Abstract
Novel complex oxides Sr2Ga1+x In1−x O5, x = 0.0–0.2 with brownmillerite-type structure were prepared in air at T = 1,273 K, 24 h. Study of the crystal structure of Sr2Ga1.1In0.9O5 refined using X-ray powder diffraction data (S.G. Icmm, a = 5.9694(1) Å, b = 15.2091(3) Å, c = 5.7122(1) Å, χ 2 = 2.48, R F 2 = 0.0504, R p = 0.0458) revealed ordering of Ga3+ and In3+ cations over tetrahedral and octahedral positions, respectively. A partial replacement of Sr2+ by La3+ according to formula Sr1−y La y Ga0.5In0.5O2.5+y/2, leads to the formation of a cubic perovskite (a = 4.0291(5) Å) for y = 0.3. No ordering of oxygen vacancies or cations was observed in Sr0.7La0.3Ga0.5In0.5O2.65 as revealed by electron diffraction study. The trace diffusion coefficient (D T) of oxygen for cubic perovskite Sr0.7La0.3Ga0.5In0.5O2.65 is in the range 2.0 × 10−9–6.3 × 10−8 cm2/s with activation energy 1.4(1) eV as determined by isotopic exchange depth profile technique using secondary ion mass spectrometry at 973–1,223 K. These values are close to those reported for Ca-doped ZrO2. High-temperature electrical conductivity of Sr0.7La0.3Ga0.5In0.5O2.65 studied by AC impedance was found to be nearly independent on oxygen partial pressure. Calculated values of activation energy at T < 1,073 K for hole and oxide-ion conductivities are 0.96 and 1.10 eV, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One the modern trends in the development of solid oxide fuel cells (SOFC) is to decrease their operation temperature down to 823–1,023 K (intermediate-temperature SOFC or IT-SOFC) [1]. However, new highly efficient materials for the components of SOFC are needed for such low working temperatures. This specially concerns cathode and electrolyte materials. Some perovskite-related oxides with group 13 elements of the Periodic Table like gallium and indium exhibit high oxide-ion conductivity at moderate temperatures and are considered as promising electrolyte materials for IT-SOFC. Thus A- and B-site doped LaGaO3 is known to exhibit high oxide-ion conductivity at 873–1,073 K with ionic transport number (t O) close to 1. The highest values of σ = 0.17 and 0.08 S/cm were achieved for La0.8Sr0.2Ga0.83Mg0.17O2.815 (LSGM) at 1,073 and 973 K, respectively [2]. Ba2In2O5 with brownmillerite-type structure exhibits a phase transition to disordered perovskite at 1,198 K after which its conductivity drastically increases [3]. This disordered perovskite structure can be stabilized by A-site substitutions. Such phases exhibit the highest oxide ion conductivity for the cubic perovskite (Ba0.3Sr0.2La0.5)2In2O5.5 with σ = 0.12 S/cm at 1,073 K [4] which was tested as an electrolyte material for solid oxide fuel cells [5]. Mixed gallium–indium phases like Ba2In2−x Ga x O5 were also studied, however, they do not exhibit superior oxide-ion conductivities [6] and x > 0.2 compositions represent perovskite-related structures different from brownmillerite or ideal perovskite [6, 7].
