Abstract
LiTi y Co 1-y O2 (y = 0, 0.02, 0.05, 0.1) thin films were deposited on metalized Si substrates using RF magnetron sputtering technique. The films were deposited at a substrate temperature of 250 °C with subsequent annealing at 600 °C for 3 h in controlled oxygen environment. The films exhibited predominant (003), (101), and (104) orientations representing single-phase hexagonal structure with R \( \overline{3} \) m space group. Two well-defined Raman peaks observed at 489 and 598 cm−1 corresponding to E g and A1g modes confirm the hexagonal layered structure. The grain size and grain distribution were observed using atomic force microscopy. The cyclic voltammetry studies on Pt//LiTi y Co1-y O2 film cathodes in aqueous region exhibited perfect redox peaks at expected potentials. The Pt//LiTi0.02Co0.98O2 film cathode exhibited better discharge capacity of about 65 μA h cm−2 μm−1 with good cycling stability compared to pure Pt//LiCoO2. The electrochemical impedance analysis revealed a lower charge transfer resistance in Ti doped LiCoO2 film cathode, resulting better discharge capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, Li ion rechargeable batteries are widely used power sources for many applications in portable electronic devices, electric vehicles, and in biomedical applications [1, 2]. Lithium transition metal oxides such as LiNiO2 [3, 4], LiMn2O4 [5], LiMnO2 [6], LiFePO4 [7, 8], and LiCoO2 have made significant role in Lithium ion-battery applications [9, 20]. Among these cathode materials, LiCoO2 has been recognized as one of the most promising material for large format lithium batteries because of high theoretical capacity (116 μA h cm−2 μm−1), high energy density (463 μW h cm−2 μm−1), high operating voltage (4 V), and good reversibility during the oxidation and reduction process [10, 11]. LiCoO2 adopt hexagonal layer structure with space group of R \( \overline{3} \) m comprising the lithium ions in the 3a sites, the transition metal ions in the 3b sites, and the oxygen ions in the 6c sites. The removal of lithium ions in LiCoO2 tends to destabilize the crystal structure during charging and discharging process which is due to increasing repulsion between the bare oxide layers that face each other. The LiCoO2 shows good reversibility and cycling stability due to its Co3+/Co4+ redox reaction. However, it suffers from structural instability during the long term cycling because the highly oxidizing Co4+ in the delithiated Li1-x CoO2 may oxidize the organic solvent of the electrolyte on the cathode surface, which causes a capacity fading. Recently, few efforts such as cation substitution, surface coatings etc. have been made to enhance the electrochemical and structural stability of LiCoO2. Ohzuku et al. [12] suggested the partial doping of cobalt with metal ions to improve the electrochemical performance and structural stability. The cation dopents such as magnesium [13], aluminum [14], and boron [15] have been studied previously and found to improve cycling performance, but it reduces the discharge capacity compared to the pure LiCoO2. According to Venkatraman et al. [16], increasing the valency of the dopant ion generates a large concentration of Co3+ ions and lower concentration of Co4+ ions which causes spontaneous deformation in Co3O4 phase and disrupts the structure stability and reduces the capacity. Gopukumar et al. [17] reported that the tetravalent ion doping at lower concentrations may improve the electrochemical performance and structural stability of LiCoO2. From this point of view, one can expect that the partial introduction of Ti ions in place of Co ions may lead to the presence of titanium in lithium sites since the ionic radii of the titanium (0.605 Å) is larger when compared to cobalt (0.545 Å). However, meager reports were found on modification of LiCoO2 thin films to enhance the structural stability and electrochemical performance in aqueous region. The structural stability and electrochemical properties mainly depends on the preparation techniques and process parameters. Thin films of LiCoO2 cathode have been deposited using various deposition techniques such as radio frequency (RF) magnetron sputtering [18], pulsed laser deposition [19], electron beam evaporation technique [20], sol-gel method [21], etc. Among these, RF magnetron sputtering technique is found to be a powerful and best suited technique for the deposition of LiCoO2 thin films with reasonable stoichiometry, crystallinity, and morphology by properly controlling the essential deposition parameters. In the present investigation, LiTi y Co1-y O2 (y = 0, 0.02, 0.05, 0.1) thin films were deposited on metalized Si substrates by RF magnetron sputtering technique and microstructural and electrochemical properties of pure and Ti doped LiCoO2 thin films were studied.
