Abstract
The gel electrolyte is an important component of the valve-regulated lead-acid (VRLA) batteries. In this study, fumed silica-based gel electrolyte systems were prepared. In this concept, several important parameters controlling the performance of the GEL-VRLA battery, such as the sulfuric acid and fumed silica concentrations, gel formulation, gelling time and rate, and different additives (Na2SO4 and MgSO4), were scientifically investigated. The gel formulations were characterized by cyclic voltammetric and electrochemical impedance spectroscopic methods. The optimum parameters were determined by using the results of anodic peak currents and redox capacities, R s and R ct values. Addition of 6 % (w/w) fumed silica to 30 % (w/w) sulfuric acid, for preparation of gelled electrolyte, increased the battery performance significantly. According to the results of the transmission electron and optic microscope images of the gel electrolytes, the three-dimensional gel structure was prepared successfully. The optimization of sulfuric acid concentration and amount of Na2SO4 and MgSO4 additives were examined for the first time in detail by cyclic voltammetry, electrochemical impedimetry, and battery test. Na2SO4 and MgSO4 additives make a good combination with a gelled-electrolyte system and improve the charge/discharge capacity according to sulfuric acid electrolytes. According to the experimental results, the fumed silica-based gel electrolyte system has a great potential for application in gelled electrolyte VRLA batteries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The valve-regulated lead-acid (VRLA) type battery is one of the best energy storage devices. Since their introduction into the market in the early 1970s, the maintenance-free, VRLA batteries have been studied extensively and widely used in large portable electrical devices and off-grid power systems [1–3].
There are two different technologies to immobilize the sulfuric acid (electrolyte) into an absorbent matter in the cells of VRLA batteries. One of them is known as absorptive glass mat (AGM), in which the electrolyte is absorbed in a separator, and the other is known as gelled electrolyte, which is obtained by mixing gelling agent and sulfuric acid [4]. When these two methods are compared, gelled electrolyte with pure silica has some advantages such as good performance at high and low temperatures [5, 6], and it is generally accepted that GEL-VRLA cells and batteries experience less electrolyte stratification than AGM-VRLA types, especially under deep-discharge cycle applications such as those experienced in remote area power supply (RAPS) operations [7–9]. Use of the gel electrolyte eliminates the numerous problems encountered in most AGM batteries, such as drainage, stratification, short circuits due to dendrites, and mostly premature capacity loss due to the release of internal cell compression [10].
Several gelling agents could be used to obtain a suitable gel structure like colloidal silica and fumed silica. Colloidal silica has a long gelling time for a good three-dimensional gel structure. However, colloidal silica has low capacity and low stability, poor thixotropy, and poor reliability under cyclic or deep-discharge condition. Gelled electrolyte which is prepared using fumed silica has a good three-dimensional web structures, good thixotrophy, and high capacity with lower internal resistance than colloidal silica-based gelled electrolyte [11–13].
Fumed silica is often used as a gel agent for GEL-VRLA batteries. Fumed silica is distributed in sulfuric acid, while the gel electrolyte is obtained. Particle size, concentration and agitation time are important factors to obtain a suitable gel structure [11]. Different gelling agents and some additives have been investigated to improve the performance and rechargeability of GEL-VRLA batteries in previous studies [6, 8, 14, 15].
In this paper, optimization of sulfuric acid concentration and optimization of the ratios of the additives were studied by cyclic voltammetric and electrochemical impedance spectroscopic methods for the first time. Different gel formulations were prepared using fumed silica, sulfuric acid, and inorganic additives (Na2SO4 and MgSO4) to achieve the best capacity and life performance. Then, performances of all the gel electrolytes were compared with battery tests according to their life cycles. Novel gelled electrolytes which contain additives are compared for performance with non-gelled and gelled electrolytes. Also, cyclic voltammetric and electrochemical impedimetric methods were performed for the first time to determine the optimum concentration of sulfuric acid and amount of Na2SO4 and MgSO4 additives.
Experimental
Preparation of electrolyte
Ten different sulfuric acid solutions (5, 10, 15, 20, 25, 30, 35, 40, 45, 50 wt%) have been prepared using concentrated sulfuric acid (95 %, Aldrich) solution to obtain optimum concentration of sulfuric acid. Appropriate amount of sulfuric acid has been determined as 30 wt% by electrochemical methods.