The brownmillerite-type structure is one of the most common among oxygen-deficient perovskites with the stoichiometry A2B2O5. It contains layers of corner-shared octahedra separated by oxygen-deficient layers with tetrahedral chains (Fig. 1). Among barium-contained complex oxides of group 13 elements only Ba2In2O5 crystallizes in the brownmillerite structure. Strontium gallate Sr2Ga2O5 is unknown and Sr2In2O5 with brownmillerite structure was prepared in the form of single-crystals from a melt of SrO-In2O3 at 2,273 K and cannot be counted as a thermodynamically stable phase [8]. Recently, some of us reported on the formation of three novel phases Sr2GaScO5, Sr10Ga6Sc4O25, and SrGa0.75Sc0.25O2.5 having, depending on Ga/Sc ratio, different perovskite-related structures [9]. One of these compounds Sr2GaScO5 represents a rare example of compounds with the brownmillerite structure where full ordering of cations over tetrahedral and octahedral positions takes place exclusively due to the size factor. Thus, Ga3+ cations (rGa3+ = 0.47 Å, coordination number (CN) = 4 and rGa3+ = 0.62 Å, CN = 6 [10]) occupy the tetrahedral sites in the structure, while large Sc3+ cations (rSc3+ = 0.747 Å, CN = 6 [10]) are located in octahedral positions. Taking into account that the ionic radius of In3+ (rIn3+ = 0.8 Å, CN = 6 [10]) in octahedral coordination is in the same size range as Sc3+, one can assume the existence of brownmillerite-like Sr2GaInO5.
In the present work, we report on the synthesis and crystal structure study of novel oxide Sr2Ga1+x In1−x O5 with ordering of In3+ and Ga3+ cations on the octahedral and tetrahedral sites of the brownmillerite structure. Partial substitution of Sr2+ by La3+ according to the formula Sr1−y La y Ga0.5In0.5O2.5+y/2 leads to the formation of a cubic perovskite phase for y = 0.3. High-temperature electrical conductivity of this compound at various oxygen partial pressures was studied. Oxygen tracer diffusion in Sr0.7La0.3Ga0.5In0.5O2.65 was examined with the help of isotopic exchange depth profile (IEDP) technique using secondary ion mass spectrometry (SIMS).
Experimental
Samples of Sr2Ga1+x In1−x O5, x = 0.0–0.2, Δx = 0.05 and Sr1−y La y Ga0.5In0.5O2.5+y/2, y = 0.1–0.5, Δy = 0.1 were prepared from stoichiometric amounts of SrCO3, Ga2O3, In2O3, and La2O3 preliminary annealed at 1,273 K, 24 h. Final heat treatment was performed in air at 1,473 K, 96 h with one intermediate regrinding after 48 h.
The phase purity of the compounds was checked by X-ray powder diffraction (XRPD) recorded in Huber G670 Guinier diffractometer (CuKα1 radiation, image foil detector). The crystal structure of Sr2Ga1.1In0.9O5 was refined by Rietveld method using GSAS program package [11].
Electron diffraction (ED) patterns were recorded with a JEOL JEM2000FX TEM with a LaB6 filament, equipped with a double tilt sample holder ±∼45°, operating at 200 kV. The sample was fixed on a copper grid with holey carbon by dipping the grid through an ethanol suspension of the sample.
Dense ceramic samples of Sr0.7La0.3Ga0.5In0.5O2.65 for the SIMS and AC impedance studies were prepared by spark plasma sintering (SPS). Approximately, 2.0–2.4 g of Sr0.7La0.3Ga0.5In0.5O2.65 powders were filled into a graphite die with an inner diameter of 12 mm. The temperature was measured with an optical pyrometer focused on the surface of the graphite die and automatically regulated from 873 to 1,523 K or 1,573 K at a heating rate of 100 K/min. The temperature of 873 K was reached within 3 min via a preset heating program. The holding time was set to 5 min at the final sintering temperature. A constant uniaxial pressure of 75 MPa was applied during the whole sintering period. After sintering, a surface layer (about 0.5 mm) of the ceramic sample was removed by polishing with sandpaper and ultrasonically cleaned in acetone to remove polish residues. Phase purity of the ceramic sample was checked by XRPD. Density of the sintered ceramic samples of Sr0.7La0.3Ga0.5In0.5O2.65, as determined by pycnometry, was 97–98 % relative to X-ray density.
