Abstract
Cobalt chromium-layered double hydroxides (CoCr-LDHs) were electrodeposited on to carbon paper by potentiostatic method from the respective cobalt and chromium ion sources. The electrodeposited CoCr-LDHs were characterized by x-ray diffraction (XRD), Fourier transferred infrared (FT-IR) spectroscopy, scanning electron microscope (SEM), energy-dispersive x-ray analysis (EDX) and x-ray fluorescence (XRF) elemental mapping. The XRD and IR data confirmed that the deposits were CoCr-LDH with carbonate and nitrate ions in the basal space. The SEM observations confirmed that the CoCr-LDH surface had distinct morphology consisting of aggregate size of about 100 nm. For the first time, the supercapacitor characteristics of the CoCr-LDHs were assessed in three-electrode configuration in 1 M KOH or two-electrode (asymmetric capacitor device with reduced graphene-oxide (RGO)). It turned out that the asymmetric capacitor consisted of the CoCr-LDH and the RGO exhibited higher energy density with excellent power density. The higher energy density and power density of the asymmetric capacitor device is believed to be due to the unique LDH morphology in addition to the Faradaic and non-Faradaic contributions. It was demonstrated that the two asymmetric capacitor devices connected in series could light an LED bulb.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to industrialization and improved life standard, the demand for energy is on increase including in developing countries. The major portion of energy demand is met by fossil fuels. While the utilization of conventional fossil fuels leads to adverse effects on environmental eco-systems, the resources for such fossil fuels are getting depleted. Thus, globally, current researches focus on developing renewable energy or greener approach. The effective use of renewable energies can be realized if stored when they are available and use them when they are non-available. In this regard, electrochemical energy storage systems such as batteries and supercapacitors are considered attractive, as they are capable of releasing the stored energy when it is required [1–3]. Compared to the batteries, the supercapacitors have several merits such as high power density, long cycle life and portability. The batteries find applications in most of the consumer electronics, while the supercapacitors find applications where the peak power is indispensable. Based on the mode of charge storage, the supercapacitors are classified into two types, viz. electrical double layer capacitors (EDLCs) and pseudocapacitors. While electrostatic charge separation (non-Faradaic) is the basis for charge storage in the former case, a definite Faradaic (redox) reaction is responsible for charge storage in the latter. Due to high specific capacitance, pseudocapacitors are considered more advantageous than EDLCs.
As an electrode for EDLC, carbon and carbon-based materials are being explored. For example, Portet et al. reported a specific capacitance of 95 Fg−1 for activated carbon [4]. Li et al. used mesoporous carbon spheres which exhibited a specific capacitance of 155 Fg−1 [5]. Lee et al. reported specific capacitance in the range of 180–250 Fg−1 for activated carbon obtained from anthracite [6]. Tao et al. used multi-branched porous carbon nanofibres which exhibited a specific capacitance of 295 Fg−1 [7]. Sun Wen et al. reported carbon nanotubes (CNTs) as EDLC material with a specific capacitance of 120 Fg−1 [8]. Quite recently, reduced graphene-oxide (RGO) has been utilized as electrode material which exhibited a specific capacitance of 200 Fg−1 [9]. On the other hand, metal oxides, metal hydroxides, polymers and their composites have been examined as electrode material for pseudocapacitors. For example, Gupta et al. used Co(OH)2 as electrode material which resulted in a capacitance of 860 Fg−1 [10]. Cao et al. reported a specific capacitance of 400 Fg−1 for Co3O4 [11]. The V2O5 exhibited a specific capacitance of 210 Fg−1, as reported by Reddy et al. [12]. Recently, an Fe2O3-graphene nanocomposite exhibited a specific capacitance of 225 Fg−1 reported by Wang et al. [13].
Quite recently, layered double hydroxides (LDHs) of general formula \( {\left[{\mathrm{M}}_{1-x}^{2+}{\mathrm{M}}_x^{3+}{(OH)}_2\right]}^{p+}{X^{n-}}_{p/n} \) ∙ yH2O (M2+ = Mg2+, Co2+, Zn2+, Ni2+; M3+ = Al3+, Fe3+, Cr3+ and X n− = \( {Cl}^{-},{\ CO}_3^{2-},\kern0.5em {\mathrm{SO}}_4^{2-} \), etc. have been considered as electrode material for supercapacitors due to their excellent redox property [14, 15]. It has been established that the LDH forms only in the narrow compositions and they crystallize in layered hexagonal crystal (hydrotalcite-like structure) with a basal length of about 7 Å. Chemical precipitation and hydrothermal and electrochemical methods are usually employed to prepare LDHs and used for supercapacitor application. There are several reports on LDHs based on CoAl [16], NiAl [17], NiMn [18], CoZn [19], CoNi [20], CoMn [21], CoFe [22], etc. To the best of our knowledge, cobalt chromium-LDH (CoCr-LDH) has not been examined for supercapacitor application so far. Cr being a multivalent cation (oxidation state varies from zero to six), a good redox behaviour is expected for Cr-based LDHs.