Experimental
Cathode preparation
LiTi y Co1-y O2 (y = 0, 0.02, 0.05, 0.1) films with a thickness of 1.2 μm were deposited on Au/Ti/SiO2/(textured) Si substrates from a 10 % Li rich, 3” pure, and mosaic LiCoO2 target. The vacuum chamber was evacuated to a base pressure of 2 × 10−6 mbar with a turbo-molecular pump backed by a rotary pump. The source to substrate distance was fixed at 5 cm. The deposition time was 90 min in O2:Ar ratio (1:9). Sputtering pressure was maintained at 5.7 × 10−3–6 × 10−3 mbar during deposition. RF power density was maintained at 2.849 W/cm2 for all depositions. The depositions were carried out at substrate temperature of 250 °C with subsequent annealing at 600 °C for 3 h in a controlled oxygen environment (2 × 10−2 mbar).
Microstructural characterizations
The structure of the deposited films was studied by a Siefert X-ray diffractometer (Siefert computerized X-ray diffractometer, model: 3003 TT) with CuK α1 radiation (λ = 1.5406 Å) source filtered by Ni film operated at a voltage of 40 KV and a current 30 mA at a scan speed of 0.03°/s in the 2θ range 10–80°. The peak positions were determined precisely using RAYFLEX-Analyze software. The Raman spectra were recorded at room temperature in the wave number region 200 to 1000 cm−1 with a Jobin Yvon (Model HR 800 UV) Raman spectrometer using an excitation wavelength of 532 nm (Nd:YAG laser). The laser poser on the sample was measured by a PM 100D console with a C-series photodiode sensor (Thorlabs GmbH). The surface morphology of the deposited films was studied by using NT-MDT Solvernext atomic force microscope. The elemental composition was carried out by energy dispersive spectroscopy (Model: Oxford instruments Inca Penta FET X3) attached with SEM (Model: EVO ma 15 manufactured by Carl Zeiss).
Electrochemical measurements
The RF-sputtered pure and Ti doped LiCoO2 films coated on metalized Si substrates were employed as working electrode (cathode), a platinum counter (anode) electrode, which acts as a reversible source and sink of lithium ions, and commercial calomel reference (Hg/Hg+) electrode by which the electrochemical analysis was calibrated in the presence of saturated Li2SO4 aqueous solution as electrolyte. The CHI 608 C (CH Instruments Inc., USA) electrochemical analyzer was used for the aqueous cell measurements. Cyclic voltametry were performed at a scan rate of 1 mV s−1 in the potential range 0.0 to 1.0 V. Chronopotentiometry studies such as charge/discharge measurements were carried out in the potential range 0.0 to 1.0 V. Electrochemical impedance spectroscopy (EIS) were also studied in the frequency range 10 mHz to 100 KHz.
Results and discussion
Microstructural studies
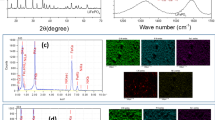
The X-ray diffraction pattern of the pure and Ti doped LiCoO2 deposited films are shown in Fig. 1. The spectra exhibited various sharp and intense peaks at 2θ = 18.9°, 37.4°, 38.2°, and 45.1° which are attributed to (003), (101), (006), and (104) planes corresponds to the α-NaFeO2 rock-salt structure with R \( \overline{3} \) m symmetry [22, 23]. From XRD results, the calculated lattice parameters and c/a ratio of LiTi y Co1-y O2 are given in the Table 1. It is observed that the average metal-metal intra-sheet distance (a hex), the average metal-metal interlayer distance (c hex), increased with increase in titanium doping concentration (as y = 0 to 0.02). It indicates well-developed layered structure and easy to migrate lithium ions. Further increasing Ti concentration (from 0.02 to 0.1), these values are observed to be decreased; it means that ion-exchange induces more cation disordering of LiCoO2 matrix.
