Abstract
A series of Co3O4 nanoplates/graphene nanosheets (GNS) composites are fabricated using a facile and green method by hydrothermal treatment and subsequent calcinations in air. The Co3O4 nanoplates with a length of 0.5–1 μm and width of 100–300 nm are homogeneously distributed on the surface of graphene nanosheets which are reduced under mild hydrothermal treatment as investigated by X-ray diffraction, scanning electron microscopy, and transmission electron microscopy. Electrochemical properties are tested by cyclic voltammetry, galvanostatic charge–discharge, and electrochemical impedance spectroscopy. The Co3O4 nanoplates/7.0 % GNS composite shows a maximum specific capacitance of 667.9 F g−1 at 1.25 A g−1 and 412.5 F g−1 at 5 A g−1 in 2 M KOH aqueous solution. The composite exhibits excellent cycling stability with 18.7 % of specific capacitance increased after 1,000 cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, tremendous attention has been focused on pseudocapacitors, which proceed with reversible faradic redox reaction of materials including transition metal oxides, transition metal hydroxides, and conducting polymers due to their high power density and long cycle life [1–4]. However, the energy density of pseudocapacitors is still low. A novel strategy incorporating nanostructured carbon materials, such as porous carbon sphere [5, 6], carbon nanotube [7–9], carbon fiber [10, 11], and graphene [12–15], has been employed to address the issue and improve the conductivity of electrode materials as well.
Since the isolation of single-layer graphene in 2004 [16], graphene has attracted much attention due to the theoretical special surface area of 2,630 m2 g−1, the high intrinsic electric conductivity, as well as the excellent mechanical strength and chemical stability. Graphene and graphene-based materials have been explored for lithium ion batteries, fuel cells, solar cells, and supercapacitors [17–20]. However, the serious agglomeration and restacking of graphene nanosheets (GNS) result in great specific surface area loss and consequently a lower capacitance than the anticipated value. For example, Ruoff and co-workers have reported graphene-based supercapacitor with specific capacitance of 166 and 135 F g−1 in ion liquid [21] and aqueous solution [22], respectively. Researchers have been committed to construct a flexible interleaved structure with transition metal nanostructure attached to GNS to boost the capacitive performance of graphene-based materials, such as GNS–Mn3O4 [23], GNS–MnO2 [24, 25], GNS–ZnO [26], GNS–SnO2 [27], and GNS–Co3O4 [28]. Among the transition metal oxides, Co3O4 is found to be one of the most promising electrode materials because of high redox activity, great reversibility, and theoretical special capacitance of 3,580 F g−1 [29]. Although there are some reports on the preparation of GNS–Co3O4, the strategies are not environmental in which graphene nanosheets is reduced using hydrazine [30] or NaBH4 [31]. It is well known that these reduction agents are toxic, explosive, and expensive. Therefore, synthesis of GNS-based materials with a facile and environment-friendly approach is still a big challenge.
In this work, we propose a facile and green method for growing cobalt oxide nanoplates on graphene nanosheets. Under hydrothermal treatment, the exfoliated graphite oxide (GO) is reduced, and simultaneously cobalt hydroxide is deposited on the surface of GNS. A sequence of Co3O4 nanoplates/GNS composites is formed by thermal decomposition of cobalt hydroxide/GNS composite. Morphology and electrochemical capacitive performance of Co3O4/GNS composite were investigated in detail and the formation mechanism of the nanostructure was also investigated.
Experiment
Preparation of Co3O4 nanoplates/GNS composite
GO was prepared from flake graphite powder (500 mesh) by Hummers method as described previously [32]. Typically, as-prepared GO sample (0.5 g) was soaked in an aqueous solution of tetramethylammonium hydroxide (TMAOH) (100 mL, 0.5 mol L−1) for a week. The brown suspension was then dialyzed with deionized water until the pH was 7 and dried in oven at 60 °C for 12 h.
A certain amount (40, 80, 240 mg) of TMAOH-soaked GO was well dispersed in 80 mL deionized water by ultrasonic agitation for 2 h. Cobalt nitrate hexahydrate, urea, and sodium citrate were dissolved in GO dispersion step by step in intervals of 10 min under vigorous stirring to give the final concentration of 40, 80, and 8 mmol L−1, respectively. The dark homogeneous solution was transferred into a 100-mL Teflon-lined stainless steel autoclave. The autoclave was heated at 180 °C for 24 h. The black products deposited at the bottom were washed by centrifugation for three times using deionized water, dried in an oven at 60 °C for 12 h, and then annealed at 350 °C for 3 h in air to form final products. The pure Co3O4 nanoplates were synthesized by the previously mentioned steps without the addition of GO.
For comparison, GNS were prepared via a hydrothermal method as reported by Xu and co-workers [33]. A total of 30 mL homogeneous GO aqueous dispersion (1 mg mL−1) was sealed in 50-mL Teflon-lined stainless steel autoclave and hydrothermally treated at 180 °C for 12 h. The cylinder-like graphene hydrogel was collected and dried in vacuum freeze–drying step.
