Abstract
The rod-like Na4Mn9O18 material was prepared by sol–gel method and compared with a similar material produced by solid-state method. Their electrochemical properties were examined in aqueous sodium-ion electrolyte cells. The resulting Na4Mn9O18 materials were characterized by SEM, TGA, and XRD techniques. The analysis shows that the rod-like Na4Mn9O18 has a small particle diameter ranging from 0.2 to 1 μm. The electrochemical performance was characterized by cyclic voltammetry and galvanostatic charge–discharge test in aqueous Na-ion cells with active carbon (AC) as counter electrode. The Na4Mn9O18 materials prepared at 800 °C show a high specific capacity of about 200 F g−1 at a current density of 200 mA g−1. The Na4Mn9O18/Na2SO4/AC Na-ion hybrid supercapacitor exhibits excellent cycle performance through 4,000 cycles with 84 % capacity remaining at 500 mA g−1 charge–discharge current density (an 18 C rate).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among various energy storage devices, Li-ion batteries have attracted most research attention mainly due to its high energy density [1, 2]. However, the high cost of lithium salt and safety issue have been the limiting factors for its broad applications in electric vehicles and grid storage [3, 4]. Based on the wide availability and low cost of sodium, ambient temperature sodium-based batteries have shown potential to meet green energy storage needs [5].
Much work has to be done in the field of Na-ion batteries in order to catch up with Li-ion technology, such as, to seek for new sodium-based energy storage or to improve the performance of known sodium compounds suitable for Na-ion cells [6]. The sodium manganese oxide has been considered as a promising cathode material since it was first reported in 1971 by Parant et al. [7]. The structure of sodium manganese oxides is dependent on the sodium content [8–11]. Orthorhombic-structured sodium manganese oxide, Na4Mn9O18, is made up of MnO5 square pyramids and MnO6 octahedra, the manganese ions are located in two different environment, leading to three available sites in which two sodium ions occupy the large S-shaped tunnels while another sodium ion occupies smaller tunnels [12, 13]. Na4Mn9O18 has been investigated as a cathode material for Li-ion battery [14], Na-ion battery [13, 15] and hybrid Li/Na-ion battery [16] in organic solvent electrolytes. The electrochemical performance of Na4Mn9O18 in aqueous Na-ion battery was first reported by Whitacre et al. in 2008 [17]. Those researches demonstrated specific capacity of about 100 mAh g−1 in organic electrolyte and 40 mAh g−1 in aqueous electrolyte at very low charge–discharge current densities. Whitacre et al. studied the aqueous hybrid capacitor Na4Mn9O18/AC (activated carbon) which demonstrated 1,000 cycles [18]. The work of Sauvage et al. showed that the pure Na4Mn9O18 displayed an initial capacity of about 80 mAh g−1 at a 0.1 C rate in NaClO4 (1 M)/PC, but only half of the initial capacity was retained after 50 cycles [15]. Cao et al. synthesized Na4Mn9O18 nanowires with a polymer-pyrolysis method and studied in Na-ion half cells which showed 128 mAh g−1 at 12 mA g−1 (0.1 C) [13]. From those studies, it has shown that crystallinity and morphology of the Na4Mn9O18 play a significant role in electrochemical performance. Further research needs to be conducted in order to improve the cycle and rate performance of Na4Mn9O18 as cathode material for Na-ion energy storage.
In this work, we synthesized the rod-like Na4Mn9O18 materials by sol–gel method. The electrochemical properties of the materials were measured in a three-electrode cell. The Na4Mn9O18 demonstrates improved electrochemical performance with a specific capacitance of over 200 F g−1 at a high current density of 200 mA g−1 (about 6 C). Its electrochemical properties were also tested in an aqueous hybrid capacitor cell composed of Na4Mn9O18/Na2SO4/AC (activated carbon). Long cycle life of over 4,000 times at an 18-C rate for the hybrid supercapacitor has been demonstrated.
Experimental
Material synthesis
Rod-like Na4Mn9O18 materials were synthesized through sol–gel method and compared with solid-state method. In solid-state synthesis route, Na2CO3 and Mn3O4 were used as starting materials. The starting materials were mixed at a Na/Mn molar ratio of 0.5:1 and mechanically milled for 12 h to obtain the solid-state precursor, and subsequently the precursor was heated at a ramp rate of 5 °C min−1 to 800 °C and held for 15 h in a muffle furnace and then cooled to ambient temperature. The samples were referred as S1 (sample 1). In the sol–gel synthesis approach, stoichiometric amount (at a Na/Mn molar ratio of 0.5:1) of Na(CH3COO)⋅3H2O and Mn(CH3COO)2⋅4H2O were dissolved separately in distilled water and then mixed together. A saturated aqueous solution of citric acid was then added at a molar ratio the same amount as the total metal ions. The pH of the mixture solution was adjusted to 7.0 by adding ammonium hydroxide solution. The mixture solution was then heated at 80 °C for about 4 h while stirring. The metal citrate precipitation was formed and was further dried in an oven in open air for one night at 60 °C to obtain sol–gel precursor. After drying, the precursor was heated at a ramp rate of 2 °C min−1 to 300 °C, and retained at this temperature for 10 h. It was further heated at a ramp rate of 5 °C min−1 to 750–850 °C for 2 h, then cooled to ambient temperature. The samples which retained at 750, 800, 850 °C through sol–gel method were referred to as S2, S3, S4. All chemicals were analytical grade and obtained from commercial sources without further purification.
