Abstract
In a novel attempt to exploit corn starch as gelling agent (in sol–gel method) and combustible fuel (in solution-assisted combustion method), high-capacity LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 cathode materials have been prepared and a comparison of electrochemical performance of the same has been made. Among the two compounds chosen for the study, LiMn1/3Ni1/3Co1/3O2 exhibits better physical and electrochemical properties. Particularly, LiMn1/3Ni1/3Co1/3O2 cathode synthesized using corn starch-assisted combustion method exhibits an appreciable capacity of 176 mAh g−1, excellent capacity retention of 93 % up to 100 cycles and susceptible to rate capability test up to 1 C rate, thus qualifying the same for high-capacity and high-rate lithium battery applications. The study demonstrates the possibility of exploiting corn starch as gelling agent and as a combustible fuel in synthesizing lithium intercalating oxide compounds with improved electrochemical behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Layered oxides with a general formula of LiMO2 (M = Mn, Co, and Ni) arguably represent themselves as one of the most successful cathode materials for rechargeable lithium batteries. Unlike geometric stabilization, wherein the structure of the native oxide is stabilized using non-‘ccp’ structure or pillars [1–3], the electronic stabilization of pristine LiMO2 oxide to arrive at LiM x Ni y Co z O2 solid solutions {x = y = z = 0.33(333) and x = y = 0.4; z = 0.2 (442)} with improved electrochemical performance has been chosen for the present study. Particularly, LiMn0.4Ni0.4Co0.2O2 (442) and LiMn1/3Ni1/3Co1/3O2 (333) compositions assume importance as they exhibit intriguing electrochemical behaviour, especially when Ni, Co and Mn adopt valence states of +2, +3 and +4, respectively [1, 4–9]. These compounds encompass advantages like high ordering of lithium and transition metals due to the presence of cobalt, and the appreciable capacity of 160–180 mAh g−1 results from that of Ni2+/Ni4+ red–ox couple. Similarly, the cost and toxicity are reduced due to the presence of manganese, which in turn addresses the issues related to structural stability upon cycling [1, 10]. In addition, LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 solid solutions are known to exhibit an admissible volume change in the range of 0 < x ≥ 0.7 [1], thus rendering enhanced electrochemical performance. Several synthesis approaches such as ceramic or acetate decomposition [11, 12], hydroxide precipitation [13], carbonate co-precipitation [14], solution combustion [15], microwave [15], inverse micro emulsion [6] and modified sol–gel [5] methods are reported for the preparation of LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds.
It is well known that synthesis procedure has an impeccable impact on cation ordering, on particle size and ultimately on the electrochemical properties [5, 16, 17] of synthesized electrode materials. Particularly, adoption of a simple and easier synthesis approach bestowed with time- and energy-saving prospects is preferred for known reasons. Towards this direction, solution-assisted sol–gel and combustion methods are preferred as they ensure uniform distribution and atomic-level mixing of reactants with the gelling agent/combustible fuel and to produce phase pure final product in the form of ultra-fine powder with reduced particle size. Again, the role of gelling agent and combustible fuel is very important as the same is responsible for the formation of transparent gel in sol–gel method and the liberation of energy to produce fluffy mass in combustion method, respectively.
Quite different from conventional approaches, a first-ever attempt to exploit corn starch that could play dual role as gelling agent in sol–gel approach and as combustible fuel in combustion method has been made due to the following reasons: Corn starch, a polysaccharide of glucose that consists of two types of polymeric chains of amylose (linear) and amylo-pectin (branched), is commonly used as a gelling agent in soap and food industries [18, 19] based on the fact that the molecular chains of corn starch unravel upon heating and colloid with each other and with precursor ions to form a gel. Considering such an advantageous segmental motion of polymeric chains in forming a gel, a novel attempt to exploit corn starch as a gelling agent in sol–gel method with acetate precursor has been made to prepare the compounds of interest, viz. LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2. Similarly, literature is replete with reports on solution-assisted combustion synthesis, wherein (rice) starch has so far been used to synthesize LiCoO2, LiMn2O4 and LiNiVO4 [20, 21] cathodes and not the compounds of interest of the present study. More interestingly, corn starch has never been exploited as a combustible component to synthesize either simple LiMO2 cathodes or LiMO2-based solid solutions to date. Hence, a novel attempt on the exploitation of corn starch as a combustible carbonaceous fuel has been made to prepare LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds, respectively.