The oxygen diffusion coefficient (D T) and the surface exchange coefficient (k) for Sr0.7La0.3Ga0.5In0.5O2.65 were determined by IEDP technique using SIMS after isotopic exchange of 18O for 16O in the dense ceramic sample prepared by SPS. For isotopic exchange studies the pellet was cut into a rectangular bar (1.0 × 2.0 × 4.0 mm). Prior to 18O exchange annealing, samples were held at 973, 1,073, 1,173, and 1,223 K in air of normal isotopic abundance for a period of time approximately one order of magnitude longer then the tracer annealing time. This was carried out to ensure that the material was in chemical equilibrium at the desired temperature and oxygen partial pressure (in this study all annealings were carried out at a nominal oxygen partial pressure of 0.2 atm). The air was subsequently removed, and labeled oxygen (95 % enriched 18O2) was introduced. Annealing times were 62 min at 973 K, 126 min at 1,073 K, 60 min at 1,173 K, and 25 min for 1,223 K. After the isotopic exchange annealing each sample was put into epoxy. The 18O concentration profiles were determined on the polished cross-sections by scanning the surface regions 0.5 mm in length using the TOF-SIMS 5 instrument operated in imaging mode, with a 45° incidence 25 keV Bi+ primary ion beam.
Netzsch 402C dilatometer operated in air (298–1,173 K, 10 K/min) was used for the thermal expansion coefficient measurements. For this purpose oxide powders of Sr2Ga1.1In0.9O5 and Sr0.7La0.3Ga0.5In0.5O2.65 were pressed into pellets of 8 mm in diameter and 5–5.5 mm in height and annealed at 1,473 K.
AC impedance measurements were used to determine electrical conductivity of dense ceramic sample of Sr0.7La0.3Ga0.5In0.5O2.65 prepared by SPS. The sintered sample was a typically made in the shape of a rectangular bar (2.6 × 7.2 × 8.7 mm). Pt paste was printed on both sides of the sample and annealed at 1,173 K for 1 h. The impedance spectroscopy measurements were performed on Z-500P (Elins) impedance analyzer in Ar/O2 mixture ( \( {P}_{\mathrm{O}}{}_{{}_2}=1{0}^{-4}-0.21\ \mathrm{atm} \)) in the 0.01 Hz to 500 kHz frequency range from 673 to 1,273 K. The oxygen partial pressure was controlled with YSZ oxygen sensor.
Results
Crystal structure of Sr2Ga1+x In1−x O5, x = 0.0–0.2
A majority of the reflections in the XRPD pattern of Sr2GaInO5 sample prepared at 1,473 K, 96 h were indexed with a I-centered orthorhombic unit cell with a = 5.987(2) Å, b = 15.234(6) Å, c = 5.735(2) Å, therefore indicating the formation of a brownmillerite-type phase. Additional reflections (max ∼3 %) observed in the XRPD pattern of Sr2GaInO5 were referred to as an admixture of SrIn2O4. Further re-annealings of this sample did not lead to a decrease of the admixture phase. This indicates that the brownmillerite phase contains slightly less amount of indium in comparison with the nominal composition of the sample. Therefore, the samples with variable amount of indium and gallium (Sr2Ga1+x In1−x O5, x = 0.05–0.2, Δx = 0.05) were prepared in the same experimental conditions. The color of the prepared samples was yellowish. Single-phase sample was prepared for Sr2Ga1.1In0.9O5 (x = 0.1). A weak reflection with d = 3.07 Å (max ∼5 % for x = 0.2 sample), which we could not refer to known phases, was observed in the XRPD patterns of the samples with x = 0.15 and 0.20. However, unit cell parameters and volume of the brownmillerite phase presented in all studied samples change concomitantly with gallium content. This indicates the existence of a solid solution Sr2Ga1+x In1−x O5. It should be noted that we were not able to prepare Sr2In2O5 and Sr2Ga2O5 with brownmillerite structures by heating stoichiometric mixture of SrCO3 and M2O3, M = Ga, In in air at 1,473 K, 96 h.