Fumed silica (Sigma-Aldrich, 7 nm) which has 7 nm particle size was used to prepare gel electrolyte. Several gelled electrolytes were prepared by mixing fumed silica, which have different ratios: 2, 4, 6, 8, 10, 12 % w/w; with 30 wt% sulfuric acid solutions.
Preparation of gelled electrolyte with additives
Different amounts of Na2SO4 (0.15, 0.20, 0.25, and 0.30 g) (8.1, 10.9, 13.6, and 16.3 g/L) (Sigma 99 %) and MgSO4 (0.03, 0.04, 0.05, and 0.06 g) (1.6, 2.2, 2.7, and 3.3 g/L) (Sigma 97 %) as additives were added in the solution including 6 wt% fumed silica and 30 wt% sulfuric acid.
Electrochemical Experiments
Three electrode systems were used for all cyclic voltammetric (CV) and electrochemical impedance spectroscopic measurements (EISs). Before every measurement, the working and counter electrodes were polished, and then the working electrode was polarized at −1.4 V versus Hg/Hg2SO4, K2SO4 (saturated) (mercury sulfate electrode (MSE)) to remove impurities.
Cyclic voltammetry studies were carried out with a CHI Potentiostat/Galvanostat Model 660D, at a 20 mV s−1 scan rate, between −1.4 and −0.8 V versus RE for all the gel electrolytes, using 0.5 cm2 lead working electrode, 0.6 cm2 lead counter electrode, and Hg/Hg2SO4, K2SO4 (saturated)as reference electrode. All the experiments were performed at room temperature of 25 °C.
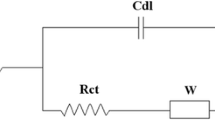
Electrochemical impedance spectroscopy experiments were performed with conventional three electrode system at open circuit potential over a 105–10−2 Hz frequency range at amplitude of 10 mV, and Fig. 1 shows an equivalent circuit model which was used at fitting process for each impedance spectrum.
Battery test
High-rate charge and discharge behaviors of the gelled electrolyte were studied with Reference 3000 series Gamry instrumentation. All battery tests were performed on one cell of a lead acid battery with two negative electrodes and one positive electrode. The dimension of each electrode was 2.5 × 3 cm. Batteries were charged and discharged with constant currents at 0.025 and 0.01 A, respectively. All experiments were done between 1.75–2.15 V.
Transmission electron microscopy (TEM)
TEM of the gel electrolytes (2, 6, and 10 % w/w fumed silica with 30 wt% sulfuric acid solutions) was performed by using FEI Company-TecnaiTM G2 Spirit/Biotwin TEM at 120 kV onto holey carbon-coated copper grids. The samples were prepared by dropping onto the grids and air-dried.
Result and discussion
Scan rate of cyclic voltammetry
Cyclic voltammetric behavior of the lead (Pb) working electrode was investigated in (30 wt%) sulfuric acid solution at different scan rates (5–100 mVs−1) to find the effect of the scan rate on the anodic peak redox capacity and current values (Fig. 2). The potential was scanned from −1.40 to −0.60 V (vs MSE). The oxidation peak at about −0.95 V (vs MSE) belongs to the formation of PbSO4 from Pb electrode. The reverse reduction process occurs with a peak potential of about −1.10 V (vs MSE). When the scan rate was increased, the anodic peak current increased and the peak redox capacity decreased. Figure 2 shows the cyclic voltammograms of sulfuric acid solution. Ideally, the difference between the peak redox capacity value and the peak current value should be as low as possible. It can be concluded that the optimum scan rate under these conditions was about 20 mV s−1. There was a considerable decrease in the redox capacity value above this scan rate.
Sulfuric acid concentration
Sulfuric acid concentration changes the discharge and charge properties of lead-acid battery [16]. The open circuit potential of a lead-acid battery cell is a function of sulfuric acid concentration according to Nernst equation. The effect of sulfuric acid concentration on the redox capacity and the current of the anodic peak have been carried out in a wide range of sulfuric acid concentrations (from 5 to 50 wt%). The peak current values were determined using cyclic voltammetry in the potential range between −1.4 and −0.6 V with a scan rate of 20 mV s−1 (Fig. 3a). The anodic peaks in the voltammograms belong to reaction of Pb to PbSO4. Pb2+ reacts with SO4 2− to form PbSO4 crystals on the electrode surface at the open circuit potentials [17]. Low sulfuric acid concentration promotes the growth of large PbSO4 crystals that are difficult to reduce [18]. The peak redox capacities and currents increased and shifted to more cathodic potentials as the concentration of sulfuric acid solution increases. The redox capacities and currents of the anodic peaks were found to increase with the increasing sulfuric acid concentrations up to 30 wt% (Fig. 3b). High concentration of sulfuric acid decreases the dissolution of PbSO4 crystals. The lower concentration of Pb2+ will then impede the subsequent electrochemical reaction from Pb2+ to Pb. Thus, PbSO4 crystals will be deposited on the surface of the electrode with increasing cycling, and the battery will fail eventually [19].There was a considerable decrease in the redox capacities and currents of the anodic peaks below and above this sulfuric acid concentration. It can be concluded that the optimum sulfuric acid concentrations under these conditions was about 30 wt%.