It is believed that the amylo-pectin branched chain dominates in forming a gel with the acetate precursor in sol–gel method and the linear amylose chain reacts predominantly with the nitrate precursor of the combustion process to liberate NO x and CO x gases to form a fluffy mass with desired porosity besides the liberation of energy resulting from the breaking of N–O bonds of nitrate precursor to cook the precursor mix effectively. As a result, phase pure and nano-crystalline LiMn x Ni y Co z O2 solid solutions are obtained from corn starch-assisted gelling and combustion techniques, which are subjected to physical and electrochemical characterization studies for comparison of results and to gain better understanding of properties as a function of corn starch-assisted synthesis methods. Further, the versatile nature of corn starch in aiding sol–gel and combustion methods to prepare LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds, known for their high capacity, reversibility and structural stability, has been demonstrated in the present study.
Experimental section
All of the chemicals (analytical grade) used in the experiments were purchased from Sigma Aldrich and used as received without further purification. In all the experiments, 15 % of excess lithium (Li/M = 1.15:1) has been used to avoid lithium loss during calcination at high temperature.
In sol–gel method, a precursor mix consisting of an appropriate amount of lithium acetate, cobalt acetate, nickel acetate and manganese acetate was dissolved in water with stirring for 1 h to get a homogeneous solution. To the solution, 3 g of corn starch was added and the process of stirring and heating (~80 °C) was continued for 2 h to get a thick gel. Similarly, in combustion method (nitrate precursor-based), a precursor mix consisting of an appropriate amount of lithium nitrate, cobalt nitrate, nickel nitrate and manganese nitrate was dissolved in water with stirring for 1 h to get a homogeneous solution. To the solution, 3 g of corn starch was added and the process of stirring and heating (~80 °C) was continued for 2–3 h to get a foamy mass. The obtained gel (sol–gel method) and foamy mass (combustion method) were dried for 12 h at 120 °C and furnace-heated to 300 °C for about 12 h followed by calcination at 850 °C for about 12 h in air using alumina crucibles. The ultra-fine powders thus obtained were ground and subjected to characterization studies.
The electrodes were prepared from a combination of 80 wt.% active material with 10 wt.% super P carbon and 10 wt.% polytetrafluoroethylene binder. The chosen powders were mixed thoroughly and rolled into thin sheet on a glass plate using isopropanol. Such a thin sheet was cut in a circular shape (14 mm) and pressed over an Al mesh (3 ton of pressure). The electrodes typically had an active material content of 8–10 mg and were dried under vacuum at 80 °C for 12 h prior to assembling the cells in an argon-filled glove box [22]. Electrochemical characterizations were carried out on freshly fabricated 2032 coin cells consisting of lithium anode, LiMn0.4Ni0.4Co0.2O2 or LiMn1/3Ni1/3Co1/3O2 cathode and a non-aqueous electrolyte containing 1 M LiPF6 (dissolved in 1:1 v/v EC/DMC) with a celgard separator.
Thermal analysis of acetate- and nitrate-based metal precursors with corn starch was studied by thermogravimetry and differential thermal analysis (TG/DTA) with a thermobalance model STA 409 PC in the temperature range of 30–800 °C using alumina crucibles under air with a heating rate of 20 °C min−1. Phase characterization was done by a powder X-ray diffraction technique on a PANalytical X’pert PRO X-ray diffractometer using Ni-filtered Cu Kα radiation (λ = 1.5406 Å) in the 2θ range of 10–90° and at a scan rate of 0.04° s−1. Surface morphology of synthesized active materials was investigated using Jeol S-3000 H scanning electron microscopy (SEM), and particle size analysis was carried out on Malvern particle size analyser. Charge–discharge study at C/10 rate (for 100 cycles) and rate capability test at different current rates (C/10, C/5, C/2 and 1 C rate) were carried out in the potential range of 2.5–4.6 V using ARBIN charge–discharge cycle life tester.