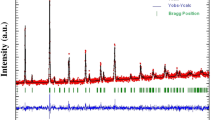
The crystal structure of brownmillerite-like Sr2Ga1.1In0.9O5 was refined using XRPD data. Since two different structural models are possible for I-centered brownmillerite, with ordered (S.G. Ibm2) or disordered (S.G. Icmm) arrangement of tetrahedral chains, they were both tested during the refinement of the crystal structure. Refinement in S.G. Icmm resulted in slightly lower R values and more reasonable interatomic distances. A summary of the refinement results, final atomic coordinates and displacement parameters for Sr2Ga1.1In0.9O5 are given in Table 1. Selected interatomic distances are present in Table 2. Observed, calculated XRPD profile intensities and their difference are shown in Fig. 2. Due to the substantial difference in scattering powers for indium and gallium (atomic number 49 and 31, respectively), it was possible to refine relative occupancies of indium and gallium in the octahedral and tetrahedral positions. It was found that the large indium cations are located only in octahedra, while the smaller gallium cations are found in tetrahedra. Bond valence sum calculations performed for the Ga tetrahedral site resulted in quite close to expected one value of 2.85. Since Sr2Ga1.1In0.9O5 contains an excess of gallium (10 %) in comparison with the amount needed to fully occupy the tetrahedral site, it was located together with indium at the octahedral site.
A study of the high-temperature expansion behavior of Sr2Ga1.1In0.9O5 in air at 298–1,273 K shows that it expands linearly with a thermal expansion coefficient (TEC) 11.8 ppm K−1. It is close to the TEC = 11.4–11.8 ppm K−1 reported for Sr and Mg-doped LaGaO3 (LSGM) [12]. No first-order phase transitions were observed in the thermal expansion curve of Sr2Ga1.1In0.9O5 in the studied temperature range. This is not surprising since a transition to disordered perovskite structure for Sr2Ga1.1In0.9O5 would involve a step with B-cation disordering, which normally happens at much higher temperatures.
Crystal structure, high-temperature electrical conductivity, and oxygen diffusion in Sr0.7La0.3Ga0.5In0.5O2.65
Preparation and crystal structure study of Sr0.7La0.3Ga0.5In0.5O2.65
Doping of Sr2GaInO5 by La3+ according to formula Sr1−y La y Ga0.5In0.5O2.5+x/2, y = 0.1–0.5, Δy = 0.1 resulted in the preparation of nearly single-phase samples only for y = 0.3. The color of the y = 0.3 sample was light brown. It should be mentioned that several re-grindings and re-heatings of the samples were necessary to achieve equilibrium, e.g., no changes in the corresponding XRPD patterns occurred after further annealing. Samples with y = 0.1 and 0.2 contain both brownmillerite and cubic perovskite (Sr,La)(In,Ga)O3−y phase, while y = 0.4 and 0.5 a cubic perovskite phase and an increasing amount of SrLaGa3O7.
A majority of the reflections in the diffraction pattern of the y = 0.3 sample were indexed with a cubic perovskite cell with a = 4.0291(5) Å (Fig. 3). Additional reflections with intensity <3 % (marked with stars in Fig. 3) were from SrIn2O4 and unidentified phase(s). No splitting of the perovskite subcell reflections was observed in the XRPD pattern of Sr0.7La0.3Ga0.5In0.5O2.65 indicating true cubic symmetry and complete disorder of cations and oxygen vacancies in the crystal structure. This result was confirmed by ED study, which showed no additional superstructure reflections on selected-area ED (SAED) patterns of Sr0.7La0.3Ga0.5In0.5O2.65 (Fig. 4).
Measurements of the thermal expansion behavior of Sr0.7La0.3Ga0.5In0.5O2.65 at 298–1,173 K in air revealed linear expansion with TEC = 12.6 ppm K−1, higher in comparison with brownmillerite-like Sr2Ga1.1In0.9O5.