Sulfuric acid concentration affects the specific resistance of the electrolyte. Figure 3c represents the electrochemical impedance spectra of the sulfuric acid solutions obtained at open circuit potential. In this work, the open circuit potential of each sample is at about −0.995 V. The results can be fitted well by the equivalent circuit of Fig. 1. A high frequency semicircle and low frequency line were obtained in all Nyquist plots. Where R s shows the ohmic resistance consisting of the resistance of the corrosion products deposited on the electrode surface, the resistance of the electrolyte, and the resistance of the electrical connections to the electrode, R ct is the charge transfer resistance of the rate-controlling electrochemical reaction of corrosion process and C d is the double-layer capacitance [20]. The plots for the sulfuric acid solutions exhibit a semicircle which indicates that the charge transfer is rate-determining step [13]. The lowest radius of semicircle (Fig. 3c), ohmic resistance, and charge transfer resistance were obtained in the 30 % sulfuric acid solution (Fig. 3d) that is supported by the cyclic voltammograms (Fig. 3a).
Fumed silica concentration
The gel formation consists of creating a three-dimensional silica gel structure in which the electrolyte is immobilized. The preferred type of silica was used to be a fumed silica with an average aggregate size of only a few nanometers in diameter [10].We used two different size fumed silica (7 and 14 nm) to obtain a suitable gel structure. Because the gel formation and the capacity of the system were good, the 7 nm particle size fumed silica was chosen for the preparation of gel system. These particles have the ability to react with the sulfate ions of the electrolyte and solidify in the desired three-dimensional gel matrix [21], the hardness of gel depending on the fumed silica content. The effect of fumed silica concentration on the performance of gelled electrolyte was determined at different fumed silica concentration, in the range of 2 to 12 wt%, in 30 wt% sulfuric acid solution. To investigate the electrochemical behavior of the gel formulations, cyclic voltammetry was carried out in the gelled electrolytes (Fig. 4a). The voltammograms of gel formulations are more stable than the voltammograms of sulfuric acid solutions having different concentration of sulfuric acid (Fig. 3a) because of peak voltage of values which were changed less. As the fumed silica concentration was increased from 2 to 5 wt%, the anodic peak currents values of these electrolytes were increased (Fig. 4b), but no good gel structure was formed. When fumed silica concentration increased especially to 6 and to 8 wt%, the gelled structure was obtained. The anodic peak current of gel formulation having 6 wt% fumed silica was higher than the gel formulation having 8 wt% fumed silica. The silica content of the electrolyte should be as low as possible in order to optimize the porosity of the colloidal particles yet sufficiently high to develop and maintain a stable gel structure [7]. The higher fumed silica concentration (10–12 wt%) led to the formation of a greater silanol-bonding network, which then resulted in a faster and firmer gelled electrolyte [22].When the impedance spectra of the gel formulations were analyzed, the values of R s and R ct were found to increase with increasing amount of fumed silica (Fig. 4c). Because the polar ions are adsorbed into the gel structure, the mobility of these ions are reduced, electron transfer becomes more difficult, and the charge transfer resistance is increased (Fig. 4d). The gel formulation having 6 wt% fumed silica has lower R s and R ct values than more concentrated gel formulations because three-dimensional structure formed properly at this concentration. According to the results of cyclic voltammetry and electrochemical impedance spectroscopy, the optimum concentration of fumed silica was determined as 6 %.