Results and discussion
Thermal analysis—TG/DTA
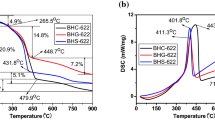
Figure 1 shows the results obtained from TG/DTA of the gels (acetate precursors) and foamy masses (nitrate precursors) corresponding to the respective precursors of LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds with corn starch. It is quite interesting to note that the thermal behaviour of sol–gel precursors corresponding to the formation of LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds is found to be identical. Likewise, the thermal behaviour of combustion precursors of 442 and 333 compounds also exhibits similarity with respect to each other, thus evidencing the fact that the thermal behaviour of each method has a major dependence on the type of precursors chosen and the same is irrespective of the type of compound being synthesized. Further, the formation temperature of LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds is found to be significantly lower for combustion method compared to sol–gel method.
Details pertinent to the thermal behaviour of acetate and nitrate precursors in sol–gel and combustion methods could be understood as follows: the acetate precursors of sol–gel method exhibit several weight loss steps corresponding to the loss of physically absorbed water (25–120 °C), decomposition of acetate precursor and corn starch (125–250 °C), and formation and dissociation of meta-stable chelating complex (380 and 450 °C, respectively) with an indication of formation of desired product viz. LiMn0.4Ni0.4Co0.2O2 at 432 °C and LiMn1/3Ni1/3Co1/3O2 at 467 °C as evident from Fig. 1. Similarly, nitrate precursors of combustion process exhibit weight loss steps corresponding to the evaporation of physically absorbed water (25–120 °C), decomposition of corn starch (200–250 °C), removal of NO x and CO x gases (250–328 °C) and completion of corn starch–nitrate combustion process (250–335 °C) to produce the final products viz. LiMn0.4Ni0.4Co0.2O2 at 328 °C and LiMn1/3Ni1/3Co1/3O2 at 335 °C, respectively.
More interestingly, the thermal behaviour of corn starch in producing mixed metal oxides such as LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 of the present study is exhibiting similarity with that of starch-assisted nitrate combustion method in preparing LiCoO2 and LiMn2O4 [20]. Hence, it is understood from TG/DTA studies that corn starch could also be exploited as an exothermic combustible fuel and as gelling agent to prepare a wide variety of lithium intercalating electrode materials with a special reference to combustion and sol–gel methods.
Phase analysis—XRD
Figure 2 shows the XRD pattern of LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds synthesized using corn starch as gelling agent (sol–gel method) and combustible fuel (combustion method), wherein all the Bragg peaks can be indexed on the α-NaFeO2 type of layered structure with R-3 m space group. Both of the compounds are found to be phase pure with the absence of cubic NiO phase at 2θ=44°, which would impede lithium ion movement due to its incompatibility with the layered structure [23]. As it is well known that the separation of (006)/(102) and (108)/(110) peaks of the hexagonal structure indicates the formation of an ordered layered structure [24], LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds of the present study are found to possess an ordered and crystalline layered structure. Also, the effect of precursors and increased cobalt content in producing an ordered layered structure could be understood from the significant splitting of peaks observed with LiMn1/3Ni1/3Co1/3O2 compound compared to that of LiMn0.4Ni0.4Co0.2O2, especially when synthesized using combustion method.
The lattice parameters a and c of the synthesized materials were calculated using unit cell software and the values are summarized in Table 1. As reported, the lattice parameter a is found to be proportional to Ni content and inversely proportional to Co content, with a constant Mn content [1, 25]. In other words, the calculated a value of LiMn1/3Ni1/3Co1/3O2 is slightly lower than that of LiMn0.4Ni0.4Co0.2O2 due to the excess concentration of cobalt in the former sample. Since the lattice parameter c is the average metal–metal interslab distance and c/a is related to trigonal distortion, a c/a value greater than 4.9 is generally preferred for a well-defined hexagonal layered structure [26, 27]. From Table 1, it is evident that the c/a ratio increases linearly with the concentration of cobalt, which in turn increases the hexagonal layeredness of LiMn1/3Ni1/3Co1/3O2 compound in a better manner than that of LiMn0.4Ni0.4Co0.2O2.
It is reported that cation mixing in layered oxides deteriorates the electrochemical performance [28, 29]. Accordingly, the intensity ratios of I (003)/I (104) (R) and (I (102) + I (006))/I (101) (R′), which are sensitive to the degree of cation mixing in lattice, have been calculated (Table 1). Basically, a higher R value (1.2) and a lower R′ value are desirable for a lower degree of cation mixing [16]. From Table 1, it is understood that an R value greater than 1.2 has been obtained for all the four compounds, which is in favour of low cation mixing [30–32]. Similarly, a lower R′ value has been obtained for combustion-derived samples, thus indicating the possibilities of better hexagonal ordering of combustion-derived products than the sol–gel-derived ones. In the light of the cited observations, it is clear that the compounds synthesized using a combination of nitrate precursor and corn starch provide the desirable layered structure than those obtained from acetate precursor and corn starch. By considering the highest R and the lowest R′ values, a combustion-synthesized LiMn1/3Ni1/3Co1/3O2 compound with increased cobalt content is expected to possess better hexagonal ordering amongst the compounds chosen for the study.