Characterizations
The crystal structure of the samples were characterized by X-ray diffraction (XRD) method using D/Max-IIIA (Rigaku Co, Japan) with Cu Kα (λ = 1.54056 Å) as radiation source at a scanning rate 2° min−1 with 2θ in the range of 10–60. Thermogravimetric analysis (TGA) was conducted on a TGA/DSC1-1F-1100 simultaneous thermal analyzer using a heating rate of 10 °C min−1 in air. The morphologies of the samples were investigated by scanning electron microscopy (SEM, FEI Quanta 200 F).
Electrochemical measurements
The rod-like Na4Mn9O18 positive electrodes were prepared by mixing 80 wt. % active material, 15 wt. % super P-Li carbon black, and 5 wt. % polyvinylidene fluoride (PVDF) binder together with N-methyl-2-pyrrolidine as the solvent. The obtained mixture slurry was pressed onto nickel grid and then dried at 80 °C for 2 h, and finally was cut into 12-mm disc shape as electrode assembly. Activated carbon (AC) with a specific surface area of about 1,700 m2 g−1 measured by BET method was obtained from Kuraray (YP-50F), Japan, and used as received without further treatment. The AC electrode assembly was prepared in the same way as the positive electrode using AC, conductive carbon, PVDF at an 85:10:5 mass ratio.
Two- and three-electrode structure cells were constructed for electrochemical measurements. A Celgard 3501 microporous membrane was used as separator, and 1 mol L−1 Na2SO4 aqueous solution as electrolyte. Cyclic voltammetry (CV) were carried out on an IM6ex (Zahner) electrochemical workstation in three-electrode cells with platinum foil as counter electrode and saturated calomel electrode (SCE) as reference electrode. The AC electrode was used as the negative electrode (counter electrode) in two-electrode and three-electrode cells in galvanostatic charge and discharge tests. Galvanostatic charge and discharge measurements were carried out using the Land (CT2001A, China) battery test system.
Results and discussion
Thermal behavior of the precursors
A TGA analysis presents the mass change of the materials during sol–gel synthesis in comparison with solid-state reaction, as shown in Fig. 1, in which the change processes of solid-state precursor (a) and sol–gel precursor (b) are displayed. From curve “a,” a weight loss of about 6 % in the temperature range of 100–700 °C was found and the formation of products was divided into three stages. The weight loss between 50 and 110 °C could be ascribed to the evaporation of crystallization water and the weight loss in the temperature range of 300–500 °C may be due to the decomposition of Na2CO3. There was a weight gain between 600 and 750 °C which could be attributed to the Na4Mn9O18 formation process in which oxygen from air was involved in the reaction. Therefore, Na4Mn9O18 can be synthesized above 750 °C. The reaction processes are formulated as follows:
In the sol–gel reactions, the formation of the precursor can involve two main processes: one is the chelation of metal ions by citric acid, and the second is the neutralization of excess citric acid with ammonia. From curve “b” in Fig. 1, the main weight loss of about 75 % in the temperature range of 100–400 °C was found to be due to the precursor decomposition in which a large amount of H2O and CO2 was lost. It is seen that the precursor decomposition stage can be divided into three regions, a slight weight loss at around 100 °C, which could be the dehydration process similarly observed in the process of solid-state precursor. The major weight loss including two stages in the temperature range of 150–400 °C could be due to the decomposition and burning of citrates. No obvious weight loss can be observed between 750 and 850 °C, therefore, Na4Mn9O18 can be formed at this temperature region. In sol–gel method, citric acid as a chelating agent and a fuel acts as a substrate for the homogeneous distribution of the metal oxide phase. Upon calcination in air, the carbonaceous substrate is oxidized to carbon dioxide, leaving behind a finely divided oxide phase [19].
