar ion laser with 514.5 nm wavelength, and 50 mW power was employed as the exciting source. When high energetic ions interact with the electrons, then they create the tracks and move very rapidly in the samples. Collision plot and different energy losses were calculated at an energy of 50 MeV; Li+3 ions irradiated ZnO thin films by using the TRIM software. Ion collision was calculated by depth versus y-axis and plots. The data points were plotted for every ion per target atom collision. The moving ions are shown in red color, and when they are stopped, they are indicated by white color as shown in Fig. 2. Phonon recoil found that the energy loss to target phonon consists of the direct creation of phonon by the ion and the additional energy loss by target recoil atoms to phonons. The creation of target vacancies was resulted from both atoms that formed the collision cascade of recoiling target atoms.
Results and discussion
Crystallographic analysis
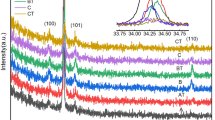
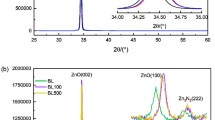
The X-ray diffraction patterns of the pristine and irradiated Mn-substituted ZnO thin films are shown in Fig. 3. The patterns consist of sharp peaks, which are matched with the common ZnO hexagonal phase, i.e., wurtzite structure [80-0074, JCPDS]. These peaks at angles (2θ) of 31.632°, 34.395°, 36.537°, 47.392°, and 62.925° corresponded to the diffractions from the (100), (002), (101), (102), and (110) planes. The (002) diffraction peak of the hexagonal (wurtzite) ZnO at angle 34.390° indicates that the nanorods for irradiated 3% Mn-substituted ZnO thin films have a significant preferential orientation in the c-axis direction [40]. For pristine ZnO and 3 and 5% Mn-substituted ZnO samples, weak peak (112) was observed. This impurity peak was disappeared just after irradiation with fluence 5 × 1012 ions/cm2 as shown in Fig. 3. This indicates that impurity phase was dissolved after SHI irradiation and sample reflects single phase as hexagonal structure due to the electron rearrangements in the sample. The lattice parameters “a” and “c” were calculated using the following relation [41].
where a and c are the lattice constants and d (hkl) is the crystallite distance for (hkl) indices. It was observed that a and c lattice parameters enhance with increasing Mn concentration. It is due to ionic radius of Mn2+ ion (0.66 Å) which is larger than Zn2+ ions (0.60 Å). The corresponding expansion of the volume of a unit cell of Mn-substituted ZnO thin films with increasing Mn doping is expected. Values of lattice parameters of Mn-substituted ZnO thin films are summarized in Table 1. The crystallite size of pure and Mn-substituted ZnO thin films was calculated using Debye–Scherrer’s formula [41].
where D is the crystallite size, λ is the wavelength of CuKα radiation (1.5406 Å), β is the full width at half maxima, and θ is the Bragg’s angle of diffraction. It is observed the increase in crystallite size with increasing Mn content in the samples. It was found that for pure ZnO, crystallite size increases after SHI irradiation with a fluence of 5 × 1012 ions/cm2. It is probably because after SHI irradiation, track formation occurred because cascade was formed in the material; this creates the defects. For 3 and 5% Mn concentrations, the increment in the crystallite size after SHI irradiation was found. It means that Mn2+ ions go to Zn2+ site to occupy the 2d crystallographic site. Thus, the XRD patterns revealed that the peak intensity is higher for irradiated samples than for pure sample. The values of crystallite size are listed in Table 1.