Oxygen diffusion in Sr0.7La0.3Ga0.5In0.5O2.65 studied by SIMS
The oxygen diffusion in Sr0.7La0.3Ga0.5In0.5O2.65 was studied by SIMS and as an example, the 18O isotopic concentration depth profile of Sr0.7La0.3Ga0.5In0.5O2.65 annealed at 1,073 K is given in Fig. 5. The oxygen tracer diffusion (D T) and surface exchange (k) coefficients were determined by non-linear least squares regression based on fitting of the diffusion profiles using the Crank relation, solution of the Fick’s second law of the diffusion, similar to the procedure described in references [13, 14]. Calculated values of D T and k at various temperatures are given in Table 3. Dependences of bulk oxygen tracer diffusion and surface exchange coefficients on reverse temperature are given in Fig. 6. They can be perfectly fitted by Arrhenius law with activation energies (E a ) 1.4(1) eV and 1.1(1) eV for the oxygen tracer diffusion and the surface exchange coefficients, respectively. The values of the oxide-ion conductivity (σ O) presented in Table 3 were calculated from D T using the Nernst–Einstein equation
where N is the concentration of oxygen vacancies in unit cell volume, e is the electron charge, D self is oxygen self-diffusion coefficient which was considered to be equal to D T oxygen tracer diffusion coefficient, k B is the Boltzmann constant, and T is the absolute temperature.
High-temperature electrical conductivity of Sr0.7La0.3Ga0.5In0.5O2.65
AC impedance spectra at T = 1,083–1,213 K and at variable oxygen partial pressure (\( {P}_{\mathrm{O}}{}_{{}_2} \)) at 1,213 K are shown at Fig. 7a and b, respectively. The impedance spectra of Pt/Sr0.7La0.5Ga0.3In0.5O2.65/Pt system are composed of one depressed semicircular arc and can successfully be described by the equivalent circuit shown in the inset of Fig. 7b. It should be noted that it is not possible to separate bulk and grain boundary resistances for the whole studied temperature range. The equivalent circuit is a series connection of resistance (R 1) and (R 2 − CPE) element. The resistance R 1 does not depend on \( {P}_{\mathrm{O}}{}_{{}_2} \), while R 2 increases significantly with the decreasing of \( {P}_{\mathrm{O}}{}_{{}_2} \). One can conclude that resistance R 1 corresponds to the total resistance of the sample and, therefore, that the semicircular arc and the difference (R 2 − R 1) correspond to the electrode impedance and electrode polarization resistance, respectively.
AC impedance data for Pt/Sr0.7La0.3Ga0.5In0.5O2.65/Pt system at variable temperature (a) and at T = 1,213 K at variable oxygen pressure (b). The equivalent circuit used to interpret the data is shown in the inset. Experimental points are shown by symbols while dashed lines correspond to the fitted by using equivalent circuit
The temperature dependence of the total conductivity of Sr0.7La0.3Ga0.5In0.5O2.65 at variable oxygen pressure is given in Fig. 8a. The values of the conductivity are nearly independent on oxygen partial pressure as can be observed from isothermal logσ versus log \( {P}_{\mathrm{O}}{}_{{}_2} \) curves shown in Fig. 8b. The dependence of logσ versus (1/T), shown in Fig. 8a, can be fitted very satisfactorily by Arrhenius law in the whole studied temperature range with activation energy (Е а) 1.08 ± 0.01 eV.
