Abstract
We reported a new method for the preparation of morphology-controllable LiMn2O4 particles. In this method, dimension-different MnO2 nanowires synthesized hydrothermally by adjusting the reaction temperature were used as the precursor. The morphology and structure of the resulting products were characterized with scanning electron microscope and X-ray diffraction, and the performances of the prepared LiMn2O4 samples as cathode material of lithium batteries were investigated by cyclic voltammetry and galvanostatic charge/discharge test. The results indicate that the morphology of LiMn2O4 transforms from tridimensional particle (TP) to unidimensional rod (UR) through quadrate lamina (QL) with increasing the diameter and length of MnO2 nanowires. Although the cyclic stabilities of LiMn2O4-TP, LiMn2O4-QL, and LiMn2O4-UR are very close (the 0.1 C capacity after 50 cycles is 101, 93, and 99 mAh g−1 at 25 °C, and 84, 78, and 82 mAh g−1 at 50 °C, respectively), LiMn2O4-QL delivers much higher rate capacity (about 70 mAh g−1 at 5 C and 30 mAh g−1 at 10 C) than LiMn2O4-TP and LiMn2O4-UR (about 20 mAh g−1 at 5 C, 3 mAh g−1 at 10 C, 25 mAh g−1 at 5 C, and 3 mAh g−1 at 10 C).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium ion battery has been attracting much attention for its application in the field of electric vehicles (EV) or hybrid electric vehicles (HEV) due to its high energy density and environmental benignity [1, 2]. Cost and rate performance are the main concerns for the application of lithium ion battery in EV/HEV [1]. Compared to other commercial cathode materials of lithium ion battery, such as LiCoO2 and LiCo1/3Ni1/3Mn1/3O2, spinel LiMn2O4 is the most competitive for EV/HEV because of its rich resource and easy preparation [1, 3–7]. However, the rate capability of LiMn2O4 needs to be improved for its application in EV/HEV.
The rate capability of LiMn2O4 is determined by the rapid insertion/desertion of lithium ions in LiMn2O4 matrix, which is seriously influenced by the particle size and morphology. Small particles can supply short pathway and large area for diffusion of lithium ions and thus favors delivering the rate capacity [8–10]. It has been found that the rate capacity of LiMn2O4 can be improved by designing nanoparticle size [11–15]. Kim et al. [11] synthesized LiMn2O4 nanorods growing in <110> that could deliver much higher capacity than the commercial sample. Lee et al. [12] reported that ultrathin LiMn2O4 nanowires delivered 102 mAh g−1 at 10 C and 62 mAh g−1 at 30 C with good capacity retention and structural stability in the region of 3.1 ~ 4.3 V. Similar result was obtained by Hosono et al. [13]. Luo et al. fabricated a series of LiMn2O4 with high rate performance, such as LiMn2O4 nanothorn microspheres, LiMn2O4 hollow nanospheres, and ordered mesoporous LiMn2O4 [14]. However, the reduced particle size of LiMn2O4 increases its contact area with electrolyte, which might lead to severe decomposition of electrolyte and the dissolution of manganese in LiMn2O4 [16, 17].
With the aim to obtain LiMn2O4 that has less surface area but exhibits good rate performance, dimension-different MnO2 nanowires were synthesized hydrothermally in this work by adjusting the reaction temperature and used as the precursors to induce the morphology-controllable synthesis of LiMn2O4 particles. A comparative study of resulting LiMn2O4 samples with different morphology was performed by using X-ray diffraction, scanning electron microscope, cyclic voltammetry, and charge/discharge test.
Experimental
Preparation
MnO2 precursor was firstly synthesized by a hydrothermal approach as reported by Wang et al. [18] and Kim et al. [11]. Specially, 10 g of manganese acetate (Mn(CH3COO)2 · 4H2O) and 9.4 g of ammonium persulfate ((NH4)2S2O8) were dissolved into 80 ml of distilled water under magnetic stirring to form a homogeneous solution. The resulting solution was transferred into 100 ml of Teflon-lined stainless autoclave and then heated at 120 °C for 12 h. After being cooled to room temperature, the product was filtrated, washed, and air-dried at 100 °C for 10 h. This product was denoted as MnO2-120. Two other samples were also prepared in the same process except for the hydrothermal temperature, 160 °C for MnO2-160 and 200 °C for MnO2-200.
