Abstract
Cadmium selenide (CdSe) thin films were grown by electrochemical technique on fluorine-doped tin oxide (FTO)-coated conducting glass substrates in the presence of organic surfactants. The influence of organic surfactants like polyethylene glycol (PEG) and polyvinylpyrrolidone (PVP) on different physico-chemical properties and its subsequent impact on photoelectrochemical (PEC) performance of CdSe thin films have been investigated. It has observed that the organic surfactants play an important role in modifying the surface morphology of CdSe thin films. The compact grain like morphology of pure CdSe is tuned to interconnected nanofibrous network on addition of PEG and to sprouting nanorods like morphology on addition of PVP. Among these nanostructures, CdSe sprouting nanorods exhibits improved power conversion efficiency of 0.55% as compared to nanofibrous (0.24%) and granular CdSe (0.16%) nanostructures. It reveals the fourfold enhancement in the PEC performance on PVP-mediated growth which can be attributed to conversion of compact dense nanostructure to porous and relatively high surface area nanostructure. This work exemplifies the ability of organic surfactant to modulate the surface morphology of the electrodeposits and pinpoints the organic surfactant that gives rise to the suitable morphology for PEC solar cell application.

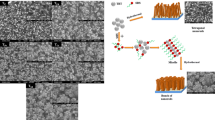
TOC: The morphology of CdSe nanostructure has successfully tuned with use of surfactants to enhance the photoelectrochemical solar cell performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium selenide (CdSe) nanostructures with its typical optical properties such as narrow direct band gap and high refractive index make it quite an interesting material in various fields like solar cells, transistors, light emitting diodes, gamma ray detectors, etc. The optoelectronic properties of CdSe nanostructures are function of their size and shape; hence, nowadays, growth of different CdSe nanostructures is highly focused [1, 2]. CdSe nanostructures are synthesized by various physical and chemical techniques such as molecular beam epitaxy, laser ablation, spray pyrolysis, solvothermal, chemical vapor deposition, and electrodeposition [3,4,5,6,7]. Among these techniques, electrodeposition is a powerful technique, as it offers various advantages over other techniques such as room temperature deposition, use of low cost raw materials and equipment, control over the surface morphology, and ability to deposit films on the complex surfaces. Hence, we have preferred electrodeposition technique for the growth of CdSe nanostructures.
As per the literature survey, various approaches such as variation in pH of solution, addition of some dopants, annealing of samples, etc. are implemented to change the size and shape of CdSe nanostructures [8,9,10]. Here, we have applied dopant-free, simple surfactant-assisted approach to alter the morphological features of pure CdSe. Here, we find that out of all above approaches, surfactant-assisted route plays an important role in tuning morphological features of materials [11,12,13,14,15,16,17,18]. Surfactants are actually molecules with single- or double-chain aliphatic, nonpolar regions, and ionic or neutral polar head groups and have been widely used to promote stable dispersion of solids in different media [16]. Surfactants strongly affect the morphology and crystal growth in association with a promotion of nucleation and current distribution [17, 18]. The tendency of surfactants to diminution of surface tension over the farming surface leads to organized placing of molecules on the precise site of the growing surface. This interprets into the formation of compact deposits with narrow pore size distributions and enhanced adhesion to the substrate. In addition, surfactants also modified materials to afford superior charge transport properties and improved the photoelectrochemical (PEC) performance [19]. Surfactants have polar head groups attached to a long chain aliphatic nonpolar tail. The head groups of surfactants get adsorbed on the electrode surface and therefore can influence the double-layer properties, kinetics, and mechanism of electrochemical processes [15]. Further, these changes in the kinetics of nucleation and crystal growth influences the morphological features of deposits, which defines the energetic and kinetics of photoelectrochemical processes at the electrode/electrolyte interface [11].
In present investigation, the surfactant-assisted morphological tuning of electrodeposited CdSe thin films is reported. The effect of organic surfactants such as polyethylene glycol (PEG) and polyvinylpyrrolidone (PVP) on different physico-chemical properties of CdSe thin films has been studied. Furthermore, a strong dependence of photoelectrochemical (PEC) performance on the shape and structure of the nano-sized CdSe is demonstrated.
Experimental
Materials
All chemicals used were of analytical grade and used as received without further purifications. Cadmium acetate and selenium dioxide were purchased from s. d. fine chemicals India.