So, in the present work, different compositions of CoCr-LDH were electrochemically deposited and their supercapacitor properties in three- and two-electrode (asymmetric, with reduced graphene-oxide (RGO)) configurations have been examined. It turned out that the asymmetric capacitor consisting of CoCr-LDH and RGO electrodes exhibited excellent power density. The detailed electrodeposition, characterization, and supercapacitor properties of CoCr-LDH and RGO are reported here.
Experimental
Electrodeposition of CoCr-LDHs and chemical synthesis of RGO
Analytical-grade chemicals cobalt nitrate (Co(NO3)2∙6H2O, Spectrum Reagents and Chemicals Pvt. Ltd.), chromium nitrate (Cr(NO3)3∙9H2O, Otto Kemi) and potassium hydroxide (KOH, Thermo Fisher Scientific) were used as received. A carbon paper (Alfa Aesar 43078 graphite foil, 0.13 mm, 0.005 in thick, 99.8 % metal basis) with a geometrical area of 1 × 1 cm2 was used for deposition of CoCr-LDHs. Prior to deposition, the carbon paper was polished and washed with acetone to avoid dust and contaminations. A three-electrode cell was used for deposition of CoCr-LDH. The cell consisted of a platinum wire (area = 3.2 cm2) as a counter electrode, mercury/mercury oxide in KOH (Hg/HgO) as a reference electrode, and the aforementioned carbon paper as working electrode. For deposition of CoCr-LDH, a mixture of cobalt nitrate and chromium nitrate solutions with different Co/Cr nitrate ratios (1:1, 2:1 and 3:1) were prepared in distilled water and used. The potentiostatic deposition was performed at a constant potential of −1.2 V vs. Hg/HgO with a deposition time of 80 s in all cases. Both sides of carbon paper were deposited with CoCr-LDH. After deposition, the carbon papers containing deposit were carefully washed with distilled water and dried naturally in air overnight. The weights of the deposits were measured by means of an electronic balance and the difference in weights before and after deposition gives the active mass on the carbon paper. The estimated weight of CoCr-LDH was 1.2 mg.
Initially, graphene-oxide (GO) was synthesized by modified Hummers method from synthetic graphite flakes as explained here. Equal amounts of graphite (1.0 g) and sodium nitrate (1.0 g) were mixed with 50 mL of concentrated sulphuric acid in a 500-mL round-bottom flask in an ice bath and kept on magnetic stirring for 30 min. To the cold solution, 6 g of potassium permanganate (KMnO4, Fischer Inorganic and Aromatics) was gradually added with constant stirring while the reaction temperature was maintained at 5 °C. After addition of KMnO4, 80-mL distilled water was slowly added with vigorous stirring and the temperature of the bath was raised to about 90 °C. Then, after 30 min of vigorous stirring, the solution was diluted further by the addition of 200-mL de-ionized (DI) water followed by 6 mL of hydrogen peroxide (30 %). The resulting mixture was again stirred for one hand filtered. The filtrate was discarded. The precipitate was washed with excess hot DI water until the pH of the filtrate was nearly neutral. The obtained sediment was redispersed in water with stirring and followed by mild sonication, giving finally a solution of exfoliated GO. Reduced graphene-oxide (RGO) was obtained from the GO through chemical reduction method by utilizing hydrazine hydrate (N2H4∙H2O) reducing agent. The above GO suspension (100 mL) was mixed with 100-mL DI water and stirred at 85 °C for 30 min. To the hot solution, 1 mL of hydrazine hydrate was added and stirred for another 2 h at 85 °C. The obtained mixture washed with hot water and ethanol to remove excess hydrazine and dried at 60 °C for overnight to obtain solid RGO.