The Raman spectroscopy has been widely used for the qualitative microstructural studies of the LiCoO2 cathodes. According to factor group analyses on the space groups R \( \overline{3} \) m and Fd3m, two (A1g + E g ) gerade modes are Raman active, and four (2A2u + 2E2u ) ungerade modes are IR active, respectively. Here, the Raman active modes A1g include only the oxygen atoms vibrate in direction parallel to the c-axis and in E g mode the oxygen atoms vibrate in perpendicular to the c-axis [24].
Figure 2 shows the Raman spectra of pure and Ti doped LiCoO2 thin films recorded in the range of 200–1000 cm−1. Pure and low concentration Ti doped LiCoO2 films exhibited two well-defined peaks located around 489 and 598 cm−1 correspond to O-Co-O bending mode (E g ) and Co-O stretching mode (A1g ) [18, 25], respectively, for various Ti doping concentrations. These results show that the deposited film possesses rock-salt like structure with R \( \overline{3} \) m symmetry which is in good agreement with the XRD studies. By increasing the Ti doping concentration above 0.05 (y ≥ 0.05), the additional Co3O4 impurity peaks were observed at 521 and 688 cm−1. These impurities obstruct the lithium ion diffusion and decrease the electron conductivity, which may greatly affect the electrochemical performance. From these microstructural analyses, it is concluded that the small amount of Ti doping is fixed as optimized condition for good structural stability and better electrochemical performance.
The grain size and grain distribution of cathode materials are also important factors for lithium ion rechargeable batteries. Figure 3 shows the two dimensional surface morphological AFM images of pure and Ti doped LiCoO2 thin films deposited on Si substrates and EDS spectrum of LiTi0.02Co0.98O2 film. From Fig. 3a–b, it is clear that the pure and low concentration Ti doped LiCoO2 films entire surface area are covered with uniformly distributed grains without any micro-cracks. The projected surface area of the film is 1 μm2. The estimated average grain size of the LiTi0.02Co0.98O2 film is about 115 nm. The average root mean square surface roughness (Rq) and arithmetic mean roughness (Ra) for LiTi0.02Co0.98O2 film was found to be 14.7 and 12.6 nm, respectively. As we know that the small grains enhance the active area of the cathode which is easy to migrate lithium ions [26]. An increase in surface roughness and grain size of the films was observed for the high concentration Ti doped LiCoO2 films which was shown in Fig. 3c, and it indicates that LiTi0.1Co0.9O2 films are inapt for cathodic films. The EDS spectrum of LiTi0.02Co0.98O2 film cathode is shown in Fig. 3d. The uniform distribution of Ti, Co, and O indicates the formation of LiTi y Co1-y O2 thin film. Lithium cannot be identified under an EDS because of its low atomic number.
Electrochemical studies
The electrochemical experiments were carried out by designing a conventional type aqueous three-electrode glass cell (Pt//LiCoO2 and Pt//LiTi0.02Co0.98O2) suffused in aqueous electrolyte. Cyclic voltammogram was employed to study the electrochemical activity of electrodes during intercalation and de-intercalation reaction. The specific discharge capacity and the cycling behavior of the prepared electrodes were determined using chronopotentiometry. Pure and Ti doped LiCoO2 positive electrodes with an active area of 0.5 cm2, a platinum (Pt) metal strip, and a saturated calomel electrode (SCE) were used as the working, counter, and reference electrodes, respectively. Though the Pt counter electrode may not be fully reversible in aqueous electrolyte during cycling experiment, it can have an active participation in the electrochemical process to meet higher discharge rates. The aqueous saturated Li2SO4 solution was used as the electrolyte. The main reason for using the saturated Li2SO4 aqueous solution is that the aqueous solution with high lithium salt concentrations processes high ionic conductivity of the electrolytes and also reduces the electrolyte/electrode interfacial resistance. The RF-sputtered LiCoO2 and Ti doped LiCoO2 thin films grown on metalized silicon substrates with subsequent post annealing at 600 °C in a controlled oxygen atmosphere were employed as positive electrodes.