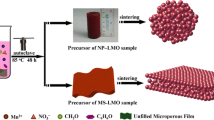
LiMn2O4 was prepared as follows. Specially, MnO2 nanowires and LiOH with the molar ratio of 2:1.05 were added into 60-ml of distilled water in order under vigorous stirring, and then the slurry was evaporated at 80 °C. The dried powder was heated to 750 °C with the heating rate of 3 °C min−1 and kept at 750 °C for 12 h. This morphology-controllable synthesis process of LiMn2O4 can be described as Scheme 1.
Characterization
Morphology of the sample was observed by scanning electron microscope (JSM-6380, Japan); X-ray diffraction was performed on a Rigaku D/max 2200 vpc diffractometer operated at 30 kV and 20 mA with Cu K α radiation. Galvanostatic charge/discharge tests were carried out on a LAND cell test (Land CT 2001A) system.
Electrochemical measurements
The electrode for electrochemical measurements was prepared as follows: 80 wt% active material (LiMn2O4), 10 wt% conductive material (acetylene black), and 10 wt% polyvinylidene fluoride binder (PVDF) were mixed in N-methyl-2-pyrrolidone. The resulting slurry was coated on an aluminum sheet and dried. The coated sheet was cut into pieces of 1 cm × 1 cm.
The electrochemical measurements were carried out on a CR2032-type coin cell with the lithium foil as the counter electrode. The electrolyte was 1 mol L−1 LiPF6 dissolved in a mixture of ethylene carbonate and dimethyl carbonate (1:1 by volume). The separator of cell was Celgard 2400. The coin cells were assembled in an Ar-filled glove box (Mikrouna, Super 1220/750/900). The assembled cells were aged overnight before testing.
Results and discussion
Morphology and structure
MnO2 is usually used as the precursor to prepare LiMn2O4 with high performance, and it has been known that morphology of MnO2 determines to some extent the morphology and performance of resultant LiMn2O4. Based on this knowledge, we try to prepare LiMn2O4 with different morphologies by adjusting the dimension of MnO2 precursor. Figure 1 presents the SEM images of the precursors and corresponding products. It can be seen from Fig. 1a, b, c that the resulting MnO2 precursors are nanowires, and their size is influenced by hydrothermal temperature, with a tendency of increased MnO2 grain size with elevating temperature. MnO2-120, MnO2-160, and MnO2-200 are 25, 45, and 75 nm in diameter and 0.5, 1.0, and 1.5 μm in length, respectively. As seen from Fig. 1d–i, the morphology of the resulting LiMn2O4 from MnO2-120, MnO2-160, and MnO2-200 is tridimensional particle (TP), quadrate lamina (QL), and unidimensional rod (UR), respectively, indicating that the morphology of LiMn2O4 can be controllable with structure-defined MnO2 precursor. Therefore, the resulting products are denoted as LiMn2O4-TP, LiMn2O4-QL, and LiMn2O4-UR, respectively. The formation mechanism of the different morphology can be explained as follows. MnO2 decomposes to Mn3O4 and O2 before lithium intercalation, and Mn3O4 expands after lithium intercalation. In the process of decomposition and expansion, MnO2 nanowires with smaller diameter are easily destroyed, thus inducing the formation of LiMn2O4 with tridimensional particles. With increasing diameter, the precursor MnO2 keeps its nanowire structure, forming LiMn2O4 particles with their size similar to rods.
The crystal structure of the resulting LiMn2O4 was determined by XRD. The XRD patterns of three products are showed in Fig. 2. It can be seen from Fig. 2 that three samples exhibit a typical feature of spinel structure with Fd3m space group [19, 20]. There are ten sharp peaks located at 18.6°, 36.2°, 37.9°, 44.0°, 48.2°, 58.2°, 64.0°, 67.3°, 75.8°, and 76.8°, corresponding to (111), (311), (222), (400), (331), (511), (440), (531), (533), and (622) planes of LiMn2O4, respectively. To obtain the lattice parameters of the three LiMn2O4 samples, XRD refinement was performed with FullProf software [21], and fitted results are showed in Fig. 2. The lattice parameter of three samples is similar; 8.231 Å for LiMn2O4-TP, 8.236 Å for LiMn2O4-QL, and 8.239 Å for LiMn2O4-UR. It has been reported that lattice parameter is tightly associated with Mn3+/Mn4+ ratio in LiMn2O4, which is important to the cyclic stability of LiMn2O4, especially at elevated temperature [22]. The similar lattice parameter suggests that three samples have similar cyclic performance, which can be confirmed by Figs. 5 and 6.