The peak intensity of all pristine and irradiated samples was different; it is due to the effect of the penetration depth of SHI irradiation as well as the thickness of the sample. The X-ray density was estimated using XRD data, and values are tabulated in Table 1. X-ray density decreases with increasing Mn content as well as fluence of Li3+ ions. Atomic packing fraction (APF) was estimated using XRD data. The APF was evaluated using the following formula [42]:
It was observed that the value of APF is almost constant for pure and Mn-substituted ZnO thin films. The standard value of APF for hexagonal is 74%, but in our case, it increases due to the creation of voids in the samples, and values of APF are outlined in Table 1. Neogi et al. [43] reported that peak intensity decreases with decreasing FWHM, which appears to be contradictory in nature concerning with the crystallinity of the samples even if irradiation impurity phases were observed. Nevertheless, in our case, it was evidently found that intensity of the peak increased as Mn content enhanced. The surface area-to-volume ratio was decreased with enhancing Mn content as well as SHI-irradiated samples due to the growth of crystallite size.
Morphological investigation
The morphological images of pure and Mn-substituted ZnO thin films with 5 × 1012 ions/cm2 fluences were obtained from SEM as shown in Fig. 4. Significant morphological changes have been observed after Li3+ ion irradiation on pure and Mn-substituted ZnO thin films. There are few studies available in the literature on Mn-substituted ZnO thin films using Li3+ SHI irradiation [43]. The pristine ZnO sample appears as discontinuous and small spherical grain over the glass substrate as shown in Fig. 4. SEM image for 5 × 1012 ion/cm2 irradiated ZnO sample clearly shows spherical shape. It is due to the high energetic ion passes through the materials, and electronic energy loss was found due to the change in morphology of the material.
Samples of 3 and 5% Mn-substituted ZnO show spherical shapes reported by Balamurali [44]. Nevertheless in our case, pristine ZnO sample shows agglomeration and irradiated pure ZnO; 3% Mn-substituted ZnO samples exhibit rod-like shapes. It confirms that change in morphology is due to SHI irradiation. A sample of 5% Mn-substituted ZnO under fluence 5 × 1012 ions/cm2 reflects nanowire-like configuration. It is due to increment in crystallite size and more nucleation of Mn into ZnO lattice structure which evinces that substitution of Mn into ZnO lattice structure.
EDAX analysis
Energy-dispersive X-ray analysis (EDX) was used to evaluate amount of elemental composition in the samples. It was explicitly found the presence of Zn, O, and Mn in the Mn-doped ZnO lattice structure. It has been observed that weight % and the atomic percentage of Mn content go on enhancing in the ZnO lattice which was expected. Au peaks are also observed due to the Au coating on the samples as shown in Fig. 5. The atomic concentration of Zn, O, and Au ions in pristine ZnO samples was determined, and the values are depicted in Table 2. Minor changes were found in pure ZnO composition of elements after SHI irradiation at fluence 5 × 1012 ions/cm2. The atomic percentage was detected in 3 and 5% Mn-substituted ZnO after SHI irradiation. It was found that the atomic percentage of Zn decreases while Mn and O atomic percentage enhance; it is due to the doping effect of Mn content. After SHI irradiation, atomic percentage of oxygen decreases; it clearly reflects that creation of oxygen vacancies owing to this frequency enhances and wavelength reduces which evinces the blue shift observed in the Raman spectra. In this article, probably we have first time compared the result of Raman spectra and our EDX results that are in good agreement with the results obtained by Raman spectra.
Electrical measurements
I–V (current–voltage) measurements of pure and Mn-substituted ZnO thin films were carried out using a two-probe method. I–V characteristics were recorded in the voltage range − 5 to 5 V. All curves of pure and Mn-substituted ZnO thin films were passed through the origin and found nearly linear, evinced ohmic nature of the films. The enhancement in conductivity of films was observed due to the effect of irradiation on the thin films. It was well understood that Mn-substituted ZnO materials irradiated by swift heavy ions; thin films after SHI irradiation lost its nuclear and electronic energies.