Morphology analysis—SEM
The surface morphology of 442 and 333 compounds prepared by corn starch as gelling agent (sol–gel method) and as combustible fuel (combustion method) has been investigated by scanning electron microscopy (Fig. 3). The presence of ultra-fine particles with definite grain boundaries is evident in the SEM images. In particular, closely arranged spherical grains of LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds with a particle size of ~0.6 μm have been synthesized by combustion method (as evident from Fig. 3b, d). On the other hand, aggregates consisting of slightly bigger particles of >0.8 μm are seen from the images of sol–gel-synthesized 442 and 333 compounds (Fig. 3a, c). Thus, the fluffy mass resulting from the combination of nitrate precursor and corn starch is found to possess reduced particle size which is desirable for improved electrochemical behaviour, which is in line with the results derived from XRD studies.
Particle size analysis
The particle size analysis results of mixed metal oxides synthesized using sol–gel and combustion methods are shown in Fig. 4. It is evident that the compounds derived from combustion method exhibit narrow distribution of particles corresponding to 0.7–0.9 μm (Fig. 4b, d). On the other hand, compounds synthesized using sol–gel method display a wide distribution of particles in the 0.7–1.4 μm range (Fig. 4a, c). Hence, it is understood from particle size analysis that the combination of nitrate-based metal precursors with corn starch as combustible fuel yields size-reduced particles, which are desired to facilitate facile intercalation and de-intercalation of lithium ions.
Electrochemical characterizations
In order to investigate the influence of corn starch-assisted solution synthesis methods on the electrochemical behaviour of currently prepared high-capacity cathode materials, 2032 coin cells containing synthesized LiMn0.4Ni0.4Co0.2O2 or LiMn1/3Ni1/3Co1/3O2 materials as cathode and lithium as anode were tested at 0.1 C rate between 2.5 and 4.6 V limit.
From Fig. 5a, b, it is evident that the initial capacity of combustion-derived LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 cathodes is found to be 182 and 176 mAh g−1, respectively. On the other hand, sol–gel-derived 442 and 333 cathodes suffer from sluggish lithium diffusion kinetics resulting from the slightly bigger particles and, due to the same reason, slightly reduced initial discharge capacity values of 176 and 171 mAh g−1 have been exhibited by them, respectively. Similarly, the irreversible capacity measured during the initial cycle (excluding the formation cycle) is found to be lower for combustion-synthesized LiMn0.4Ni0.4Co0.2O2 (1.8 %) and LiMn1/3Ni1/3Co1/3O2 (1.7 %) cathodes compared to those of sol–gel-synthesized 442 (3.5 %) and 333 (2.0 %) cathodes. Further, maintenance of discharge plateau at 3.8 V and the absence of plateau around 4.0 V exhibited up to 100 cycles confirm the absence of possible structural changes of layered 442 and 333 cathodes into spinel-related phases [23, 24]. Such an improved structural stabilization is attributed to the presence of Mn4+ that maintains the oxide network favourable for facile lithium intercalation/de-intercalation upon extended cycling.