Electrochemical behavior
The electrochemical reversibility of three LiMn2O4 samples were investigated by cyclic voltammetry, and Fig. 3 shows the cyclic voltammograms of LiMn2O4-TP, LiMn2O4-QL, and LiMn2O4-UR at a scanning rate of 0.1 mV s−1. Two pairs of redox peaks can be clearly identified at around 4.0 and 4.1 V. As we know, the ratio of the cathodic peak current to anodic peak current (I pc/I pa) and the difference between cathodic peak potential and anodic peak potential (E pc − E pa) are the key indicatives of electrochemical reversibility. For LiMn2O4-TP, LiMn2O4-QL, and LiMn2O4-UR, the I pc/I pa values at 4.0 and 4.1 V are 0.93 and 0.86, 0.92 and 0.88, and 0.83 and 0.86, and the (E pc − E pa) values are 161 and 141 mV, 134 and 132 mV, and 145 and 144 mV, respectively. It is evident that LiMn2O4-QL has the minimum value of E pc − E pa and the closest value of I pc/I p to 1, implying that LiMn2O4-QL has the best electrochemical reversibility. The difference in electrochemical reversibility among three samples should be related to their different morphology.
The charge/discharge curves of LiMn2O4-TP, LiMn2O4-QL, and LiMn2O4-UR are given in Fig. 4. There are two plateaus located at 4.0 and 4.1 V, corresponding to the two pairs of redox peaks of Fig. 3, which reflect the two steps for the insertion/desertion of lithium ions in LiMn2O4 matrix [23, 24]. In the first step, lithium ions occupy half of available tetrahedral sites (8a) in spinel LiMn2O4 structure, corresponding to Li x Mn2O4 (0 < x < 0.5) in lithium-defected state. In the second step, lithium ions further occupy the remaining empty 8a sites, assigned to Li x Mn2O4 (0.5 ≤ x ≤ 1) in lithium-rich state. The repulsive interaction of lithium ions in lithium-rich state is much stronger than that in lithium-defected state, causing larger polarization for insertion/desertion of lithium ions in the second step [25]. It seems that no significant difference in charge/discharge performance can be identified among three samples at thus low rate (0.1 C).
One of problems for the use of LiMn2O4 as cathode of lithium battery is capacity decay with cycling, which results from the destruction of spinel structure due to the continuous expansion and contraction with the insertion and desertion of lithium ions [26]. The cyclic performance of our prepared LiMn2O4 samples was investigated at 0.1 C under 25 and 50 °C between 3.5 and 4.5 V. Figures 5 and 6 present the capacity variation of the samples with cycling. The 0.1 C capacity of LiMn2O4-TP, LiMn2O4-QL, and LiMn2O4-UR at 50th cycle is 101, 93, and 99 mAh g−1 at 25 °C, and 84, 78, and 82 mAh g−1 at 50 °C, respectively. The capacity decay can be observed for all the samples and becomes worse at elevated temperature that causes easily the severe dissolution of manganese and decomposition of electrolyte [17, 27]. It can be noted that there is no significant difference in the cyclic stability among three samples.
Interestingly, although three samples have similar low rate capacity and cyclic stability, their high rate performance is different. Figure 7 presents the capacity variation of three samples with rates. The significant difference in capacity can be observed at higher rates (5 and 10 C). The discharge capacity of LiMn2O4-TP at high rate is instable, decreasing from 82 to 72 mAh g−1 in 10 cycles at 1 C, and from 33 to 13 mAh g−1 in 10 cycles at 5 C. LiMn2O4-UR shows better stability at higher rate but still delivers low rate capacity; 90 mAh g−1 at first cycle and 85 mAh g−1at tenth cycle for 1C, 28 mAh g−1at first cycle and 22 mAh g−1 at tenth cycle for 5 C. Differently, LiMn2O4-QL shows good stability and rate performance; 88 mAh g−1 at first cycle and 87 mAh g−1 at tenth cycle for 1 C, 68 mAh g−1 at first cycle and 67 mAh g−1 at tenth cycle for 5 C. When the rate rises to 10 C, the capacities of LiMn2O4-TP and LiMn2O4-UR are negligible (about 3 mAh g−1), but the capacity of LiMn2O4-QL retains about 30 mAh g−1, indicative of the superior rate performance of LiMn2O4-QL. The superior rate performance of LiMn2O4-QL should be related to its good electrochemical reversibility.