Characterization and electrochemical tests
The crystal structures of the electrodeposited CoCr-LDHs and the synthesized RGO were examined by x-ray diffraction using diffractometer (Bruker D8 Advance) equipped with Cu K ∝ radiation (λ = 1.5418 Å). The XRD measurement was performed in the 2θ range of 10–80° at a scan rate of 4° min−1. The infrared spectra of hydroxide deposits scraped from the carbon paper or synthesized RGO were recorded by using a Fourier transformed infrared (FT-IR) spectroscopy. The surface morphology of all the deposits and RGO was investigated using field emission scanning electron microscope equipped with elemental micro analysis (Zeiss Gemini Supra 55VP or Bruker, FESCAN). Elemental mapping on the CoCr-LDH samples was done using x-ray fluorescence (Horiba XGT 5200). The RGO electrode (active mass = 2 mg) on carbon paper was fabricated by using slurry of the RGO (90 wt%) and polyvinylidene fluoride (PVDF) as a binder (10 wt%) with the help of N-Methyl-2-pyrrolidone (NMP) as a solvent. All the electrochemical tests were performed with Biologics electrochemical workstation (SP-300). The cyclic voltammetric and galvanostatic charge-discharge studies were carried out in a three- or two-electrode (CoCr-LDH and RGO) cell in 1 M KOH. The cyclic voltammograms (CVs) were recorded for CoCr-LDH electrodes in a potential range of −0.1 to 0.5 V vs. Hg/HgO. The potential window examined for RGO was −0.9 to 0.1 V, while the potential window used for the asymmetric capacitor (CoCr-LDH and RGO) was 0 to 1.6 V. In all cases, the scan rates were 5, 10, 20, 30, 40 and 50 mVs−1. The galvanostatic charge-discharge (CD) cycles were carried out at current densities of 0.5, 1, 2, 3, 4 and 5 Ag−1 in the respective potential range. The cycle life data were collected at a current density of 5 Ag−1. Electrochemical impedance of the electrodes was recorded in the frequency range of 100 kHz to 0.01 Hz using the same electrochemical workstation.
Results and discussion
Crystal structure and morphology of CoCr-LDH and RGO
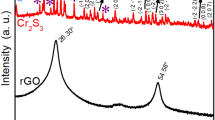
The crystal structure of the electrodeposited CoCr-LDHs on the carbon paper was examined by XRD studies. Figure 1 shows the XRD patterns of the representative (2:1) CoCr-LDH on the carbon paper and the bare carbon paper substrate. The patterns consist of two Bragg peaks located at 26.8° and 54.9° which are assigned to carbon. No Bragg peaks for CoCr-LDH were seen. This means that the electrodeposited CoCr-LDH is amorphous in nature. Thus, to identify the phase and the crystal structure of CoCr-LDHs, electrodeposition was done separately on stainless steel (SS) under the aforementioned conditions. Figure 2 shows the XRD patterns of the electrodeposited CoCr-LDHs on the SS substrate. In each case, apart from the Bragg peaks for SS substrate, distinct Bragg peaks were present at 10.21°, 20.12°, 33.82°, 43.88° and 60.08°. According to Ref [23], these set of Bragg peaks correspond to the XRD pattern of CoCr-LDH. The interlayer distance obtained from (003) peak is about 8.66 Å which is slightly higher than that of the previously reported value of 7.56 Å [24]. The larger interlayer distance could be due to presence of larger number of interlayer species such as \( {\mathrm{NO}}_3^{-} \), \( {CO}_3^{2-}, \) H2O, etc. in the basal space. It is noteworthy that the d spacings of (003), (006) and (012) peaks are 8.66, 4.35 and 2.64 Å, respectively, and are in the order of d (003) ≃ 2d (006) ≃ 3d (009) which is characteristic of a layered structure. In addition, the appearance of an asymmetric peak at 33.82° confirms turbostatic nature present in the LDH, as has been reported previously [25]. It is seen that the base line in the XRD patterns is non-uniform and noisy. Such background is usually observed for Co-based materials if Cu x-ray source is used.
Figure 3 shows IR spectra of CoCr-LDH powder samples scraped from the deposited electrodes. In all cases, a broad peak is seen in range of 3200–3400 cm−1 which is ascribed to hydrogen-bonded OH of LDH. The absence of sharp peak in this range confirms characteristics of α-Co(OH)2 nature which has bonded hydroxyl group. The peaks present at 1597 and 1382 cm−1 are assigned to carbonate (CO3 2−) and nitrate (NO3 −) ions, respectively. While \( {\mathrm{NO}}_3^{-} \) would have resulted from the nitrate deposition bath, \( {CO}_3^{2-} \) would have come from the atmosphere. As per previous reports [23], during synthesis of the hydroxides, generally, CO2 from the atmosphere are trapped in the form of CO3 2− in the interlayers. Besides, the bending vibration of H2O also usually appears at 1597 cm−1. Thus, the peak observed at 1597 cm−1 is attributed to overlapping peaks of CO3 2− and bending vibration of H2O. The peak at 1110 cm−1 is due to C–O stretching vibration of carbonate. The peak observed at 820 cm−1 is assigned to out of plane bending vibration of H2O (δ OH) [26]. The peak appeared at 565 cm−1 is due to Co–O and Cr–O bonds. The Cr–O bond also has additional peak at 624 cm−1.