A comparison of the cycling behaviour of LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 cathodes up to 100 cycles (0.1 C rate) and coulombic efficiency details are furnished in Fig. 6a. The observed discharge capacity value (Q dc) of initial, 50th and 100th cycle and the corresponding capacity fade behaviour of LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 cathodes are summarized in Table 2. It is evident from Fig. 6a and Table 2 that corn starch-assisted combustion-synthesized LiCo1/3Ni1/3Mn1/3O2 cathode has delivered a capacity as high as 176 mAh g−1 with an appreciable capacity retention and a negligible capacity fade of 3 and 7 % corresponding to 50 and 100 cycles, which is either comparable with or superior than the recent reports [2, 12, 15]. However, the same cathode, when synthesized using corn starch-assisted sol–gel method, exhibits a reduced capacity of 171 mAh g−1 with a capacity fade of 9 and 17 % after the completion of 50 and 100 cycles, respectively. On the other hand, combustion-derived LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 cathodes are found to suffer from significant capacity fade to the extent of 14–26 %, especially upon extended cycling. Further, the cycling efficiency of combustion-synthesized LiMn1/3Ni1/3Co1/3O2 cathode subjected to 100 cycles is found to be ~98 % (Fig. 6a), which is noteworthy. Based on these observations, combustion-synthesized LiMn1/3Ni1/3Co1/3O2 cathode is regarded as a superior cathode material and combustion as the better synthesis method. Such an improved electrochemical performance could be endorsed to the synergistic effect of an increased concentration of cobalt present in LiCo1/3Ni1/3Mn1/3O2 cathode and the presence of ultra-fine particles (~0.6 μm) obtained from combustion method. Hence, the role of synthesis method and the selection of compound in realizing better electrochemical behaviour could be understood.
a Discharge behaviour and coulombic efficiency of LiMn1/3Ni1/3Co1/3O2 and LiMn0.4Ni0.4Co0.2O2 cathodes at C/10 rate. b Rate capability behaviour of LiMn1/3Ni1/3Co1/3O2 and LiMn0.4Ni0.4Co0.2O2 cathodes synthesized using corn starch as a gelling agent (sol–gel method) and as combustible fuel (combustion method)
In order to investigate the suitability of corn starch-assisted sol–gel- and combustion-synthesized cathodes for high-rate lithium intercalation and de-intercalation applications, a rate capability test was performed and a comparison of the results has been made (Fig. 6b). The chosen cathodes were subjected to 0.1, 0.2, 0.5 and 1 C rate discharge conditions, wherein the effect of each rate has been studied for ten subsequent cycles. Prior to the end of the rate capability test, the cells were discharged at C/10 rate with a view to examine the extent of regaining the initial capacity, which is a measure of capacity retention behaviour, too. As expected, the rate capability and capacity retention behaviour of combustion-synthesized LiMn1/3Ni1/3Co1/3O2 cathode is found to be superior to the rest of the cathodes under the influence of varied discharge current values. Interestingly, specific capacity values of 175, 156, 148 and 122 mAh g−1 have been observed for combustion-synthesized LiMn1/3Ni1/3Co1/3O2 cathode after 10, 20, 30 and 40 cycles corresponding to 0.1, 0.2, 0.5 and 1 C rate. In other words, the discharge capacity (122 mAh g−1) exhibited by the compound at 1 C rate (or after completing 40 cycles) is found to be more than 80 % of the initial capacity of 176 mAh g−1. Further, excellent capacity retention of 93 % has been exhibited by combustion-synthesized LiMn1/3Ni1/3Co1/3O2 cathode even after the completion of 1 C rate discharge conditions, which is also superior than the rest of the cathodes considered for rate capability test. Thus, the suitability of combustion-synthesized LiMn1/3Ni1/3Co1/3O2 cathode for high-rate lithium intercalation and de-intercalation applications has been demonstrated through the current study.
Conclusion
Layered LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2 compounds have been synthesized using a novel corn starch-assisted sol–gel and combustion methods, wherein the gelling and combustible nature of corn starch with acetate and nitrate precursors, respectively, has been exploited for the formation of phase pure and crystalline products with desired physical as well as electrochemical properties. From the series of compounds synthesized in the current study, LiCo1/3Mn1/3Ni1/3O2 obtained from corn starch-assisted combustion method with a narrow distribution of particles (~0.6 μm) has delivered an appreciable discharge capacity of 176 mAh g−1, better capacity retention (93 %) up to 100 cycles and an appreciable rate capability behaviour at 1 C rate, which is superior compared to the rest of the cathodes. This study demonstrates the feasibility of deploying corn starch as a gelling agent and as combustible fuel in sol–gel and combustion methods to prepare high-capacity mixed oxide cathode materials such as LiMn0.4Ni0.4Co0.2O2 and LiMn1/3Ni1/3Co1/3O2. Further, this study recommends that corn starch-assisted combustion-synthesized LiMn1/3Ni1/3Co1/3O2 cathode with improved electrochemical behaviour could be considered favourably for high-capacity and high-rate lithium battery applications.