Cyclic voltammetric and electrochemical impedimetric behavior of the lead working electrode in gelled electrolytes containing 30 wt% sulfuric acid with different concentration of fumed silica (2, 4, 6, 8, 10, and 12 % w/w): a cyclic voltammograms, b the anodic peak capacities and currents, c impedance spectra, and d R s and R ct values; transmission electron microscope images of gel electrolytes 2, 6, and 10 % w/w (e, f, g, respectively) fumed silica with 30 wt% sulfuric acid solutions); and h stereo microscopic photograph of gelled electrolyte
Transmission electron microscope images of gel electrolytes (2, 6, and 10 % w/w fumed silica with 30 wt% sulfuric acid solutions) were shown in Fig. 4e, f, g, respectively. The best three-dimensional structure of gel electrolyte system was obtained 6 % w/w fumed silica with 30 wt% sulfuric acid solutions (Fig. 4f). In this system, the gel electrolyte was formed by nano-sized rings. When the concentration of fumed silica was lower than 6 % w/w fumed silica, more diluted and less viscous system formed (Fig. 4e). If the fumed silica concentration is higher than 6 % w/w, the more compact and more viscous system was obtained (Fig. 4f). The ions of sulfuric acid were not organized well in this compact system.
Stereo microscopic photograph of the gel electrolyte containing 6 % fumed silica and 30 % sulfuric acid is presented in Fig. 4h. The three-dimensional gelled structure can be seen easily in this picture.
Stirring rate
The effect of stirring rate on the formation of gelled electrolyte was investigated at stirring rates of 250, 500, 1,000, and 1,400 rpm. The cyclic voltammograms and EIS results for gelled electrolytes are shown in Fig. 5. This figure shows that the peak currents and capacities were affected by the stirring rate. As the stirring rate increase up to 500 rpm, the diffusion rate of the electrolyte increases and the fumed silica nanoparticles are distributed more homogeneously to form three-dimensional gel structure. The highest anodic peak current and redox capacity and the lowest internal resistance were observed at the 500 rpm stirring rate (Fig. 5b). There was a considerable decrease in the peak current and capacity below and above the 500 rpm stirring rate. It can be concluded that the optimum stirring rate under these conditions was about 500 rpm.
Cyclic voltammetric and electrochemical impedimetric behavior of the lead working electrode in gelled electrolytes containing 6 wt% fumed silica and 30 wt% sulfuric acid with different stirring rates (250, 500, 1,000, and 1,400 rpm): a cyclic voltammograms, b the anodic peak capacities and currents, c impedance spectra, and d R s and R ct values
Figure 5c shows the EIS spectra for gelled electrolyte prepared at different stirring rates. The mobility of the ions increased in the well-organized gel structure with increasing diffusion rate, and the lowest values of R s and R ct were obtained at 500 rpm stirring rate (Fig. 5d). This result is well-adjusted with the voltammetric results.
Agitation time
The agitation time and stirring rate are important factors in the formation of suitable gel structure. These parameters play an important role in the adsorption of the electrolyte into three-dimensional gel structure. In this part, cyclic voltammetry and electrochemical impedance spectroscopy were used to determine the performance of the gelled electrolytes containing 6 wt% fumed silica and 30 wt% sulfuric acid with 500 rpm stirring rate and with different agitation times (30, 60, 90, and 120 min) at 25 °C.
Figure 6a shows the cyclic voltammograms for the lead electrode in gelled electrolytes. The peak currents and redox capacities decrease with increasing agitation time. The highest anodic peak current and anodic redox capacity were observed at the end of 30-min agitation time. Figure 6b shows that a higher or lower agitation time will lead to a sharp decrease in the peak currents and redox capacities.
Cyclic voltammetric and electrochemical impedimetric behavior of the lead working electrode in gelled electrolytes containing 6 wt% fumed silica and 30 wt% sulfuric acid with different agitation times (30, 60, 90, and 120 min): a cyclic voltammograms, b the anodic peak capacities and currents, c impedance spectra, and d R s and R ct values
The impedance spectra for gelled electrolyte prepared at a stirring rate of 500 rpm is shown in Fig. 6c. The R s and R ct values increase with increasing agitation time. The lowest R s and R ct were observed at the end of 30-min agitation time (Fig. 6d.). According to the results of cyclic voltammetry and electrochemical impedance spectrum, the optimum agitation time for stable three-dimensional gel structures containing 6 wt% fumed silica and 30 wt% sulfuric acid was determined as 30 min.
The effects of Na2SO4 and MgSO4 on the performance of gel electrolyte
Na2SO4 and MgSO4 were used as additives to increase the solubility of PbSO4 in the electrolyte and improve the lead-acid battery rechargeability. Several gel formulations were prepared using sulfuric acid (30 wt%), fumed silica (6 wt%), and different amount of Na2SO4 and MgSO4.