Figure 6 shows the I–V characteristics of pristine and SHI-irradiated ZnO and Mn-substituted ZnO materials. Senos et al. [45] reported that the conductivity decreases with the increasing in the Mn concentration and with increasing the sintering temperature, especially at 110 °C. However, in our case, we have found that the crystallite size of SHI-irradiated pure and Mn-doped ZnO samples were increased while the surface-to-volume ratio decreased. The electron carrier mobility was increased, while the resistivity of the as-synthesized material decreased which reveals the enhancement in conductivity of the material. Since the Mn acts as a deep donor in ZnO, which modifies the concentrations of intrinsic defects at the grain boundaries by increasing the donor concentration, it means that Mn significantly suppresses the concentration of charge carriers like intrinsic donors, interstitial zinc, or oxygen vacancy [46]. SHI irradiation results clearly show enhancement in conductivity as compared to pristine sample; it is due to high energetic ion passing through the material it interacts with the electrons due to the inelastic collision. It was clearly confirmed the SHI irradiation effect on pure and Mn-doped ZnO samples, which gives conductivity. Hence, this material can be used for fabrication of the devices such as radiation detectors or sensors.
Raman spectroscopy
Raman scattering technique is one of the most powerful techniques, which is used to estimate the disorder, crystalline properties, and presence of a defect in the materials. The Raman spectra of pure and Mn-substituted ZnO thin films ranging from 200 to 1000 cm−1 are shown in Fig. 7a–c. The ZnO crystals have hexagonal (wurtzite) structure with space symmetry \( {\mathrm{C}}_{6\mathrm{v}}^4 \) (P 63 mc) which was intervened using Halford method [47]. The optical phonon irreducible representation of the pure and Mn-doped ZnO thin films was investigated using the following equation [48]:
where A 1 and E 1 are the polar modes, and they can be splitted into transverse optical (TO) and longitudinal optical (LO) phonons, respectively; these are Raman active modes. The non-polar E 2 mode is Raman active, while the B 1 mode is Raman inactive. Two formula units per primitive cell give six Raman active phonon modes at 101 (E 2, low), 381 (A 1, TO), 407 (E 1, TO), 437 (E 2, high), 554 (A 1, LO), 583 cm−1 (E 1, LO), and 776 (E 2, high) of first order, respectively [49].
Figure 7 represents the comparison between the Raman spectra of pure and Mn-substituted ZnO thin films and SHI irradiation effect. High intense spectra line was used for the Gaussian fit; it intervened which gives three Raman bands. These bands indicate peak positions of the 437.63, 550.87, and 776.01 which are characteristics of the hexagonal (wurtzite) structure of ZnO, which are assigned for lattice vibration of oxygen atoms. When ZnO thin film was irradiated at fluence 5 × 1012 ions/cm2, it was observed that phonon modes are shifted from 437.63 to 439.36 cm−1 (E 1 high) which exhibit blue shift. This mode can be used to characterize the stress in ZnO lattice. Singh et al. [50] reported that 437.8 cm−1 peak shifted to 347.1 cm−1 is due to enhancement of Mn content. However, in our case, peak shifted from 437.63 to 439.36 cm−1 after SHI irradiation and the explicitly blue shift was observed in Raman modes. It outlines that Mn content is not responsible for the blue shift. Nevertheless, SHI irradiation is responsible for the band shifting. It was intervened that blue shifting of Raman modes slightly shifted to higher energy and band broadened as irradiated Li3+ ions. The band was shifted from 550 to 565 cm−1 of pristine, and SHI-irradiated thin film aroused due to the oxygen deficiency such as oxygen vacancies reported by Du [51].
For SHI-irradiated and pristine 3% Mn-substituted ZnO thin films, blue shifting was also observed, but one slight mode was appeared at 331.34 and 335.14 cm−1, respectively; it was aroused due to E 2 (high) and E 2 (low) multiple scattering phonons. The band 571.50 cm−1 can be assigned to E 1 transverse optical mode (TO) and A 1 (LO) mode due to the presence of zinc interstitial defects. It was evidently observed that band intensity enhances for 3% Mn-substituted ZnO thin film after irradiation which is much higher than pristine. It may be owing to electron cascade formation after SHI irradiation in the sample for 5% irradiated Mn-substituted ZnO thin films, Raman mode was observed at 330.75, 435.18, 525.57, and 568.86 cm−1. When Mn2+ was substituted into Zn2+ ions, new lattice defects induced or intrinsic host lattice defects were created. Mn-substituted ZnO pristine and irradiated thin films are revealing that the intensity from 568.86 cm−1 shifted to 568.89 cm−1 at A 1 (LO) mode; shifting of mode was found higher in 3% Mn-doped ZnO sample because of the presence of defect in ZnO lattice after SHI irradiation. It was found that Raman mode was shifted to a higher frequency for irradiated samples as compared to pristine, which gives blue shift as shown in Fig. 7.