The cyclic voltammogram (CV) of pure and Ti doped LiCoO2 deposited films were studied in 1 M Li2SO4 aqueous electrolyte region at a scan rate of 1 mV s−1 in a voltage range of 0.0 to 1.0 V. Figure 4a–b shows pure and low concentration Ti doped LiCoO2 film cathodes, which exhibited a single pair of perfect oxidation and reduction peaks at around 0.586 and 0.522 V indicating the lithium intercalation and de-intercalation of the host matrix. These results are in good agreement with our earlier reports on pure LiCoO2 sputtered films by Jeevan et.al [27]. The observed oxidation and reductions peaks for LiTi0.02Co0.98O2 are sharp when compared to pure LiCoO2 cathode. A small peak separation of 30 mV represents the fast kinetics of Li+ ions in the LiTi0.02Co0.98O2 films with high intercalation/de-intercalation rates. The kinetics of Li+ ion in the LiTi0.02Co0.98O2 film host structure is the key factor to understand the whole battery operation. The set of peaks associated with the Co3+/Co4+ redox couple according to the following electrochemical process:
The Li+ ion diffusion co-efficient of LiTi0.02Co0.98O2 film was calculated from the CV studies, and the average Li+ ion diffusion co-efficient in the electrochemical reaction can be obtained by the following equation [27, 28].
Where I p is the peak current, A is the surface area of the cathode, n is stoichiometric number of electrons involved in the reaction, C o is the Li+ concentration in the electrode, D L is the diffusion co-efficient of lithium ions, and υ is the scan rate. Using the above Eq. (1), the calculated Li+ diffusion co-efficients of Pt//LiCoO2 and Pt//LiTi0.02Co0.98O2 electrochemical cells were 1.8 × 10−13 and 2.21 × 10−12 cm2 s−1, respectively [27, 29]. From the above result, the diffusion of Li ions in the LiTi0.02Co0.98O2 does not bring much variation on its hexagonal framework at the film surface in the aqueous electrolyte. The cyclic voltammogram studies were also performed for Pt//LiTi0.1Co0.9O2 film cathode as shown in Fig. 4c. The CV exhibited well-defined three peaks at 0.06, 0.62, and 0.71 V during the oxidation process and two peaks during reduction process at 0.49 and 0.77 V indicating the order and disorder of Li ions from the host matrix, which is in good agreement with microstructural analysis and in consistence with the previous reports [30].
The Pt//LiCoO2 and Pt//LiTi0.02Co0.98O2 electrochemical cells were charged and discharged at a current rate of 60 μA in the voltage range of 0.0 to 1.0 V at room temperature. The charge/discharge curves of pure and low concentration Ti doped LiCoO2 films at various cycles are shown in Fig. 5. The pure Pt//LiCoO2 aqueous cell exhibited an initial discharge capacity of about 58.8 μA h cm−2 μm−1, whereas the Pt//LiTi0.02Co0.98O2 aqueous cell exhibited better discharge capacity 65 μA h cm−2 μm−1 when compared to pure Pt//LiCoO2 cell. Further higher concentration Ti doped films exhibited lower discharge capacity. This behavior can be explained as follows: the electrons produced by the oxidation reaction (Co3+ → Co4+ +1e−) probably migrate through the d orbital in the transition metal layer, and the octahedral site containing Co3+ would be surrounded by octahedral site containing Ti4+ (d°). The high concentration of Ti4+ (d°) would arrest the current path, hence the increased electronic resistance of the LiTi y Co1-y O2 (y ≥ 0.05) resulting in the capacity loss. The low electronic conductivity would be one reason for the poor performance. From this consideration, the lower capacity of LiTi y Co1-y O2 (y ≥ 0.05) film might be ascribed to the blocking of the electron conduction through the octahedral sites occupied by Ti4+ during electrochemical Li+ de-intercalation. The difficulty of the electron transport consequently led to the large resistance [31]
The cycling performance of pure and low concentration Ti doped Pt//LiCoO2 electrochemical cells at 60 μA constant current rates for 25 cycles is shown in Fig. 6. The discharge capacity of pure LiCoO2 cathode is observed to be 54.6 μA h cm−2 μm−1 after 25 cycles with a capacity retention of 93 %. Whereas, for LiTi0.02Co0.98O2 film cathode, the discharge capacity 63.6 μA h cm−2 μm−1 even after 25 cycles with a high capacity retention of 98 %. The electrochemical properties were also carried out in a non-aqueous medium using Li metal as counter electrode which exhibited good cycling stability even after 75 cycles along the good capacity retention as shown in Fig. 6. It was observed that the discharge capacity fading rate of low concentration Ti doped films is low when compared to pure LiCoO2.