Sample preparation
CdSe thin films were deposited on FTO-coated substrates by potentiostatic mode of electrodeposition at −0.79 mV/SCE for 45 min. Equimolar solutions (0.1 M) of cadmium acetate and selenium dioxide were used as precursors of Cd and Se, respectively. Furthermore, the pH of solution is adjusted to ∼2 by the addition of hydrochloric acid and employed as a precursor solution. In order to study the effect of organic surfactants on CdSe growth and their properties, two different surfactants, polyethylene glycol (PEG; MW = 646.87 g mol−1) and polyvinylpyrrolidone (PVP; MW = 140,000 g mol−1), were added separately in definite proportions to the aqueous precursor solution. The concentration of organic surfactants was kept constant at 1 wt% in final solution. All the experiments were performed in quiescent solution at room temperature. Films prepared with PEG and PVP surfactant are denoted as C-PEG and C-PVP respectively where the films prepared without surfactant is denoted as C-PURE.
Measurements
Cyclic voltammetry study is performed using scanning potentiostat EG and G Versa stat-II model PAR-362. The crystal structure of the films was examined using X-ray diffraction (XRD Philips PW3710 X-ray diffractometer with Cr K α (λ = 2.28970 Å)) radiation operating at 40 kV and 30 mA. Compositional study was carried out by energy dispersive X-ray analysis technique (EDAX) Horiba 7700H. The surface morphology was observed by scanning electron microscopy (JEOL JSM 6360 with an accelerating voltage 20 kV). The UV-VIS absorption spectrum of the samples was recorded on systronics-119 model spectrophotometer. Photoelectrochemical (PEC) study was performed in a conventional three electrode arrangement with the deposited film as a working electrode, a graphite counter electrode and saturated calomel electrode (SCE) as a reference electrode. The PEC solar cell performance was studied under constant illumination of 30 mW cm−2 from tungsten filament lamp with an active area of 1 cm2 CdSe film electrode. The 1 M polysulphide (Na2S + NaOH + S) aqueous solution was used as an electrolyte.
Results and discussion
The reactions culminating the electrochemical growth of CdSe thin film can be illustrated as below [14,15,16,17,18,19,20]. Selenium dioxide was dissolved in H2O.
Cadmium has tendency to easily undergo dissolution in acidic media which can be written as
And this is followed by final reduction reaction given as,
Also, the reduction of H2SeO3 to elemental Se takes place, in following way
Therefore, simultaneous formation of CdSe and deposition of elemental Se is possible. Thus, reaction (5) may be the source of the excess selenium and it explains the presence of excess Se in the film as depicted from EDAX in further measurement [19].
The cyclic voltammogram (CV) recorded for films deposited with and without surfactant shown in Fig. 1 is nearly same. In CV’s curves for all the CdSe films, there is sharp increase in cathodic current from 0.6 V to 0.8 V/SCE showing simultaneous reduction of Cd and Se. The crossover between anodic and cathodic current curves appears on the reverse potential sweep which indicates that nucleation process exists on the substrate in an electrolytic bath [20].
X-ray diffraction (XRD) patterns of CdSe films deposited at optimized preparative parameters are shown in Fig. 2. The diffracting angle 2θ was varied between 10o and 100o. A broad peak in XRD Patterns at 38.26o belongs to (111) plane of cubic CdSe (powder diffraction file no. 65–2891). The peaks marked with star belong to FTO substrate. In addition to this, XRD patterns of CdSe films with and without surfactants are same which confirm that addition of surfactant does not change the crystal structure of electrodeposits. Quantitative elemental analysis of the film was carried out using the EDAX technique for as deposited film. The inset of Fig. 3 shows typical EDAX pattern of CdSe film. The elemental analysis was carried out only for Cd and Se; the average atomic percentage of Cd to Se was 43.73:56.27, showing that the film is slightly rich in selenium.
The optical absorption spectra for the CdSe films were recorded in the wavelength range of 350 to 850 nm at room temperature. The optical absorption data were analyzed using the following Tauc’s relation [21] of optical absorption in semiconductor near band edge
Where E g is the separation between the conduction band minima and valence band maxima, hυ is the photon energy, and n is a constant equal to one for direct gap semiconductors and four for indirect gap materials. The variation of (αhυ) 2 versus (hυ) is linear as seen from Fig. 3, which means that the mode of transition in these films is of direct nature. Extrapolation of these curves on energy axis for zero absorption coefficient value gives the optical band gap energy. The band gap was found to be 2.08, 2.05, and 2.0 eV for C-PURE, C-PEG, and C-PVP films, respectively. The observed band gap values are higher than bulk CdSe (1.7 eV) which may be attributed to the nanocrystalline nature of CdSe. In general, high band gap energy values were reported for chemically deposited CdSe films [22].