Optical property investigation
Figure 8 shows optical absorbance spectra for pure and 3 and 5% Mn-doped ZnO thin films in the wavelength range of 420–550 nm at room temperature. The absorption in UV region was formed due to the transition of valence band electron into conduction band by absorbing the energy from optical radiation. The absorption spectra of the thin films were expected to depend on several factors, such as the size of the particle, band gap, defects, and impurity centers [52]. For pristine ZnO sample, the absorption peak was observed at 432.75 nm, and after SHI irradiation at fluence 5 × 1012 ions/cm2, the absorption peak was decreased by 0.53 nm. It may be due to the creation of Coulomb explosion in the materials. For 3% Mn-doped ZnO, pristine sample was observed at absorption peak at 450.48 nm, but after SHI irradiation, it was reduced by 12.04 nm. For 5% Mn-doped ZnO, exciton absorption peak was obtained at 450.12 nm for the pristine sample; after SHI irradiation, it was 445.58 nm that was reduced to 4.54 nm as compared to the pristine. The energy band gap of pristine and SHI-irradiated Mn-doped ZnO samples was estimated by the following equation [52]:
where α is the absorption coefficient, A is the absorbance, and d is the thickness. The energy band gap was calculated considering a direct allowed transition between valence and conduction bands using the Tauc’s equation.
where B is the constant and E g is the energy band gap of the material. The plots of (αhυ)2 versus hυ are shown in Fig. 8 for the pristine and SHI-irradiated Mn x Zn1 − x O thin films.
The energy band gap of the pristine ZnO sample was evaluated as 3.371 eV, but after SHI irradiation, it was found to be 3.301 eV that decreases the band gap after SHI irradiation due to the formation of vacancies of oxygen. For 3% Mn-doped ZnO pristine thin films, it was 2.50 eV, but after SHI irradiation, it was obtained as 2.47 eV. Similarly, for 5% Mn-doped ZnO sample, for pristine, it was 2.35 eV, and after SHI irradiation, it was 2.24 eV. The reduction in energy band gap of pristine 3 and 5% Mn-doped ZnO as compared to the pure ZnO thin films is higher. It is due to the substitution of Mn ion into ZnO lattice and hence causing the sp–d interaction between the d-electrons of Mn ions and band electrons of ZnO in its tetrahedral crystal field and the clustering. The effect of SHI irradiation clearly revealed the reduction in the band gap. It confirms red shifting of the absorption edge due to the increase in crystallite size and generation of defect level. This property may be applicable in various optoelectronic devices and achieving to improve photocatalytic efficiency. In designing modern optoelectronic device, it is also interesting to know the refractive index as a function of wavelength to predict the photoelectric behavior of a device. The refractive index (n) of pristine and irradiated Mn-doped ZnO thin film sample was calculated by using Herve–Vandamme [53].
Here, A = 13.6 eV and B = 3.4 eV are constants, and E g is the optical band gap energy, and the relation is well suited for most materials usually used in optoelectronic devices.
Refractive index of ZnO nanomaterial optical constant value is n = 2.0034, but in our case, pristine ZnO sample has refractive index value of 2.2418; after SHI irradiation, it was enhanced to 2.2625; it is due to the electron–ion interaction in the material. The refractive index for pristine and irradiated 3% Mn-doped ZnO sample was enhanced from 2.3089 to 2.5234, and refractive index was calculated for 5% Mn pristine and irradiated ZnO samples, and it was found that the value of refractive index increases from 2.5679 to 2.6105. It was explicitly fortified the enhancement in the refractive index after SHI irradiation, and the values of refractive index are listed in Table 3.