Thus, from the aforementioned XRD and IR data, it is confirmed that the electrodeposited sample is CoCr-LDH having nitrate and carbonate ions in the basal space. The following nitrate reduction reaction and its associated reactions are responsible for LDH deposition [23, 24] and [27]. The nitrate ion (\( {\mathrm{NO}}_3^{-} \)) undergoes reduction-producing ammonia and hydroxyl ions (Eq. 1). The protonation of ammonia generates ammonium ions and also hydroxyl ions (Eq. 2). The generation of hydroxyl ion increases the local pH at the electrode/electrolyte interface, leading to LDH formation (Eq. 3). It was observed that the colour of CoCr-LDH was brownish green.
The charge balancing is compensated by the presence of appropriate anion (X n−), where X n− = NO3 −, CO3 2−, etc. It is noted that the applied potential was −1.2 V vs. Hg/HgO (E 0 = 0.2 V) which is close to hydrogen evolution reaction. Such gas evolution will usually lead to porous morphology. The potentiostatic method has been preferred over potentiodynamic to prepare LDHs because the LDH materials form only in the narrow composition range (0.2–0.4). In the potentiostatic method, the time and potential are key parameters. In the present work, constant deposition time of 80 s was kept constant to have uniform deposited mass and film thickness. It is well known that increasing the holding time will have higher mass deposition and the thicker film. However, we have not varied the deposition time as this was not the focus of this work.
Figure 4 (left) shows the FE-SEM images taken on the surface of CoCr-LDH deposits. It can be seen that distinct morphologies for each of (1:1), (2:1) and (3:1) electrodeposited CoCr-LDH samples. The surface of (1:1) CoCr-LDH sample is rather denser consisting of spherical (average size of 30 nm) and flaky morphology, the surface of (3:1) CoCr-LDH sample is too porous consisting of mainly flakes (average size of 10 nm). While the too denser electrode matrix will hinder the electrolyte interaction, the too porous electrode matrix will have lesser reactive sites because of its nature. It can be seen that the surface morphology of (2:1) CoCr-LDH sample is neither denser nor porous. The surface consists of aggregates of nanospheres consisting of ultrafine grains and pores throughout the electrode matrix. The estimated average grain size and pore size are 100 and 250 nm, respectively, for the (2:1) sample. This distinct porous nanosphere morphology would lead to effective interaction with the KOH electrolyte and would bring about better electrochemical performance. The composition of CoCr in each of the LDH samples was examined by energy-dispersive x-ray analysis (EDX). Figure 4 (right) shows the EDX profiles of all the three CoCr-LDH samples. Detailed observed elemental compositions are shown in Table 1. The presence of Co and Cr in all three samples is clearly seen. In each case, the obtained Co/Cr ratios nearly match with that of the nominal composition. The EDX data also confirmed the presence of O and C. While the source of O is from the metal hydroxides, the source of C could be from the carbon substrate used for deposition. This observation has been substantiated by elemental mapping done by x-ray fluorescence (XRF) (Fig. 5). The elemental mapping clearly shows that Co and Cr are homogeneously distributed throughout the electrode matrix. It is noteworthy that clear increase of Co concentration visibly seen as we move from (1:1) sample to (3:1) sample. In addition, the Co/Cr compositions in each sample match with that of the XRF profile intensities.
Figure 6a shows the XRD pattern obtained for the RGO. It can be seen that the pattern consists of a large intense broad peak centred at 23° and a low intense asymmetric peak at 43° which are characteristics of RGO. The broad peak implies the poor ordering of the graphene sheets along the stacking direction confirming that the RGO is comprised of a few layers of graphene. The asymmetric peak at 43° confirms the turbostatic nature of disordered carbon layer in RGO. A similar observation has been reported elsewhere [28]. To substantiate the XRD data, Raman spectrum of RGO was taken and is shown in Fig. 6b. The Raman spectrum consists of two bands located at 1330 and 1592 cm−1 which correspond to the D and G bands of RGO, respectively, as reported by Zhao et al. [29]. While the appearance of D band is originated from the edges of defected carbon layers due to breakdown of translational symmetry, the G band is attributed to the second-order scattering of graphitic nature of carbon. It is well known that the disorder in the RGO is measured with the intensity ratio of D and G bands. In the present case, the I D/I G is found to be 1.3 which confirms that extensive oxidation and reduction have resulted, leading to large disorders in the RGO. Figure 6c shows the SEM image taken on the surface of the RGO sample. The morphology of the RGO seemed like folded curtain, forming overlapped compact structure rather than agglomerated sheets. The thickness of sheets is about 250 nm with a large dimension of over 5 μm. The folded structure with the large dimension of sheets is the typical morphology usually obtained for RGO [29].