Scanning electron micrographs (SEM) of CdSe films with and without surfactants are shown in Fig. 4. The surface of the C-PURE film is compact with uniform coverage of nanograins. Further, the addition of PEG in an electrolytic solution shows considerable morphological change. The surface of CdSe is completely changed from compact grains to the interconnected nano-fibrous network. The ordered chain structure of PEG adsorbs easily at metal chalcogenide surface, which reduces the activity of growing deposit greatly and hence inhibits the growth in certain directions [23,24,25]. Therefore, the addition of PEG modifies the growth kinetics of deposit, which leads to anisotropic growth of the crystals. The CdSe films deposited in presence of PVP shows uniformly distributed sprouting rods of about 200–400 nm size. The PVP structure consists of polyvinyl skeleton with polar groups; the donated loan pairs of both nitrogen and oxygen atoms in polar group of one PVP unit may occupy the orbital of metal ion [26]. Zhang et al. and Wei et al. have reported the formation of co-ordinate bond between PVP and metal ions [27, 28]. In some reports, co-ordination of PVP with metal ions has been proposed by shift of the 〉C = O stretching in IR spectra of PVP surrounding nanoparticles, also carbonyl group of PVP partly co-ordinates with surface of metal atoms [29,30,31,32,33]. Here, we likewise believe that co-ordination of cadmium with PVP may alter the electrochemical growth kinetics, which can take part in controlling the grain size and morphology of CdSe by reducing the reaction rate. The surface adsorption of surfactant molecules on growing CdSe surface significantly modifies the growth of CdSe. This observation leads to a clear conclusion that the polymers (PVP and PEG) acts as a template during electro deposition of CdSe and alters the way in which CdSe grows on to the substrate upon cathodic polarization.
The photocurrent-voltage (I-V) curves for the films deposited with and without surfactant are shown in Fig. 4 and Fig. S1 (see Electronic Supplementary Material). The Fig. S1 shows the photoelectrochemical conversion properties of the CdSe thin films deposited with different surfactant concentrations. The CdSe thin films deposited with 1 wt% of surfactant concentrations in the final solution shows highest photoconversion efficiency. Therefore, in Fig. 4, the PEC performance of pure and 1 wt% surfactant-added CdSe thin film was presented. From Fig. 4, it is clear that the highest photoconversion efficiency (η) of 0.55% is obtained for the film deposited with PVP surfactant. This is a significant improvement as compared to CdSe film (η = 0.16%) deposited without surfactant and film deposited with PEG surfactant (η = 0.25%). Moreover, the PEC performance in our study is superior to earlier reports [34, 35]. The enhanced efficiency of CdSe-PVP film can be attributed to highly porous and uniformly dispersed sprouting rods like morphology with microvoids which permits electrolyte to percolate inside the porous structure and increase the electrode-electrolyte interface. The morphology of CdSe-PEG film is also porous but fibrous structure of film is not found to be uniformly distributed on substrate surface which may results to poor electrical contact and thereby the less efficiency. The compact surface of pure CdSe film results to reduced electrode-electrolyte interface and hence the poor solar cell performance.
Conclusions
In conclusion, we have successfully tuned the morphology of CdSe thin film using different organic surfactants such as PEG and PVP. The organic surfactants greatly modify the surface morphological features, suitable for an intended application. The compact grain-like morphology of CdSe films is converted into interconnected nano-fibrous network on addition of PEG while sprouting rods with micro voids are formed on the addition of PVP. This morphological tuning causes nearly fourfold increase in photo conversion efficiency of CdSe from 0.16 to 0.55%. This morphological modification enhances the active surface area, which results into improvement in PEC performance. Thus, present investigation opens up simple and easy way of morphological tuning which can be applicable for other metal chalcogenides.