Conclusions
Pure and Mn-substituted ZnO thin films using glass substrate have been successfully synthesized by dip coating technique. It was confirmed that swift heavy ion beam irradiation modified the surface morphology and structural, electrical, and optical property of pure and Mn-substituted ZnO thin films. XRD data explicitly show that the particle size enhances with increasing Mn content and gives a stable structure and impurity peak was disappeared after SHI irradiation due to the electron rearrangements. Surface morphology of irradiated samples at fluence 5 × 1012 ions/cm2 reveals a rod-like nature with 3% Mn-substituted ZnO thin film and wire-like configuration for 5% Mn-substituted ZnO sample. EDX has revealed the atomic percentage of Zn, Mn, and O, which confirms that Mn substituted into ZnO lattice and after SHI irradiation creation of oxygen vacancies in the sample. I–V characteristics showed that the materials are more conducting after SHI irradiation due to the ion–electron interaction, which creates the electron cascade. Raman spectra revealed that peak shifted to higher frequency owing to this wavelength decreases after SHI irradiation, which gives a blue shift. The energy band gap of the pure and Mn-doped ZnO thin films was reduced after SHI irradiation, and Mn content increased owing to the Coulomb explosion, which was aroused after SHI irradiation in the materials. Hence, one may evidently say that the swift heavy ion is a useful technique to modify the properties of the materials.
References
Kim K, Park Y (2003) Optical absorption and electronic structure of Zn1-xMnxO alloys studied by spectroscopic ellipsometry. J Appl Phys 94(2):867–869. https://doi.org/10.1063/1.1582395
Mondal S, Bhattacharyya S, Mitra P (2013) Preparation of manganese doped ZnO thin films and their characterization. Bull Mater Sci 36(2):223–229. https://doi.org/10.1007/s12034-013-0462-3
Lu F, Wang J, Lang M, Toulemonde M, Namavar F, Trautmann C (2012) Amorphization of nanocrystalline monoclinic ZrO2 by swift heavy ion irradiation. Phys. Chem. Chem Phys 14:12295
Choudhury N, Singh F, Sarma B (2013) Effect of swift heavy ion irradiation on lead sulphide quantum dots embedded in polyvinyl alcohol. Radiat Eff Defects Solids 168(7-8):498–503. https://doi.org/10.1080/10420150.2012.761995
Kumar S, Katharria Y, Batra Y, Kanjilal D (2007) Influence of swift heavy ion irradiation on electrical characteristics of Au/n-Si (1 0 0) Schottky barrier structure. J Phys D Appl Phys 40(22):6892–6897. https://doi.org/10.1088/0022-3727/40/22/006
Gawai U, Khawal A, Shripathi T, Dole B (2016) A study on the synthesis, pair distribution function and diverse properties of cobalt doped ZnS nanowires. Cryst eng comm 18(8):1439–1445. https://doi.org/10.1039/C5CE02253C
Wojnarowicz J, Mukhovskyi R, Pietrzykowska E, Kusnieruk S, Mizeracki J, Lojkowski M (2016) Microwave solvothermal synthesis and characterization of manganese doped ZnO nanoparticles. Beilstein J Nanotechnol 7:721–732
Sharma R, Patel S, Pargaien K, Sharma R, Pargaien K (2012) Synthesis, characterization and properties of Mn doped ZnO nanocrystals. Adv Nat Sci Nanosci Nanotechnol 3:035005