References
Ngala JK, Chernova NA, Ma M, Mamak M, Zavalij PY, Wittingham MS (2004) J Mater Chem 14:214–220
Doeff MM, Peng MY, Ma Y, Visco SJ and DeJonghe LC (1996) US Pat 558961
Armstrong AR, Huang H, Jennings RA, Bruce PG (1998) J Mater Chem 8:255–259
Ohzuku T, Makimura Y (2001) Chem Lett 8:744–745
Huang ZD, Liu XM, Oh SW, Zhang B, Ma PC, Kim JK (2011) J Mater Chem 21:10777–10784
Sinha NN, Munichandraiah N (2009) ACS Appl Mater Interfaces 6:1241–1249
Luo W, Li X, Dahn JR (2010) Chem Mater 22:5065–5073
Wu F, Wang M, Su Y, Chen S (2009) J Power Sources 189:743–747
Yang S, Song Y, Ngala K, Zavalij PY, Whittingham MS (2003) J Power Sources 119:239–246
Kim JM, Chung HT (2004) Electrochim Acta 49:937–944
Yabuuchi N, Ohzuku T (2003) J Power Sources 119:171–174
Guo J, Jiao LF, Yuan HT, Li HX, Zhang M, Wang YM (2006) Electrochim Acta 51:3731–3735
Shaju KM, Subba Rao GV, Chowdari BVR (2002) Electrochim Acta 48:145–151
Cho TH, Park SM, Yoshia M, Hirai T, Hideshima Y (2005) J Power Sources 142:306–312
Fey GTK, Chang CS, Prem Kumar T (2010) J Solid State Electrochem 14:17–26
Zhang CF, Yang P, Dai X, Xiong X, Zhan J, Zhang YL (2009) Trans Nonferrous Met Soc Chaina 19:635–641
He P, Wang HR, Qi L, Osaka T (2006) J Power Sources 160:627–632
Lacerda LG, da Carnvatho S, Flho MA, Demiate IM, Bannnach G, Ionashiro M, Schnitzler E (2008) J Therm Anal Calorim 93:445–449
Games ME, Sikavitas VI, Behravesh E, Reis RL, Mikos AG (2003) J Biomed Mate Res Part A 67:87–95
Kalyani P, Kalaiselvi N, Muniyandi N (2002) J Power Sources 111:232–238
Kalyani P (2009) Int J Electrochem Sci 4:30–42
Gangulibabu, Bhuvaneswari D, Kalaiselvi N, Jayaprakash N and Periasamy P (2009) J Sol-Gel Sci Technol 49:137-144
Zhang L, Wang X, Muta T, Li D, Noguchi H, Yoshio M, Ma R, Takada K, Sasaki T (2006) J Power Sources 162:629–635
Wang J, Yao X, Zhou X, Liu Z (2011) J Mater Chem 21:2544–2549
Macneil DD, Lu Z, Dahn JR (2002) J Electrochem Soc 149:A1332–A1336
Carley AF, Jackson SD, O’Shea JN, Roberts MW (1999) Surf Sci 440:L868–L874
Sathiya M, Prakash AS, Ramesha K, Shukla AK (2009) Mater Res Bull 44:1990–1994
Paulsen JM, Thomas CL, Dahn JR (2000) J Electrochem Soc 147:861–868
Peres JP, Delmas C, Rougier A, Broussels M, Perton F, Biensan P, Willmann P (1996) J Phys Chem Solids 57:1057–1060
Reale P, Privitera D, Panero S, Scrosati B (2007) Solid State Ionics 178:1390–1397
Wangm LQ, Jiao LF, Yuan HT, Guo J, Zhao M, Li HX, Wang YM (2006) J Power Sources 162:1367–1372
Idemoto Y, Matsui T (2008) Solid State Ionics 179:625–635
Acknowledgments
Among the authors, Gangulibabu and D. Bhuvaneswari are thankful to the Council of Scientific & Industrial Research (CSIR) for the financial support through senior research fellowship. N. Kalaiselvi is grateful to CSIR for financial assistance through CSIR-EMPOWER project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gangulibabu, Bhuvaneswari, D. & Kalaiselvi, N. Comparison of corn starch-assisted sol–gel and combustion methods to prepare LiMn x Co y Ni z O2 compounds. J Solid State Electrochem 17, 9–17 (2013). https://doi.org/10.1007/s10008-012-1851-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1851-z