The electrochemical impedance spectroscopy (EIS) was performed to understand electrode kinetics of Li ions in the films by analyzing the variation in impedances associated with the different processes during lithium intercalation/de-intercalation reaction. The impedance measurements were carried out in the frequency range from 10 mHz to 100 KHz as shown in Fig. 7. Pure and Ti doped LiCoO2 film cathodes exhibit depressed semicircles in the high frequency region and a straight line in the low frequency region. The equivalent circuit (Zview-softwere) is used to fit the experimental impedance spectrum is shown in insert of Fig. 7. In this equivalent circuit, Rs represent the Ohmic resistance of the aqueous solution, and it is almost same for the two electrodes. The charge transfer resistance is observed to be increased with higher Ti doping. The estimated charge transfer resistance from impedance spectra analysis for Pt//LiCoO2, Pt//LiTi0.02Co0.98O2, and Pt//LiTi0.1Co0.9O2 are 156, 33, and 1400 Ω, respectively. It means the charge transfer resistance (R ct) of the electrode/electrolyte interface is very high than Pt//LiTi0.02Co0.98O2, and (C dl) represent the double layer capacitance between the electrode and electrolyte, and the quasi-straight line in the low frequency is assigned to Warburg impedance (Z w) which refer to the impedance of Li+ diffusion in bulk material [32, 33]. This suggested that Ti doping can significantly increase the charge transfer rate of LiCoO2, and consequently enhance the electrochemical performance.
Conclusions
Pure and Ti doped LiCoO2 thin films were deposited on metalized Si substrates using RF magnetron sputtering technique. From XRD and Raman studies, single-phase hexagonal layered structure with R \( \overline{3} \) m symmetry was observed for pure and lower titanium doped LiCoO2 films. From AFM, the calculated grain size, RMS, and AM was 115, 14.7, and 12.6 nm, respectively, for LiTi0.02Co0.98O2 films. The LiTi0.02Co0.98O2 cathodic film exhibited excellent electrochemical performance, with an initial discharge capacity of 65 μA h cm−2 μm−1 and with a capacity retention of 98 % after 25 cycles. From EIS studies, the charge transfer resistance was decreased for lower Ti doped LiCoO2 films which helps is obtaining better electrochemical performance.