Na2SO4 was added to the gelled electrolyte system containing 6 wt% fumed silica and 30 wt% sulfuric acid with the amount of 0.15, 0.20, 0.25, and 0.30 g. Figure 7a shows the cyclic voltammograms of the gelled electrolyte with and without Na2SO4. The anodic peak current and redox capacity values increase with the addition of Na2SO4. This result indicates that Na2SO4 makes a good combination with gelled electrolyte system and improves the charge/discharge capacity according to sulfuric acid electrolyte. The highest values were obtained with the addition of 0.25 g Na2SO4 (Fig. 7b). The peak current and capacity values decreased below and above the 0.25 g of Na2SO4. The optimum amount of Na2SO4 was determined as 0.25 g under these conditions. When 0.25 g of Na2SO4 was added to the gel electrolyte system, high R s value and the lowest R ct value were observed (Fig. 7d). The addition of Na2SO4 reduces the resistance of PbSO4 film, then increase the conductivity ability. R ct value was the lowest because the concentration of SO4 2− is high in the gel system (Fig. 7d).
Cyclic voltammetric and electrochemical impedimetric behavior of the lead working electrode in gelled electrolytes containing 6 wt% fumed silica and 30 wt% sulfuric acid with different amounts of Na2SO4 (0.15, 0.20, 0.25, and 0.30 g): a cyclic voltammograms, b the anodic peak capacities and currents, c impedance spectra, and d R s and R ct values
A 0.03, 0.04, 0.05, and 0.06 g of MgSO4 were added to the gelled electrolyte system containing 6 wt% fumed silica and 30 wt% sulfuric acid. Figure 8a indicates the cyclic voltammograms of the gelled electrolyte with and without MgSO4. When MgSO4 was added to the gel system, the anodic peak redox capacity increased and peak currents decreased (Fig. 8b). As the concentrations of ions increased, the mobility of the ions decreased. The optimum value of MgSO4 was observed as 0.04 g. When 0.04 g of MgSO4 was added to the system, the high R s value was observed because PbSO4 occurred on the surface of lead electrode (Fig. 8d).
Cyclic voltammetric and electrochemical impedimetric behavior of the lead working electrode in gelled electrolytes containing 6 wt% fumed silica and 30 wt% sulfuric acid with different amounts of MgSO4 (0.03, 0.04, 0.05, and 0.06 g): a cyclic voltammograms, b the anodic peak capacities and currents, c impedance spectra, and d R s and R ct values
Battery tests
The charge-discharge tests were performed for gel VRLA batteries prepared with 6 % (w/w) SiO2 and 30 % sulfuric acid and Na2SO4, MgSO4 additives as 100 cycles (Fig. 9). The batteries were charged and discharged with constant currents at 0.025 and 0.01 A, respectively. All the batteries were discharged until 80 % state of charge condition during the cycling tests. In most cases, the discharge capacities of the gel VRLA batteries decreased in the first cycle and then gradually increased and remained stable throughout the remaining test period. The discharge capacity of the gel VRLA batteries increased with increasing numbers of discharge cycles. The highest discharge capacity of the gel VRLA batteries may be attributed to the better distribution of the gelled electrolyte throughout the battery cells during the first few charge/discharge cycles due to the thixotropic properties of the gelled electrolytes [7]. The distribution of nano-sized silica pores may restrict the diffusions of active species and prevent the early corrosion of positive and negative active materials. According to the results, the lowest discharge capacity values were observed for non-gelled system. The addition of Na2SO4 and MgSO4 reduced the battery performance. The gelled battery with MgSO4 additive has shown good initial capacity according to the non-gelled and gelled battery with Na2SO4 cells. However, the discharge capacities of Na2SO4 gelled battery cell increased with the increasing cycle number.
The gelled electrolyte system has the highest discharge capacity, and when the additives are added into the gelled system, the rechargeability of the electrolyte system increased. The effects of these additives in gel formulations can be explained by their structures. Because Na+ and Mg2+ ions are the hard acids, the reaction of these metals with SO4 2−, which is a hard base, occurs easier. It is a part of the charge and discharge reactions of lead acid batteries;
When the concentrations of Na+ and Mg2+ ions increase in the gelled electrolyte, PbSO4 dissolves easier because sulfate ions preferred more to react with Na+ and Mg2+ ions. If the SO4 2− ions react with these ions, the concentration of the free Pb2+ ions increase in media, and the charge reaction of battery occurs easier. In the battery tests, Na2SO4 and MgSO4 additives lead to decrease the discharge capacities when the cells are not fully discharged (80 % state of charge condition) due to the formation of HSO4 − (causes H2 and O2 evolution) and/or early corrosion of active materials (formation of PbSO4) [7, 15, 23]. The reformation of Pb layers on negative active material affected by Na+ and Mg2+ ions in deep discharge conditions.