The close to independent total conductivity of Sr0.7La0.3Ga0.5In0.5O2.65 with oxygen partial pressure indicates that oxide-ion conductivity pre-dominate at the studied experimental conditions. However, one can notice a very weak oxygen partial pressure dependence of the conductivity, reflected as a slight increase of the log σ with increasing log \( {P}_{\mathrm{O}}{}_{{}_2} \) at low temperatures (Fig. 8b). This can be explained by the creation of holes (h •) according to the following mechanism:
where V • •O is an oxygen vacancy, O xO is an oxygen atom at regular position according to Kröger–Vink notations. Therefore, the total conductivity (σ tot) of Sr0.7La0.3Ga0.5In0.5O2.65 can be expressed as a sum of oxide-ion (σo) and hole (\( {\sigma}_{{\mathrm{h}}^{\bullet }} \)) conductivities and expressed by the following Eq. [15]:
Since only \( {\sigma}_{{\mathrm{h}}^{\bullet }} \) shows a \( {P}_{\mathrm{O}}{}_{{}_2} \) dependence, Eq. 2 allows a separation values of σ O and \( {\sigma}_{{\mathrm{h}}^{\bullet }} \) at a fixed temperature. The temperature dependences of the total, hole, and oxide-ion conductivities for Sr0.7La0.3Ga0.5In0.5O2.65 are shown in Fig. 9. Below 1,073 K, the temperature dependences of the logarithm of the hole and oxide-ion conductivities versus (1/T) are linear with activation energies 0.96 and 1.10 eV, respectively. It should be noted that the value of the activation energy for oxide-ion conductivity is close to that one obtained by SIMS (1.4(1) eV). For T > 1,073 K the oxide-ion conductivity continues to increase, while the p-type conductivity saturates. This leads to an increased transport number for the oxide-ion conductivity (t O) to values close to 1 in the high-temperature region (Fig. 10). The values of the oxide-ion conductivity of Sr0.7La0.3Ga0.5In0.5O2.65 as calculated from oxygen tracer diffusion coefficients D T using Nernst–Einstein equation, see Table 3, are substantially lower in comparison with those one determined from AC impedance data (Fig. 9). Disagreement between values of oxide-ion conductivity obtained by different techniques is also reported in the literature, see, for example, reference [17].
Temperature dependence of the total, oxide-ion, and hole conductivities for Sr0.7La0.3Ga0.5In0.5O2.65. Values for the conductivity of (ZrO2)0.9(Y2O3)0.1 from reference [16] are given for the comparison. Values of oxide-ion conductivity of Sr0.7La0.3Ga0.5In0.5O2.65 calculated from oxygen tracer diffusion coefficients determined by SIMS using Nernst–Einstein equation are also present
Discussion
In spite of the thermodynamic instability of brownmillerite-type Sr2In2O5, this structure can be stabilized for the complex oxides Sr2Ga1+x In1−x O5 with x close to 0 and with the Ga3+ and In3+ cations ordered in the tetrahedral and octahedral positions, respectively. The most plausible explanation for the instability of Sr2In2O5 compared to Ba2In2O5 is the misfit between the long In-O interatomic distances (d(In-O)average = 2.01 Å), due to the large ionic radius of In3+, in the InO4 tetrahedra, and the short Sr-O distances (d(Sr-O)average = 2.70 Å) [8] for this structure type. The mismatch is obviously smaller in Ba2In2O5, where the corresponding distances are d(In-O)average = 2.05 Å and d(Ba-O)average = 2.83 Å, respectively [18]. The complete substitution of In3+ in the tetrahedra by the smaller Ga3+, as in Sr2Ga1+x In1−x O5, resolves this problem making brownmillerite structure stable. A specific feature of the brownmillerite structure (Fig. 1) is the presence of distorted MO6 octahedra with substantially elongated axial M-O bonds. The appearance of GaO4 tetrahedra resulted in an increasing axial distortion of the InO6 octahedra, which happens due to the necessity to compensate shrinking of the tetrahedra because of the much smaller size of Ga3+ cation in comparison with In3+ one. The axial distortion of InO6 in Sr2Ga1.1In0.9O5, defined as the difference between the axial and equatorial bond lengths normalized by their average is 0.12. This value is substantially larger in comparison with both Ba2In2O5 (0.08) and Sr2In2O5 (0.06).