References
Quan Z, Iwase K, Sonoyama N (2011) J Power Sources 196:6762–6767
Kosuri YR, Penki TR, Nookala M, Morgen P, Gowravaram MR (2013) Adv Mater Lett 4(8):615–620
Song MY, Lee R (2002) J Power Sources 111:97–103
Sun Y, Wan P, Pan J, Xu C, Liu X (2006) Solid State Ionics 177:1173–1177
Jayanth Babu K, Jeevan Kumar P, Hussain OM, Julien CM (2012) J Solid State Electrochem 16(10):3383–3390
Ji H, Yang G, Miao X, Hong A (2010) Electrochim Acta 55:3392–3397
Rosaiah P, Jeevan Kumar P, Jayanth Babu K, Hussain OM (2013) Appl Phys A 113:603–611
Trudeau ML, Laul D, Veillette R, Serventi AM, Mauger A, Julien CM, Zaghib K (2011) J Power Sources 196:7383–7394
Jung K-T, Cho G-B, Kim K-W, Nam T-H, Jeong H-M, Huh S-C, Chung H-S, Noh J-P (2013) Thin Solid Films 546:414–417
Qiu X-Y, Zhuang Q-C, Zhang Q-Q, Cao R, Ying P-Z, Qiang Y-H, Sun S-G (2012) Phys Chem Chem Phys 14:2617–2630
Mei T, Tang K, Zhu Y, Qian Y (2011) Dalton Trans 40:7645–7650
Ohzuku T, Ueda A, Nagayama M, Iwakoshi Y, Komori H (1993) Electrochim Acta 38:1159–1167
Tukamoto H, West AR (1997) J Electrochem Soc 144:3164–3168
Jang YI, Huang B, Wang H, Sadoway DR, Ceder G, Chiang YM, Liu H, Tamura H (1999) J Electrochem Soc 146:862–868
Alcantare R, Lavela P, Tirado JL, Stoyanova R, Zhecheva E (1997) J Solid State Chem 134:265–273
Venkatraman S, Subramanian V, Gopu Kumar S, Renganathan NG, Muniyandi N (2000) Electrochem Commun 2:18–22
Gopukumar S, Jeong Y, Kim KB (2003) Solid State Ionics 159:223–232
Jeevan Kumar P, Jayanth babu K, Hussain OM (2014) Mater Chem Phys 143:536–544
Svoukis E, Athanasopoulos GI, Moradpour A, Schneegans O, Revcolevschi A, Giapintzakis J (2012) Appl Surf Sci 258:9366–9369
Kalai Vani V, Hussain OM (2007) Ionics 13:473–477
Patil V, Patil A, Choi J-W, Lee Y-P, Yoon Y, Kim H-J, Yoon S-J (2009) J Electroceram 23:214–218
Liao C-L, Lee Y-H, Yu H-C, Fung K-Z (2004) Electrochim Acta 50:461–466
Pan H, Yang Y (2009) J Power Sources 189:633–637
Baddour-Hadjean R, Pereira-Ramos J-P (2010) Chem Rev 110:1278–1319
Julien C, Camacho-Lopez MA, Escobar-Alarcon L, Haro-Poniatowski E (2001) Mater Chem Phys 68:210–216
Hwang KH, Lee SH, Joo SK (1995) J Power Sources 54:224–227
Kumar PJ, Babu KJ, Hussain OM (2012) Adv Sci Eng Med 4:190–199
Wang GJ, Qu QT, Wang B, Shi Y, Tian S, Wu YP, Holze R (2009) Electrochim Acta 54:1199–1203
Koichi N, Hideki O, Kazuhiro O, Yoshitaka M, Ichiro N, Tatsuo K (2000) Solid State Ionics 135:143–147
Kanamura K, Toriyama S, Shiraishi S, Ohashi M, Takehara Z-i (1996) J Electroanal Chem 419:77–84
Ji-Ae C, Lee SR, Won-II C, Byung-Won C (2011) Met Mater Int 17:649–654
Shenouda AY, Murali KR (2008) J Power Sources 176:332–339
Kim JH, Park CW, Sun YK (2003) Solid State Ionics 164:43–49
Acknowledgments
One of the authors, Mr. K. Sivajee Ganesh, would like to gratefully acknowledge the University Grants Commission (UGC), New Delhi, for awarding UGC – BSR fellowship in sciences for meritorious students
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivajee Ganesh, K., Purusottam Reddy, B., Jeevan Kumar, P. et al. Microstructural and electrochemical properties of LiTi y Co 1-y O2 film cathodes prepared by RF sputtering. J Solid State Electrochem 19, 3621–3627 (2015). https://doi.org/10.1007/s10008-015-2963-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-2963-z