Conclusions
The effects of the sulfuric acid and fumed silica concentrations, agitation time, stirring rate, and Na2SO4 and MgSO4 additives on the voltammetric and electrochemical impedimetric behaviors and the battery performance were investigated. The optimum parameters were determined by using the results of anodic peak currents and redox capacities, R s and R ct values. Addition of 6 % (w/w) fumed silica to 30 % (w/w) sulfuric acid, for the preparation of gelled electrolyte, increased the battery performance significantly. The three-dimensional gel structure was prepared successfully. Na2SO4 and MgSO4 additives make a good combination with gelled electrolyte system and improve the charge/discharge capacity according to sulfuric acid electrolyte. The optimization of sulfuric acid concentration and amount of Na2SO4 and MgSO4 additives were examined in detail by cyclic voltammetric and electrochemical impedimetric methods. According to the experimental results, the fumed silica-based gel electrolyte system has a great potential for application in the gelled electrolyte VRLA batteries.
References
Wagner R (2005) J Power Sources 144:494–504
Chang Y, Mao X, Zhao Y, Feng S, Chen H, Finlow D (2009) J Power Sources 191:176–183
Soria ML, Hernández JC, Valenciano J, Sánchez A, Trinidad F (2005) J Power Sources 144:473–485
Tantichanakul T, Chailapakul O, Tantavichet N (2011) J Power Sources 196:8764–8772
Martha SK, Hariprakash B, Gaffoor SA, Shukla AK (2003) Bull Mater Sci 26:465–469
Tang Z, Wang J, Mao X, Shao H, Chen Q, Xu Z, Zhang J (2007) J Power Sources 168:49–57
Lambert DWH, Greenwood PHJ, Reed MC (2002) J Power Sources 107:173–179
Tuphorn H (1992) J Power Sources 40:47–61
Newnham RH (1994) J Power Sources 52:149–153
Valerie T (2006) J Power Sources 158:1124–1132
Chen MQ, Chen HY, Shu D, Li AJ, Finlow DE (2008) J Power Sources 181:161–171
Wu L, Chen HY, Jiang X (2002) J Power Sources 107:162–166
Pan K, Shi G, Li A, Li H, Zhao R, Wang F, Zhang W, Chen Q, Chen H, Xiong Z, Finlow D (2012) J Power Sources 209:262–268
Tang Z, Wang J, Mao X, Chen Q, Shen C, Zhang J (2007) J Appl Electrochem 37:1163–1169
Hernández JC, Soria ML, González M, García-Quismondo E, Muñoz A, Trinidad F (2006) J Power Sources 162:851–863
Pavlov D, Petkov G, Rogachev T (2008) J Power Sources 175:586–594
Li H, Liu H, Wang Q, Chen H, Rend A, Hud J (2010) Electrochim Acta 56:663–666
Guo Y, Niu L, Zhang S, Chen S (2000) J Power Sources 85:38–43
Xiang J, Ding P, Zhang H, Wu X, Chen J, Yang Y (2013) J Power Sources 241:150–158
Vinod MP, Vijayamohanan K (2000) J Power Sources 89:88–92
Jones RW (1989) Fundamental principles of sol–gel technology. The Institute of Metals, London
Tantichanakul T, Chailapakul O, Tantavichet N (2013) J Ind Eng Chem 19:2085–2091
Yazda MS, Molazemia A, Moayed MH (2006) J Power Sources 158:705–709
Acknowledgments
This work was supported by the SAN-TEZ program (No. 00897.STZ.2011-1) of Ministry of Science, Industry and Technology, Republic of Turkey, with Anadolu University, and Ericsson Turkey. Y. Şahin thanks to Oktay Uysal for his support to this study. The authors would like to thank AUBIBAM for the TEM analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gençten, M., Dönmez, K.B., Şahin, Y. et al. Voltammetric and electrochemical impedimetric behavior of silica-based gel electrolyte for valve-regulated lead-acid battery. J Solid State Electrochem 18, 2469–2479 (2014). https://doi.org/10.1007/s10008-014-2507-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-014-2507-y