One of the ways to create disorder in the oxygen sublattice in brownmillerite-like compounds and thereby stabilizing a cubic perovskite structure is a partial heterovalent substitution of cations resulting in an increase of the oxygen content. In the case of brownmillerite-type Sr2Ga1+x In1−x O5 it was achieved by partial replacement of Sr2+ by La3+ according to chemical formula Sr1−y La y Ga0.5In0.5O2.5+x/2. Surprisingly, such compound was only obtained for y = 0.3 and with no or very narrow homogeneity range. As expected, the introduction of additional oxygen atoms in tetrahedral layers of brownmillerite structure does result in the formation of the cubic perovskite Sr0.7La0.3Ga0.5In0.5O2.65 with complete disorder in both anion and cation sublattices. Obtained by SIMS values of the oxygen trace diffusion coefficient D T at 1,073 K and E a(D) for Sr0.7La0.3Ga0.5In0.5O2.65 (6.5 × 10−9 cm2/s and 1.4(1) eV) are close to those ones reported for Ca-doped ZrO2 (Zr0.858Ca0.142O1.858)—7.54 × 10−9 cm2/s and 1.53 eV [19]. However, D T values for typical SOFC electrolytes like YSZ (Zr0.81Y0.19O1.905) [20], (Y2O3)2(Sc2O3)9(ZrO2)89 [17], and GDC (Ce0.9Gd0.1O1.95) [20] are by one order of magnitude higher—6.2 × 10−8, 1.84 × 10−7, and 2.7 × 10−8 cm2/s (1,073 K), respectively. Moreover, LSGM (La0.8Sr0.2Ga0.8Mg0.2O2.8) shows an even higher value of D T = 4.13 × 10−7 cm2/s (1,073 K) [21]. The origin of the lower oxide-ion conductivity in Sr0.7La0.3Ga0.5In0.5O2.65 seems to be the very high preference of Ga3+ for the tetrahedral environment. One can speculate about the localization of oxygen vacancies near Ga3+ resulting in formation of GaO4 tetrahedra at the local level in Sr0.7La0.3Ga0.5In0.5O2.65. Such a local ordering of oxygen vacancies was revealed by an EXAFS study of Ba2In2−x GaxO5 [6]. Therefore, the increased activation energy of oxide-ion diffusion from InO6 octahedra to GaO4 tetrahedra may be expected. This conclusion can be supported by potential energy surface study in Ga and Sr doped Ba2In2O5 [22]. In Sr0.7La0.3Ga0.5In0.5O2.65 the situation is even more complicated due to low stability of In3+ with CN < 6 in complex oxides with small A-cations like Sr2+. It should be also noted that Ba0.3Sr0.2La0.5InO2.75 exhibits much higher value of oxygen conductivity in contrast to Sr0.7La0.3Ga0.5In0.5O2.65: 0.12 S/cm [4] and 5.0 × 10−3 S/cm at 1,073 K, respectively. The possible explanation of this difference can be larger bottleneck space for oxide-ion diffusion in the case of Ba-contained phase.
It should be noted that in comparison with barium and indium mixed oxides like Ba2In2O5 [3] or Ba4In6O13 [23], Sr0.7La0.3Ga0.5In0.5O2.65 does not show significant p-type conductivity. A similar result was obtained in reference [6], where Ba2In1.5Ga0.5O5 was found to exhibit nearly pure oxide-ion conductivity in contrast to what is observed for un-doped Ba2In2O5. The reason can be the presence of randomly distributed Ga3+ in the perovskite structure. As a result the oxide-ion transport number (Fig. 10) approaches 1 in the high-temperature region.
Conclusions
Sr2Ga1+x In1−x O5 system shows an example of the stabilization of the brownmillerite structure by B-cation size effect, due partial replacement of large In3+ cations by smaller Ga3+ ones. A study of the crystal structure of Sr2Ga1.1In0.9O5 reveals ordering of Ga3+ and In3+ cations over tetrahedral and octahedral positions, respectively. A partial replacement of Sr2+ by La3+ according to the formula Sr1−y La y Ga0.5In0.5O2.5+x/2 leads to the formation of cubic perovskite for y = 0.3. Study of high-temperature electrical transport of Sr0.7La0.3Ga0.5In0.5O2.65 reveals nearly pure oxide-ion conductivity at high temperature. However, the value of oxide-ion conductivity is about two orders of magnitude lower as compared to Ba0.3Sr0.2La0.5InO2.75 and LSGM. This is most likely due to higher oxygen vacancy concentration in Sr0.7La0.3Ga0.5In0.5O2.65 and their localization near Ga3+ cations resulting in formation of stable GaO4 tetrahedra.