Supercapacitor performance of CoCr-LDH
To examine the electrochemical activity, initially cyclic voltammograms of each of CoCr-LDH electrodes in three-electrode configuration were recorded in 1 M KOH in the potential range of −0.1 to 0.5 V at a scan rate of 5 mVs−1. The CV of bare carbon paper was also done. The obtained CV curves have been shown in Fig. 7a. The carbon paper did not show any peak and the current generated also nearly zero. Undoubtedly, it can be seen that a pair of redox peak appears in all case. An anodic peak occurs at ∼0.21 V and a cathodic peak appeared at 0.15 V with a potential difference of about 60 mV which is closer to the potential difference of an ideal reversible reaction (59 mV). The appearance of redox peaks and non-rectangular shape CV curves indicate that the deposited CoCr-LDHs are electrochemically active and the capacitor characteristics are mainly governed by Faradaic nature. The following reversible reaction is responsible for the appearance of the redox peak in CV.
It is noted that the anodic peak currents of (1:1), (2:1) and (3:1) CoCr-LDH samples are 2.9, 26.2 and 4.3 mA, respectively. Evidently, the (2:1) CoCr-LDH electrode exhibits highest peak current compared to other compositions. This implies that the (2:1) CoCr-LDH electrode is capable of storing large charge. The specific capacitance was calculated from the CV curves by using the following formula:
where C is specific capacitance (Fg−1), Q is charge (coulomb), v is potential window (V) and m is active mass (g). It turns out that the estimated specific capacitances of (1:1), (2:1) and (3:1) CoCr-LDH electrodes are 140, 265 and 145 Fg−1, respectively. Figure 7b shows the galvanostatic charge-discharge (CD) profiles of each of (1:1), (2:1) and (3:1) CoCr-LDH electrodes recorded at current of 1 Ag−1. It can be seen that in all cases, the CD profiles are mirror images which confirm the redox reactions are highly reversible. It is noted that most of the charge storage occurs in the potential range of 0.16 to 0.26 V which is in accordance with the CV data discussed above. A notable CD time was exhibited by the (2:1) CoCr-LDH electrode, substantiating the CV data. Figure 7c shows the comparison of the specific capacitances of each of CoCr-LDH electrodes estimated from the CD curves using the following formula:
where C is capacitance (Fg−1), I is current (A), t is discharge time(s), ∆v is potential window (V) and m is active mass (g). It is evidently seen that the (2:1) CoCr-LDH electrode exhibits a specific capacitance of as high as 240 Fg−1, while other electrodes exhibit a specific capacitance of about 140 Fg−1. The electrochemical activity of the prepared compositions seems dependent on the surface morphology. It can be seen that the (1:1) composition electrode surface consists of uniformly dense matrix. Such dense material would lead to less effective reaction sites for reaction with OH− (due to poor surface area). Undoubtedly, the (3:1) composition has more porous morphology. Such porous morphology would have also lead to less effective reaction sites leading to medium electrochemical activity. In the case of the (2:1) electrode, the optimum morphology would have led to more reaction sites leading to better electrochemical activity. It is believed that for the (2:1) composition, the optimum morphology seemed to result which would have facilitated the electrolyte interaction effectively (full utilization of active material) leading to better electrochemical activity and hence high peak current/specific capacitance. Since the (2:1) CoCr-LDH electrode exhibited a highest specific capacitance, its further supercapacitor properties were examined in detail.
Figure 8a shows the CV curves recorded for the (2:1) CoCr-LDH electrode in 1 M KOH in the potential range of −0.1 to 0.5 V at various scan rates. The CV curves of other compositions are shown in supplementary material (Fig. S1). It is seen that in all scan rates, redox peak appears which confirms that the supercapacitor characteristic is dominantly Faradaic in nature. With the increase of scan rate, the peak (both anodic and cathodic) currents increase which is usually observed. It was found that as scan rate increases the specific capacitance decreases (supplementary material, Fig. S2) which is also widely observed. Figure 8b shows the galvanostatic CD profiles of the (2:1) CoCr-LDH electrode recorded at different currents. In each current, the CD curves are mirror images confirming high reversibility of charging and discharging processes even at a high current of 5 Ag−1. The specific capacitance estimated from the CD curves are given in Fig. 8c. The specific capacitance decreases as the discharge current increases. It is noted that at a current of 0.5 Ag−1, the (2:1) CoCr-LDH electrode exhibited a specific capacitance of 265 Fg−1. Even at high current of 5 Ag−1, the LDH exhibited a specific capacitance of 190 Fg−1. This means that the (2:1) CoCr-LDH is capable of discharging even at high current with passable specific capacitance. The charge transport mechanism in the CoCr-LDH is governed by the following reaction:
During the fast chagrining/discharging rates, the H+ transportation in the solid matrix is limited; thereby, the ability of H+ to react with OH− is also limited. This results in low specific capacitance at high discharge rate. This has been usually observed in the batteries and supercapacitors. Thus, the low specific capacitance exhibited at high current is due to the decrease in the efficiency of utilization of active material. At low current density, the full utilization of the electroactive surface of LDH leads to high specific capacitance [30].