García M, Ruiz-González M, Quesada A, Costa-Krämer J, Fernández J, Khatib S (2005) Interface double-exchange ferromagnetism in the MnZnO system: new class of biphase magnetism. Phys Rev Lett 94(21):217206. https://doi.org/10.1103/PhysRevLett.94.217206
Li X, Xu X, Quan Z, Guo J, Wu H, Gehring G (2009) Role of donor defects in enhancing ferromagnetism of Cu-doped ZnO films. J Appl Phys 105(10):103914. https://doi.org/10.1063/1.3130104
Aumayr F, Facsko S, El-Said A, Trautmann C, Schleberger M (2011) Single ion induced surface nanostructures: a comparison between slow highly charged and swift heavy ions. J Phys Condens Matter 23(39):393001. https://doi.org/10.1088/0953-8984/23/39/393001
Matsunami N, Itoh M, Takai Y, Tazawa M, Sataka M (2003) Ion beam modification of ZnO thin films on MgO. Nucl Instrum Methods Phys Res, Sect B 206:282–286. https://doi.org/10.1016/S0168-583X(03)00745-6
Dhawan S, Kumar D, Ram M, Chandra S, Trivedi D (1997) Application of conducting polyaniline as sensor material for ammonia. Sensors Actuators B Chem 40(2-3):99–103. https://doi.org/10.1016/S0925-4005(97)80247-X
Bodke M, Khawal H, Gawai U, Dole B (2015) Synthesis and characterization of chromium doped zinc sulfide nanoparticles. Oalib 02(05):1–8. https://doi.org/10.4236/oalib.1101549
Sunitha D, Nagabhushana H, Sharma S, Singh F, Nagabhushana B, Dhananjaya N (2013) Structural, iono and thermo luminescence properties of heavy ion (100 MeV Si7+) bombarded Zn2SiO4:Sm3+ nanophosphor. J Lumin 143:409–417. https://doi.org/10.1016/j.jlumin.2013.04.025
Mote V, Huse V, Dole B (2012) Synthesis and characterization of Cr doped ZnO nanocrystals. World J Condens Matter Phys 02:208–211
Singh P, Kaushal A, Kaur D (2009) Mn doped ZnO nanocrystalline thin films prepared by ultrasonic spray pyrolysis. J Alloys Compd 471(1-2):11–15. https://doi.org/10.1016/j.jallcom.2008.03.123
Sharma P, Gupta A, Rao K, Owens F, Sharma R, Ahuja R (2003) Ferromagnetism above room temperature in bulk and transparent thin films of Mn-doped ZnO. Nat Mater 2(10):673–677. https://doi.org/10.1038/nmat984
Kumar V, Kumar R, Lochab S, Singh N (2007) Effect of swift heavy ion irradiation on nanocrystalline CaS:Bi phosphors: structural, optical and luminescence studies. Nucl Instrum Methods Phys Res, Sect B 262(2):194–200. https://doi.org/10.1016/j.nimb.2007.06.006
Polat I, Aksu S, Altunbaş M, Yılmaz S, Bacaksz E (2011) The influence of diffusion temperature on the structural, optical and magnetic properties of manganes doped zinc oxysulfide thin films. J Solid State Chem 184(10):2683–2689. https://doi.org/10.1016/j.jssc.2011.07.017
Kumar P, Kartha C, Vijayakumar K, Singh F, Avasthi D (2008) An investigation on the variations in properties of Ni+ irradiated ZnO thin films. Radiat Eff Defects Solids 163(7):635–644. https://doi.org/10.1080/10420150701589776
Mallick P, Rath C, Dash J, Biswal R, Agarwal D, Behera D (2010) Observation of grain growth in swift heavy ion irradiated NiO thin films, Indian. J Phys 84:1399–1404
Amekura H, Ishikawa N, Okubo N, Ridgway M, Giulian R, Mitsuishi K (2011) Zn nanoparticles irradiated with swift heavy ions at low fluences: optically-detected shape elongation induced by nonoverlapping ion tracks. Phys Rev B 83(20):205401. https://doi.org/10.1103/PhysRevB.83.205401