References
Steele BCH (2001) J Mater Sci 36:1053–1068
Huang K, Tichy RS, Goodenough JB (1998) J Am Ceram Soc 81:2565–2575
Goodenough J, Ruez-Diaz J, Zhen Y (1990) Solid State Ionics 44:21–31
Kakinuma K, Yamamura H, Haneda H, Atake T (2002) Solid State Ionics 154–155:571–576
Kakinuma K, Arisaka T, Yamamura H, Atake T (2004) Solid State Ionics 175:139–143
Yao T, Uchimoto Y, Kinuhata M, Inagaki T, Yoshida H (2000) Solid State Ionics 132:189–198
Hashimoto T, Yoshinaga M, Nakano K, Omoto K, Sugimoto T, Tanaka M, Yashima MJ (2009) Ceram Soc Jpn 117:56–59
von Schenk R, Mueller-Buschbaum H (1973) Z Anorg Allg Chem 395:280–286
Chernov SV, Dobrovolsky YA, Istomin SY, Antipov EV, Grins J, Svensson G, Tarakina NV, Abakumov AM, Van Tendeloo G, Erikson SG, Rahman SMH (2012) Inorg Chem 51:1094–1103
Shannon RD (1976) Acta Crystallogr A32:751–767
Larson AC, Von Dreele RB (2000) General structure analysis system (GSAS). Los Alamos National Laboratory Report LA-UR-86-748; Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J Appl Cryst 34:210–213
Ullmann H, Trofimenko N, Tietz F, Stoever D, Ahmad-Khanlou A (2000) Solid State Ionics 138:79–90
Boehm E, Bassat J-M, Steil MC, Dordor P, Mauvy F, Grenier J-C (2003) Solid State Sci 5:973–981
Kilner A, De Souza RA, Fullarton IC (1996) Solid State Ionics 86–88:703–709
Song S-J, Wachsman ED, Doris SE, Balachandran UJ (2003) Electrochem Soc 150:A790–A795
Minh NQ, Takashi T (1995) Science and technology of ceramic fuel cells. Elsevier, Amsterdam
Raj ES, Atkinson A, Kilner JA (2009) Solid State Ionics 180:952–955
Berastegui P, Hull S, Garcia-Garcia FJ, Erikson S-GJ (2002) Solid State Chem 164:119–130
Simpson LA, Carter REJ (1966) Am Ceram Soc 49:139–144
Manning PS, Sirman JD, Kilner JA (1997) Solid State Ionics 93:125–132
Ishihara T, Kilner JA, Honda M, Sakai N, Yokokawa H, Takita Y (1998) Solid State Ionics 113–115:593–600
Mohn CE, Allan NL, Stølen S (2006) Solid State Ionics 177:223–228
Abakumov AM, Rossell MD, Gutnikova OY, Drozhzhin OA, Leonova LS, Dobrovolsky YA, Istomin SY, Van Tendeloo G, Antipov EV (2008) Chem Mater 20:4457–4467
Acknowledgments
This work was partially supported by Ministry of Science and Education of Russian Federation (state contract 14.740.12.1358), Russian Foundation for Basic Research (grant no. 11-08-01159a and 11-03-01225), and MSU-development Program up to 2020. This work is financially supported by the Swedish Research Council (VR) and the Baltic Sea/Visby program from the Swedish Institute. The electron microscopy facility was supported by Knut and Alice Wallenberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Istomin, S.Y., Antipov, E.V., Fedotov, Y.S. et al. Crystal structure and high-temperature electrical conductivity of novel perovskite-related gallium and indium oxides. J Solid State Electrochem 18, 1415–1423 (2014). https://doi.org/10.1007/s10008-013-2190-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2190-4