The cycle life of the (2:1) CoCr-LDH electrode was examined for 1000 charge cycles at a current rate of 5 Ag−1. The obtained data have been shown in Fig. 8d. The insert of Fig. 8d shows the CD curves recorded at the first three cycles and the last three cycles. It can be seen that at the first cycle, the LDH exhibited a specific capacitance of 190 Fg−1. At the 1000th cycle, the obtained specific capacitance has been as high as 150 Fg−1. This means that the LDH retained as high as 80 % of charge even at 1000th cycle. The CD profiles shown in Fig. 8d insert confirm that the CoCr-LDH is highly stable. Such a high stability could be due to well stabilized unique lamellar structure of the LDH with the presence of Cr. It should be noted that this is the first report on CoCr-LDH material for supercapacitor application. Table 2 shows the comparison of specific capacitances of several Co-based LDHs reported in the literature. It can be seen that the CoNi-LDH has the highest specific capacitance, while the other Co-based LDHs have specific capacitance in the range of 160–200 Fg−1. The CoCr-LDH showed specific capacitance of 240 Fg−1 which is better than most of the Co-based LDHs, except CoNi-LDH. The merits of the CoCr-LDH over other hydroxides or LDHs are its excellent stability in KOH and non-dissolution of metal ions in the electrolyte which are essential for developing practical device (see supplementary material about Cr health issue).
To substantiate the aforementioned potentiodynamic and galvanostatic studies, electrochemical impeadance was done on each electrodes in 1 M KOH. The obtained Nyquist plots are shown in Fig. 9. In all the cases, the Nyquist plot consists of small semicircle at high frequency region and a spike at low frequency region. While the diameter of the high-frequency region gives the electrode resistance, the observed spike at low-frequency region confirms diffusion controlled Warburg component. It is noteworthy that the magnitude of the Warburg component of the (2:1) composition is lower than that of the other samples, implying lower charge-transfer resistance. Such lower resistance would have resulted in the effective electrochemical reaction-high supercapacitor performance. Thus, the impedance data substantiate the aforementioned potentiodynamic and galvanostatic studies.
EDLC performances of RGO
The EDLC property of the RGO was initially assessed by CV studies in the potential range of −0.9 to 0.1 V at different scan rates in 1 M KOH. The obtained CV curves have been shown in Fig. 10a. A clear rectangle shape CV curves have been observed in all the scan rates. The appearance of rectangular shape CV and absence of redox peak confirm that the capacitor behaviour is mainly contributed by double layer formation (non-Faradaic). The specific capacitance obtained from CV curves have been given in supplementary material (Fig. S2). A specific capacitance of about 140 Fg−1 has been obtained at 10 mV s−1. Figure 10b shows the CD profiles of RGO recorded at different currents. It is seen that in each case, the CD profiles are almost mirror image which is characteristics of EDLCs. Figure 10c shows the variation of specific capacitance on the discharge current. As the discharge current increases, the specific capacitance decreases, as has been observed for any supercapacitor material. It is noted that a specific capacitance of 145 Fg−1 has been exhibited at 1 Ag−1. This value is in the range of specific capacitance value reported elsewhere. For example, recently, 125 Fg−1 has been reported for RGO on stainless steel mesh [31]. Figure 10d shows the cycle life data of RGO obtained at a current of 5 Ag−1 for 1000 CD cycles. The RGO exhibited specific capacitance of about 145 Fg−1 in all the cycles with retention of over 97 % efficiency even at the 1000th CD cycle. This indicates excellent stability of the RGO as EDLC material. The specific capacitances of RGO at 1st and 1000th cycles remained nearly same (99 Fg−1); no degradation has resulted. The insert of the CD profiles shown in Fig. 10d implies excellent stability. Such a high stability could be due to the highly folded curtain-like and non-agglomerated graphene sheets associated with the RGO.