Structure characterization
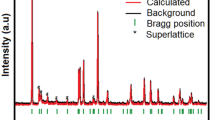
The XRD data of Na4Mn9O18 synthesized by different methods and conditions are shown in Fig. 2. The diffraction peaks of the four samples are in agreement with the JCPDF file (Pbam space group, JCPDS card no. 27–0750), indicating that Na4Mn9O18 crystallites are formed. The samples synthesized from the sol–gel method contain certain impurity phases. A diffractogram peak located at 15.8° is ascribed to the impurity of Na0.7MnO2.05 (JCPDS card no. 27–0751). It is found that S3 synthesized at 800 °C has the lowest impurity content. Optimization of the Na/Mn molar ratio of starting materials could result in purer materials. SEM analysis representing morphology of Na4Mn9O18 samples are shown in Fig. 3; it can be seen that all materials display similar rod-like crystallite morphology. The diameter of the sample synthesized from solid-state method was larger than that from the sol–gel method. The morphologies of the three samples prepared by sol–gel method were slightly different from each other. S2 burned at 750 °C does not form an integral rod-like structure. With the rise of sintering temperature the particle size increases. The diameter of the rod-like sample S3 synthesized from sol–gel method at 800 °C ranges from 0.2 to 1 μm.
Electrochemical characterization
The electrochemical behaviors of the four Na4Mn9O18 samples were characterized by CV technique using three-electrode cells at a sweep rate of 1 mV s−1 between 0 and 0.9 V vs. SCE. The CV profiles of the four samples are shown in Fig. 4 in which similar oxidation and reduction symmetrical peaks are displayed indicating that all four materials have same redox reactions with excellent reversibility in aqueous electrolyte. The curves show three main pairs of oxidation and reduction peaks at approximately 0.1, 0.34, and 0.46 V vs. SCE. Those peaks were similarly reported by Sauvage et al. for Na4Mn9O18 in an organic electrolyte [15] and Whitacre et al. for Na4Mn9O18 in aqueous electrolyte [18, 20].
Figure 5a shows the first charge and discharge curves of the Na4Mn9O18 materials collected from a three-electrode cell, with the Na4Mn9O18 of S1–S4 as working electrode and activated carbon as counter and SCE as reference electrodes. There were two obvious plateaus and one small couple of plateaus displayed on charge–discharge curves. The plateaus are also consistent with the CV data. The S1 and S3 electrodes presented higher first discharge-specific capacitance of 200 F g−1 or 44.5 mAh g−1 through a potential range of 0.8 V at a current rate of 200 mA g−1 (approximately 6 C). S2 and S4 show a relative lower specific capacitance which may be due to the fact that the S2 and S4 samples contain a small amount of impurities. The theoretical capacity of Na4Mn9O18 is 122 mAh g−1 when sodium ions are completely intercalated into Na4Mn9O18. In experiments, just a portion of sodium ions can be cycled reversibly which leads to a lower practical capacity [16, 20]. In Fig. 5b, S2 and S3 show a better cyclic behavior with a capacity retention of over 93 % through 500 cycles, while S1 and S4 only show a capacity retention of less than 80 %. S3 exhibits a higher specific capacity and retention rate, owing to the integrated rod-like morphology and proper particle size. Small changes in synthesis condition may lead to different outcomes, thus, the factors of degree of purity, composition uniformity, particle morphology, and size distribution can affect the electrochemical properties of Na4Mn9O18 material.
Two-electrode hybrid asymmetric supercapacitors were examined with S1 or S3 as positive electrode and AC as negative electrode. Since the Na4Mn9O18 material has a higher specific capacity than that of the AC material, a combination of a thicker AC laminate and a thinner Na4Mn9O18 film was adopted selecting a mass ratio of AC to Na4Mn9O18 of 2.5 in this study. Cycle stability of the hybrid system in aqueous Na2SO4 solution is displayed in Fig. 6a. In this case, over 4,000 charge–discharge cycles was imposed on the Na4Mn9O18/AC capacitor using S3 as positive electrode through a voltage window of 0–1.7 V at 500 mA g−1 (18 C) charge–discharge rate. The hybrid capacitor exhibits excellent cycle behavior. The capacity retention was 84 % through the 4,000 cycles and the coulombic efficiency was nearly 100 %. Figure 6b inset shows the 1,000th charge–discharge curves of the hybrid capacitor at a current density of 500 mA g−1 through a voltage range of 0–1.7 V. The discharge curve deviates slightly from the ideal liner shape, which indicates that the faradic reaction occurred. The discharge profile contains a very small sudden voltage drop (iR drop) which is due to the small internal resistance of the hybrid capacitor cell. The time duration of charge and discharge process is almost equal implying high coulombic efficiency in the cycles.