Xiang X, Zu X, Bao J, Zhu S, Wang L (2005) Optical properties and structure characterization of sapphire after Ni ion implantation and annealing. J Appl Phys 98(7):073524. https://doi.org/10.1063/1.2084314
Nakajima A, Nakao H, Ueno H, Futatsugi T, Yokoyama N (1998) Coulomb blockade in Sb nanocrystals formed in thin, thermally grown SiO2 layers by low-energy ion implantation. Appl Phys Lett 73(8):1071–1073. https://doi.org/10.1063/1.122087
Dolia S, Sharma P, Dhawan M, Kumar S, Prasad A, Samariya A (2012) Swift heavy ion irradiation induced modifications in magnetic and dielectric properties of Mn–Ca ferrite. Appl Surf Sci 258(9):4207–4211. https://doi.org/10.1016/j.apsusc.2011.06.011
Leino A, Djurabekova F, Nordlund K (2014) Radiation effects in nanoclusters embedded in solids. Eur Phys J B 87:50359
Avasthi D, Singh J, Biswas A, Bose S (1998) Study on evolution of gases from Mylar under ion irradiation. Nucl Instrum Methods Phys Res, Sect B 146(1-4):504–508. https://doi.org/10.1016/S0168-583X(98)00464-9
Alaria P, Turek M, Bernard M, Bouloudenine A, Berbadj N (2005) No ferromagnetism in Mn doped ZnO semiconductors. Chem Phys Lett 415(4-6):337–341. https://doi.org/10.1016/j.cplett.2005.09.003
Kumar S, Kumar R, Singh D (2009) Swift heavy ion induced modifications in cobalt doped ZnO thin films: structural and optical studies. Appl Surf Sci 255(18):8014–8018. https://doi.org/10.1016/j.apsusc.2009.05.005
Agarwal D, Avasthi D, Singh F, Kabiraj D, Kulariya P, Sulania I (2009) Swift heavy ion induced structural modification of atom beam sputtered ZnO thin film. Surf Coat Technol 203(17-18):2427–2431. https://doi.org/10.1016/j.surfcoat.2009.02.109
Link S, Sayed E (1999) Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J Phys Chem B 103:8410–8426
Kluth P, Johannessen B, Giraud V, Cheung A, Glover C, Azevedo G (2004) Bond length contraction in Au nanocrystals formed by ion implantation into thin SiO2. Appl Phys Lett 85(16):3561–3563. https://doi.org/10.1063/1.1803619
Yang T, Li Y, Zhu M, Li Y, Huang J, Jin H (2010) Room-temperature ferromagnetic Mn-doped ZnO nanocrystal synthesized by hydrothermal method under high magnetic field. Mater Sci Eng B 170(1-3):129–132. https://doi.org/10.1016/j.mseb.2010.03.037
Jiang H, Rühle M, Lavernia E (1999) On the applicability of the x-ray diffraction line profile analysis in extracting grain size and micro strain in nanocrystalline materials. J Mater Res 14(02):549–559. https://doi.org/10.1557/JMR.1999.0079
Khawal H, Gawai U, Asokan K, Dole B (2016) Modified structural, surface morphological and optical studies of Li3+ swift heavy ion irradiation on zinc oxide nanoparticles. RSC Adv 6(54):49068–49075. https://doi.org/10.1039/C6RA04803J
Zhu Q, Xie C, Li H, Yang C, Zhang S, Zeng D (2014) Selectively enhanced UV and NIR photoluminescence from a degenerate ZnO nanorod array film. J Mater Chem C 2(23):4566. https://doi.org/10.1039/c4tc00011k
Cerqueira M, Rolo A, Viseu T, Ayres J, de Lacerda-Arôso T, Oliveira F (2010) Raman study of doped ZnO thin films grown by rf sputtering. Phys Status Solidi C 7(9):2290–2293. https://doi.org/10.1002/pssc.200983747
Lal C, Jain R, Jain I (2007) Structural and electrical properties of swift heavy ion beam irradiated Fe/Si interface. Bull Mater Sci 30(2):153–156. https://doi.org/10.1007/s12034-007-0027-4