Asymmetric supercapacitor device performances of CoCr-LDH and RGO
To enhance the energy density and power density of CoCr-LDH, asymmetric supercapacitor device was constructed by using the aforementioned (2:1) CoCr-LDH and RGO electrodes in a gel-type electrolyte. The gel electrolyte was prepared by mixing 1 M KOH with polyvinyl alcohol (PVA) in ratio of 2:1. Figure 11a shows the CV curves recorded for the asymmetric capacitor in the potential window of 0 to 1.6 V at different scan rates. The CV curves recorded in other potential windows are shown in supplementary materials (Fig. S3). It is evidently seen that in all scan rates, the CV curves have the Faradaic as well as non-Faradaic contributions, confirming perfect asymmetric capacitor. A specific capacitance of 120 Fg−1 has been obtained at a scan rate of 10 mVs−1. Figure 11b shows the CD profiles recorded at different currents for the asymmetric capacitor. It is seen that the CD curves are in accordance with the CV curves discussed above. An asymmetric supercapacitor device was assembled by using CoCr-LDH as positive electrode and RGO as negative electrode material, respectively. To ensure equal charge (Q+ = Q−, where Q+ and Q− are the charges of the positive and negative electrodes, respectively), the two electrode masses were balanced. The mass loading of positive electrode material and negative electrode material were calculated by the following formula [32]:
wherein, C − and C + represent the specific capacitance (Fg−1), V − and V + represent the potential window for the galvanostatic charge-discharge process and m − and m + are the mass loadings of active material on the negative and positive electrodes. Figure 11c shows the specific capacitance obtained at different current densities. The specific capacitance as high as 160 Fg−1 has been obtained at a current of 1 Ag−1. Even at a high current of 5 Ag−1, the asymmetric capacitor exhibits a specific capacitance of 80 Fg−1. This implies the excellent capability of the device to undergo discharge at high current density. The stability of asymmetric capacitor was examined for 1000 CD cycles at a current density of 2 Ag−1. The obtained data have been shown in Fig. 11d. The device loses only about 20 % of its specific capacitance up to 300 cycles. Evidently, nearly 70 % of charge has been stabilized for the device beyond 300 cycles.
The Energy density (E) and power density (P) of the asymmetric device were calculated using the following formulae [33]:
where C (= I t/V M) is specific capacitance, I is discharge current (A), t is discharge time (s), V is potential window during discharging process (1.6 V) and M is total mass of the two electrodes (4 mg). Figure 12a shows the comparison of Ragone plots obtained for the (2:1) CoCr-LDH, RGO and the asymmetric capacitor device. It is noteworthy that this is the first report on asymmetric capacitor consisting of CoCr-LDH and RGO which exhibit high energy density of 14.2 Wh kg−1 and a maximum power density of 4860 W kg−1 with a wide potential window of 0 to 1.6 V. In addition, the asymmetric capacitor exhibits higher energy density than the CoCr-LDH electrode and higher power density than the RGO. The higher energy density of the asymmetric capacitor device is due to contributions from Faradaic and non-Faradaic capacitances with high operating voltage (1.6 V). Recently, an asymmetric capacitor consists of cobalt oxide and 3D nanoporous carbon has been reported by Rahul et al. with a specific energy of 15 Wh kg−1 [34].
Two asymmetric capacitor devices were fabricated and connected in series to examine the capability of the resultant device to light an LED bulb (6.4 mW). It turned out that the asymmetric capacitor could light the LED bulb for about 5 min (shown in Fig. 12b and also Fig. S4) on one charge (4 mA for powering 5 min). Thus, the aforementioned studies showed that the CoCr-LDH in combination with the RGO could be a potential material for practical supercapacitor device. Works are under progress to make large area device as well as flexible devices using the aforementioned materials.
Conclusions
The electrodeposited amorphous CoCr-LDHs were found to be electrochemically active in 1 M KOH, exhibiting Faradaic capacitance of as high as 250 Fg−1. The high specific capacitance of the (2:1) CoCr-LDH electrode seemed to be due to the unique morphology consisting of aggregates having ultrafine grains and pores. The RGO synthesized in this work exhibited a specific capacitance of about 145 Fg−1. The asymmetric capacitor consisting of the (2:1) CoCr-LDH and the RGO electrodes resulted in excellent specific capacitance with high power density and excellent stability due to a combination of Faradaic and non-Faradaic reactions. It was demonstrated that the two asymmetric capacitors connected in series could light the LED bulb. Thus, the CoCr-LDH or the CoCr-LDH with RGO could be a potential material for practical supercapacitor application.