The power performance of the Na4Mn9O18 (S3)/AC hybrid supercapacitor was further studied, as it is shown in Table 1, specific capacitance was measured as a function of charge/discharge current density. The data of specific capacitance, energy and power density are the average value and was based on the weight of active positive electrode material. The hybrid capacitor was capable of delivering high energy and power performance. The specific energy was 34.8 Wh kg−1 at a power density of 62.0 W kg−1, and at a higher power density of 377.4 W kg−1, the specific energy was retained at 21.0 Wh kg−1 value. A specific capacity of 119.2 F g−1 can be extracted at a current density of 100 mA g−1. With the increase of current density, the capacitance value decreases, but the capacitance is still able to reach 61.1 F g−1 at a current density of 500 mA g−1 (an 18-C rate). The capacity fading at high rates may be due to the intrinsic structure of Na4Mn9O18 discussed in the “Introduction” section [12, 13]. The sluggish kinetics of Na-ion diffusion limited the rate performance: the sodium ions in small tunnels almost cannot deintercalate–intercalate at high charge–discharge current density, simultaneously, Na+ in the S-shaped tunnels also result in sluggish kinetics of the sodium ion diffusion process in the solid-state material. To improve the rate performance, methods such as carbon coating, doping, and optimization of particle size will be necessary.
Figure 7 shows the impedance spectra of the two hybrid supercapacitor with S1 and S3 positive electrodes at open circuit voltage, frequency was scanned from 105 to 10−2 Hz and an a.c. voltage oscillation of 5 mV spectra displays a small semicircle in the high frequency and followed by a straight line in the low frequency region. The straight line with a slope is related to the diffusion control process, and the semicircle part at high frequency corresponds to the charge transfer process at the electrode–electrolyte interface and is known as Faradaic resistance. As shown in Fig. 7, the impedance curves of the two systems show a similar shape, but S1 shows a smaller resistance with an electrolyte resistance of 0.84 Ω and a charge transfer resistance of 1.46 Ω. The small charge transfer resistance would give rise to high power performance of the hybrid system.
Conclusions
Rod-like Na4Mn9O18 materials were synthesized employing sol–gel method and compared with the solid-state method. The structure of the Na4Mn9O18 samples was characterized by XRD showing an orthorhombic structure. Na4Mn9O18 material with rod-like morphology shows a small particle size with a diameter ranging from 0.2 to 1 μm. This material exhibits improved electrochemical performance in aqueous electrolyte, the hybrid capacitor composed of Na4Mn9O18 as positive electrode and AC as negative electrode also show an excellent cyclic lifespan and power performance. The hybrid cell exhibits 4,000 cycles at 500 mA g−1 (an 18-C rate) with 84 % capacity remaining.
Reference
Marom R, Amalraj SF, Leifer N, Jacob D, Aurbach D (2011) J Mater Chem 21:9938–9954
Fergus JW (2010) J Power Sources 195:939–954
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-González J, Rojo T (2012) Energy Environ Sci 5:5884–5901
Kim SW, Seo DH, Ma X, Ceder G, Kang K (2012) Adv Energy Mater 2:701–721
Slater MD, Kim D, Lee E, Johnson CS (2013) Adv Funct Mater 23:947-958
Ellis BL, Nazar LF (2012) Curr Opin in Solid State Mater Sci 16:168–177
Parant JP, Olazcuaga R, Devalette M, Fouassier C, Hagenmuller P (1971) J Solid State Chem 3:1–11
Hu F, Doeff MM (2004) J Power Sources 129:296–302
Li Y, Wu Y (2009) Nano Res 2:54–60
Guenne LB, Deniard P, Biensan P, Siret C, Brec R (2000) J Mater Chem 10:2201–2206
Bach S, Pereiraramos J, Willmann P (2006) Electrochim Acta 52:504–510
Doeff MM, Ding L, De Jonghe LC (1995) MRS Online Proc Library 393:107
Cao Y, Xiao L, Wang W, Choi D, Nie Z, Yu J, Saraf LV, Yang Z, Liu J (2011) Adv Mater 23:3155–3160
Hosono E, Matsuda H, Honma I, Fujihara S, Ichihara M, Zhou H (2008) J Power Sources 182:349–352
Sauvage F, Laffont L, Tarascon JM, Baudrin E (2007) Inorg Chem 46:3289–3294
Doeff MM, Peng MY, Ma Y, De Jonghe LC (1994) J Electrochem Soc 141:L145–L147
Whitacre JF, Tevar AD, Sharma S (2008) 214th ECS Meet Abstr MA2008-02:642
Whitacre JF, Tevar A, Sharma S (2010) Electrochem Commun 12:463–466
Hao YJ, Wang L, Lai QY (2011) J Solid State Electrochem 15:1901–1907
Tevar AD, Whitacre JF (2010) J Electrochem Soc 157:A870–A875
Acknowledgments
The authors are grateful for funding from the Ministry of Science and Technology of China (863 Program) (no. 2011AA11A235).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Zhang, N., Ni, J. et al. Improved electrochemical performance of sol–gel method prepared Na4Mn9O18 in aqueous hybrid Na-ion supercapacitor. J Solid State Electrochem 17, 1939–1944 (2013). https://doi.org/10.1007/s10008-013-2044-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2044-0