Mondal S, Mitra P (2012) Preparation of cadmium-doped ZnO thin films by SILAR and their characterization. Bull Mater Sci 35(5):751–757. https://doi.org/10.1007/s12034-012-0350-2
Mote V, Dargad J, Purushotham Y, Dole B (2015) Effect of doping on structural, physical, morphological and optical properties of Zn1−xMnxO nano-particles. Ceram Int 41(10):15153–15161. https://doi.org/10.1016/j.ceramint.2015.08.088
Mote V, Purushotham Y, Shinde R, Salunke S, Dole B (2015) Structural, optical and antibacterial properties of yttrium doped ZnO nanoparticles. Cerâmica 61(360):457–461. https://doi.org/10.1590/0366-69132015613601932
Neogi S, Chattopadhyay S, Banerjee A, Bandyopadhyay S, Sarkar A, Kumar R (2011) Effect of 50 MeV Li3+ irradiation on structural and electrical properties of Mn doped ZnO. J Phys Condens Matter 23(20):205801. https://doi.org/10.1088/0953-8984/23/20/205801
Balamurali S, Chandramohan R, Suriyamurthy N, Parameswaran P, Karunakaran M, Dhanasekaran V (2012) Optical and magnetic properties of Mn doped ZnO thin films grown by SILAR method. J Mater Sci Mater Electron 24:1782–1787
Han J, Mantas P, Senos A (2011) Effect of Al and Mn doping on the electrical conductivity of ZnO. J Eur Ceram Soc 21:1883–1886
Han J, Mantas P, Senos A (2002) Defect chemistry and electrical characteristics of undoped and Mn-doped ZnO. J Eur Ceram Soc 22(1):49–59. https://doi.org/10.1016/S0955-2219(01)00241-2
Wu D, Huang Z, Yin G, Yao Y, Liao X, Han D (2010) Preparation, structure and properties of Mn-doped ZnO rod arrays. Cryst Eng Comm 12:192–198
Gültekin D, Akbulut H (2016) Raman studies of ZnO products synthesized by solution based methods. Acta Phys Pol A 129:16–19
Ridhuan N, Abdul Razak K, Lockman Z, Abdul A (2012) Structural and morphology of ZnO nanorods synthesized using ZnO seeded growth hydrothermal method and its properties as UV sensing. PLoS One 7:50405
Singh A, Thool G, Bangal P, Madhavendra S, Singh S (2014) Low temperature Mn doped ZnO nanorod array: synthesis and its photoluminescence behavior. Ind Eng Chem Res 53(22):9383–9390. https://doi.org/10.1021/ie500077v
Guo S, Du Z, Dai S (2009) Analysis of Raman modes in Mn-doped ZnO nanocrystals. Phys Status Solidi B 246(10):2329–2332. https://doi.org/10.1002/pssb.200945192
Sangeetha R, Muthu Kumaran S, Ashok Kumar M (2015) Structural, optical, dielectric and antibacterial studies of Mn doped Zn0.96Cu0.04O nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 144:1–7. https://doi.org/10.1016/j.saa.2015.02.056
Hervé P, Vandamme L (1994) General relation between refractive index and energy gap in semiconductors. Infrared Phys Technol 35(4):609–615. https://doi.org/10.1016/1350-4495(94)90026-4
Acknowledgements
The authors are grateful to IUAC, Project No. IUAC/XIII.3A/UFR No. 52309, New Delhi, for providing the ion beam irradiation facilities and Prof. S. S. Shah for his continuous encouragement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 809 kb)
Rights and permissions
About this article
Cite this article
Khawal, H.A., Mote, V.D., Asokan, K. et al. Formation of defect, oxygen vacancy creation, and shifting of phonon mode by Li3+ swift heavy ion irradiation on Zn1 − x Mn x O thin films. J Solid State Electrochem 22, 1237–1248 (2018). https://doi.org/10.1007/s10008-017-3833-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3833-7