Conclusions
Morphology-controllable synthesis of LiMn2O4 can be realized by using MnO2 nanowires with different dimensions as the precursor. The MnO2 nanowires can be easily obtained by adjusting the reaction temperature in a hydrothermal approach. The cyclic stability of the prepared LiMn2O4 samples with tridimensional particle, quadrate lamina, and unidimensional rod are similar at low rate (0.1 C), but LiMn2O4 with quadrate lamina exhibits better cyclic stability and discharge capacity at high rate (>1 C). The better rate performance of LiMn2O4 with quadrate lamina can be attributed to its better electrochemical reversibility.
References
Park OK, Cho Y, Lee S, Yoo HC, Song HK, Cho J (2011) Energy Environ Sci 4:1621–1633
Luo JY, Wang YG, Xiong HM, Xia YY (2007) Chem Mater 19:4791–4795
Xiong LL, Xu YL, Zhang C, Zhang ZW, Li JB (2011) J Solid State Electrochem 15:1263–1269
Qing CB, Bai Y, Yang JM, Zhang WF (2011) Electrochim Acta 56:6612–6618
Wang YP, Wang XY, Yang SY, Shu HB, Wei QL, Wu Q, Bai YS, Hu BA (2012) J Solid State Electrochem 16:2913–2920
Tan CL, Zhou HJ, Li WS, Lu DS, Hou XH, Xu MQ, Huang QM (2008) J Power Sources 184:408–413
Zeng RH, Li WS, Lu DS, Huang QM (2007) J Power Sources 174:592–597
Okubo M, Mizuno Y, Yamada H, Kim J, Hosono E, Zhou HS, Kudo T, Honma I (2010) ACS Nano 4:741–752
Lu CH, Lin SW (2001) J Power Sources 97–98:458–460
Crain D, Zheng JP, Sulyma C, Goia C, Goia D, Roy D (2012) J Solid State Electrochem 16:2605–2615
Kim DK, Muralidharan P, Lee HW, Ruffo R, Yang Y, Chan CK, Peng H, Huggins RA, Cui Y (2008) Nano Lett 8:3948–3952
Lee HW, Muralidharan P, Ruffo R, Mari CM, Cui Y, Kim DK (2010) Nano Lett 10:3852–3856
Hosono E, Kudo T, Honma I, Matsuda H, Zhou HS (2009) Nano Lett 9:1045–1051
Luo JY, Xiong HM, Xia YY (2008) J Phys Chem C 112:12051–12057
Bao SJ, Li CM, Li HL, Luong JHT (2007) J Power Sources 164:885–889
Matsuda K, Taniguchi I (2004) J Power Sources 132:156–160
Amatucci G, Pasquier AD, Blyr A, Zheng T, Tarascon JM (1999) Electrochim Acta 45:255–271
Wang X, Li YD (2002) J Am Chem Soc 124:2880–2881
Jayalakshmi M, Rao MM, Scholz F (2003) Langmuir 19:8403–8408
Shaju KM, Bruce PG (2008) Chem Mater 20:5557–5562
Rodriguez-Carvajal J (1990) In: Satellite meeting on powder diffraction of the XV Congress of the IUCr 127; International Union of Crystallography: Toulouse, France
Chung H-T, Myung S-T, Cho T-H, Son J-T (2001) J Power Sources 97–98:454–457
Liu W, Farrington GC, Chaput F, Dunn B (1996) J Electrochem Soc 143:879–884
Liu W, Wowal K, Farrington GC (1998) J Electrochem Soc 145:459–465
Lu DS, Li WS, Zuo XX, Yuan ZZ, Huang QM (2007) J Phys Chem C 111:12067–12074
Song MY, Ahn DS, Park HR (1999) J Power Sources 83:57–60
Chung KY, Lee HS, Yoon WS, McBreen J, Yang XQ (2006) J Electrochem Soc 153:A774–A780
Acknowledgments
The authors are highly grateful for the financial support from the joint project of National Natural Science Foundation of China and Natural Science Foundation of Guangdong Province (Grant No. U1134002), the National Natural Science Foundation (Grant No. 21273084), the Natural Science Fund of Guangdong Province (Grant No. 10351063101000001), and the key project of Science and Technology in Guangdong Province (Grant No. 2012A010702003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, X., Fu, Z. & Li, W. Morphology-controllable synthesis of LiMn2O4 particles as cathode materials of lithium batteries. J Solid State Electrochem 17, 1201–1206 (2013). https://doi.org/10.1007/s10008-012-1980-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1980-4