References
Conway BE (1999) Electrochemical supercapacitors: scientific fundamentals and technological applications. Kluwer Academic/Plenum Press, New York
Hsu YK, Chen YC, Lin YG (2012) J Electroanal Chem 673:43–47
Fang DL, Chen ZD, Liu X, Wu ZF, Zheng CH (2012) J Electrochim Acta 81:321–329
Portet C, Taberna PL, Simon P, Flahaut E, Robert CL (2005) Electrochim Acta 50:4174–4181
Li W, Chen D, Li Z, Shi Y, Wan Y, Wang G, Jiang Z, Zhao D (2007) Carbon 45:1757–1763
Lee HC, Byamba-Ochir N, Shim WG, Balathanigaimani MS, Moon H (2015) J Power Sources 275:668–674
Tao XY, Zhang XB, Zhang L, Cheng JP, Liu F, Luo JH, Luo ZQ, Geise HJ (2006) Carbon 44:1425–1428
Wen S, Jung M, Joo OS, Mho SL (2006) Curr Appl Phys 6:1012–1015
Wang Y, Shi ZQ, Huang Y, Ma YF, Wang CY, Chen MM, Chen YS (2009) J Phys Chem C 113:13103–13107
Gupta V, Kusahara T, Toyama H, Gupta S, Miura N (2007) Electrochem Commun 9:2315–2319
Cao L, Lu M, Li HL (2005) J Electrochem Soc 152:A871–A875
Ravinder Reddy N, Ramana Reddy G (2006) J Power Sources 156:700–704
Wang Z, Ma C, Wang H, Liu Z, Hao Z (2013) J Alloys Compd 552:486–491
Huang S, Zhu GN, Zhang C, Tjiu WW, Xia YY, Liu T (2012) ACS Appl Mater Interfaces 4:2242–2249
Guo X, Zhang F, Evans DG, Duan X (2010) Chem Commun 46:5197–5210
Wang J, You J, Li Z, Yang P, Jing X, Zhang M (2008) J Electroanalytical Chem 624:241–244
Wimalasiri Y, Fan R, Zhao XS, Zou L (2014) Electrochim Acta 134:127–135
Sim H, Jo C, Yu T, Lim E, Yoon S, Lee JH, Yoo J, Lee J, Lim B (2014) Chem Eur J 20:14880–14884
Woo MA, Song MS, Kim TW, Kim IY, Ju JY, Lee YS, Kim SJ, Choya JH, Hwang SJ (2011) J Mater Chem 21:4286–4292
Liu XM, Zhang YH, Zang XG, Fu SY (2004) Electrochim Acta 49:3137–3134
Lia M, Cheng JP, Wang J, Liua F, Zhang XB (2016) Electrochim Acta 206:108–115
Xu ZP, Li L, Cheng CY, Ding R, Zhou C (2013) Appl Clay Sci 74:102–108
Vinothbabu P, Elumalai P (2014) J Solid State Electrochem 19:813–820
Dixit M, Kamath PV (1995) J Power Sources 56:97–100
Song Y, Moon HS (1998) Clay Miner 33:285–296
Jaswal VS, Arora AK, Kinger M, Gupta VD, Singh J (2014) Orient J Chem 30:559–566
Vinothbabu P, Elumalai P (2014) RSC Adv 4:31219–31225
Li ZQ, Lu CJ, Xia ZP, Zhou Y, Luo Z (2007) Carbon 45:1686–1695
Zhao Y, Song X, Song Q, Yin Z (2012) Cryst Eng Comm 14:6710–6719
Karthikeyan K, Kalpana D, Amaresha S, Lee YS (2012) RSC Adv 2:12322–12328
Subramani K, Jeyakumar D, Sathish M (2014) Phys Chem Chem Phys 16:4952–4961
Hong W, Wang J, Li Z, Yang S (2015) Energy 93:435–441
Sun K, Peng H, Mu J, Ma G, Zhao G, Lei Z (2015) Ionics 21:2309–2317
Salunkhe RR, Tang J, Kamachi Y, Nakato T, Kim JH, Yamauchi Y (2015) ACS Nano 9:6288–6296
Acknowledgments
The authors acknowledge the central electrochemical research institute (CECRI), Karaikudi and central instrumentation facility (CIF) of Pondicherry University for Raman and SEM analysis. PE thanks CSIR, New Delhi, Govt. of India for the research scheme (01/2532/11/EMR-II).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiran, S.K., Padmini, M., Das, H.T. et al. Performance of asymmetric supercapacitor using CoCr-layered double hydroxide and reduced graphene-oxide. J Solid State Electrochem 21, 927–938 (2017). https://doi.org/10.1007/s10008-016-3436-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3436-8