References
Erata S, Ari M, Metina H (2008) Mater Chem Phy 111:114–120
Khandelwal A, Jena D, Grebinski J, Hull K, Kuno M (2006) J Electron Mater 35:170–172
Chang S, Hsiao Y, Li T (2014) J Electron Mater 43:3077–3081
Zarghami V, Mohammad M, Fray D (2012) J Electron Mater 41:3050–3055
Kurtz E, Schmidt M, Don B, Wachter S, Litvinov D, Gerthsen D (2001) J Cryst Growth 227:630–633
Meteleva YU, Radychev N, Novikov G (2007) Inorg Mater 43:455–465
Liu C, Wu P, Sun T, Dai L (2009) J Phys Chem C 113:14478–14481
Shinde S, Dubal D, Ghodake G, Fulari V (2014) J Electroanal Chem 727:179–183
Shinde S, Dubal D, Ghodake G, Lee D, Lohar G, Rath M, Fulari V (2014) Mater Lett 132:243–246
Xue J, Shen Q, Yang F, Liang W, Liu X (2014) J Alloys Compd 607:163–168
Inamdar A, Mujawar S, Ganesan V, Patil P (2008) Nanotechnology 19:325706–325713
Shen C, Zhang X, Li H (2001) Mater Sci Eng B 84:265–270
Fu J, Gao D, Xu Y, Xue D (2008) Electrochim Acta 53:54645468
Shen CM, Zhang XG, Li HL (2005) Appl Surf Sci 240:34–41
Devaraj S, Munichandraiah N (2007) J Electrochem Soc 154:A901–A909
Vanalakar SA, Suryawanshi MP, Mali SS, Moholkar AV, Kim JY, Patil PS, Kim JH (2014) Curr App Phy 14:1669–1676
Ghaemi M, Khosravi-Fard L, Neshati J (2005) J Power Sources 141:340–350
Vanalakar SA, Mali SS, Jo EA, Kim JY, Kim JY, Patil PS, Kim JH (2014) Solid State Sci 36:41–46
Vanalakar SA, Kamble AS, Shin SW, Mali SS, Agawane GL, Patil VL, Kim JY, Patil PS, Kim JH (2015) Sol Energy 122:1146–1153
Lokhande CD, Lee EH, Jung KD, Joo OS (2005) Mater Chem Phys 93:399–403
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Phys Status Solidi 15:627
Pawar SM, Moholkar AV, Rajpure KY, Bhosale CH (2006) J Phys Chem Solids 67:2386–2391
Lokhande C, Lee EH, Jung KD, Joo OS (2005) Mater Chem Phys 91:200–204
Dobryszycki J, Biallozar S (2001) Corros Sci 43:1309–1319
Vanalakar SA, Gang MG, Patil PS, Kim JY, Kim JH (2016) Indian J Eng Mater Sci 23:139–142
Vanalakar SA, Kim JH, Patil PS (2016) In: Thomas S (ed) Advanced polymeric materials: from macro- to nano-length scales, 1st edn. CRC Press, New Jerrcy
Lui X, Yang J, Wang L, Yang X, Lude L, Wang X (2000) Mater Sci Eng A 289:241–245
Zhang Z, Zhao B, Hu L (1996) J Solid State Chem 121:105–110
Tao D, Qian W, Huang Y, Wei F (2004) J Cryst Growth 271:353–357
Goel A, Rani N (2012) Open J Inorg Chem 2:67–73
Tan Y, Dai X, Li Y, Zhu D (2003) J Mater Chem 13:1069–1075
Toshima N, Nakata K, Kitoh H (1997) Inorg Chim Acta 265:149–153
Teranishi T, Miyake M (1998) Chem Mater 10:594–600
Gudage YG, Deshpande NG, Sagade AA, Sharma RP, Pawar SM, Bhosale CH (2007) Bull Mater Sci 30:321–327
Raut VS, Lokhande CD, Killedar VV (2017) J Mater Sci: Mater Electron 28:140–3150
Acknowledgements
One of the authors B. B. Sinha is grateful to the Department of Science and Technology (DST), India for award of Inspire fellowship. One of the authors S. A. Vanalakar is thankful to the University Grant Commission (UGC), New Delhi, India for providing fellowships under Raman Fellowship for Post-Doc Research in USA scheme (File No. 5/155/2016 (IC)). This work was partly supported by the converging research center program funded by the Ministry of science, ICT and Future planning (2013K000407) and Human Resource Development of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Knowledge Economy (no. 20124010203180).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(PDF 213 kb).
Rights and permissions
About this article
Cite this article
Kamble, A.S., Patil, V.L., Sinha, B.B. et al. Influence of surfactants on electrochemical growth of CdSe nanostructures and their photoelectrochemical performance. J Solid State Electrochem 21, 2649–2653 (2017). https://doi.org/10.1007/s10008-017-3651-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3651-y