Materials characterization
The crystal structure was determined by X-ray diffractometer (XRD, Rigaku D/max 2550VB+) from 5 ° to 70 ° with CuKα radiation (λ = 1.54056 Å). The surface morphology was characterized by scanning electron microscope (SEM; JEOL JSM-6360 LV), transmission electron microscope (TEM; JEOL JEM-2100 F), and atom force microscope (AFM; Vecco Dimension). The Fourier transform infrared (FT-IR) spectra were recorded on Nicolet560 spectroscopy with KBr pellet technique. Raman spectra were tested with Dior LABRAM-1B instrument. The graphene content of GNS/Co3O4 composites was measured by C-S800-infrared-carbon sulfur analyzer. The graphene content of as-prepared composites adding 40, 80, and 240 mg GO were determined to be 4.1, 7.0, and 12.3 %, respectively.
Electrochemical measurement
Cycle voltammetry (CV) test, galvanostatic charge–discharge (CD) test, and electrochemical impedance spectroscopy (EIS) were carried out on a CHI660C electrochemistry workstation. The working electrode was prepared by mixing 85 wt.% active materials, 10 wt.% acetylene black as conductive agent, and 5 wt.% poly(tetrafluoroethylene) (PTFE) as binder to form a slurry and pressing the slurry onto a nickel grid (1 cm2). The electrodes were dried under vacuum at 110 °C for 12 h. To fabricate the graphene working electrode, freeze-dried graphene hydrogel was mixed with PTFE in a mass ratio of 95:5. The fabrication of electrode is just the same as detailed earlier. All electrochemical measurements were finished in a three-compartment cell: a working electrode, a platinum plate as counter-electrode, and a saturated calomel electrode (SCE) as reference electrode. The electrolyte was 2 M KOH aqueous solution.
Results and discussion
Microstructure characterizations
Figure 1 shows the XRD patterns of GO, GNS, and Co3O4 nanoplates/GNS composite. The intense peak of GO at 2θ ≈ 11 ° corresponds to the (001) reflection. GO exhibits a layered structure with a higher d-spacing (0.806 nm) than that of natural graphite (0.336 nm), which demonstrates complete oxidation of graphite into graphite oxide [34]. For GNS, the (001) peak at 11 ° disappears and a broad (002) peak appears at 23.6 °, confirming the conversion of GO to graphene. Diffraction lines, assigned to the crystal planes of (111), (220), (311), (222), (400), (422), (511), and (440), are observed for the 7 % GNS/Co3O4 composite, which are well indexed to face-centered cubic structured as Co3O4 (JCPDS card no. 43–1003, Fd3m, a 0 = 0.808 nm). The additive peaks at 28.3 ° (002) and 41.5 ° (100) can be attributed to graphene. The (002) peak and (100) peak are weaker than that of GNS, implying less restacking and agglomeration of GNS in composites [35]. In comparison, all diffraction peaks observed in Co3O4/12.3 % GNS composite were still in good accordance with those of cubic spinel Co3O4.
Figure 2 presents the FT-IR spectra of the as-prepared samples. The intensive characteristic peaks of GO, consisting of O–H stretching mode at 3,450 cm−1 and C = O stretching vibrations of carboxyl groups at 1,730 cm−1, disappear in the spectrum of GNS. The result indicates that the major oxygen-containing functional groups of GO have been removed after reduction [36]. The remaining absorption at around 1,600 and 1,060 cm−1 can be assigned to skeletal vibrations of un-oxidized graphite domains and C–O stretching vibrations of the residual epoxy groups, respectively. For the composite, the absorption peaks at 570 and 660 cm−1 is attributed to the vibration of the Co–O [37].
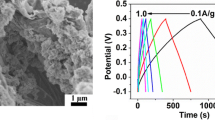
The morphology and microstructure of Co3O4/7.0 % GNS composites are shown in Fig. 3. The cross-sectional AFM images in Fig. 3a, b provide evidence that single-atom layer GO sheet and GNS were obtained with the thickness of 0.80 and 3.54 nm, respectively. The GNS exhibits interconnected 3D porous network in SEM image (Fig. 3c) and crumpled silk-liked structure in TEM image (Fig. 5a). Figure 3d–f shows the SEM images and corresponding EDS pattern of Co3O4/7.0 % GNS composite. As can be seen, the irregular and thin plates with a length of 0.5–1 μm and width of 100–300 nm are decorated on the surface of graphene nanosheets, which could prevent the restacking of graphene nanosheets. The EDS pattern of Co3O4/7.0 % GNS composite confirms the existence of the elements including Co, O, and C on the surface of graphene nanosheets. For the Co3O4/4.1 % GNS composite, many stacked irregular platelets were observed in the image (Fig. 4a). It is hard to see scrolled graphene nanosheets. When the content of graphene in composite is 12.3 %, the graphene nanosheets agglomerate to form clusters (Fig. 4b). Few cobalt oxide nanoflakes were loosely decorated on the graphene nanosheets.
To investigate the structural information of Co3O4 nanoplates on graphene nanosheets, TEM, high-resolution transmission electron microscopy (HRTEM), and selected area electron diffraction (SAED) results are presented in Fig. 5. TEM image in Fig. 5b shows the porous nanoplates consisting of particles with a diameter of around 5–20 nm which is probably because of the elimination of H2O molecules during calcination [38]. It can be obviously observed that Co3O4 nanoplates are wrapped by graphene sheets. The SAED pattern in the inset of Fig. 5c reveals the poly-crystalline nature of the nanoplates. HRTEM image (as presented in Fig. 5d) shows a clear cubic lattice. The HRTEM image is recorded through the (−1 1 2) direction. The (2–2 2) and (2 2 0) lattice spacing are 0.233 and 0.286 nm, respectively. By using the formula for a cubic lattice: a 0 = d (h 2 + k 2 + l 2)1/2, a 0 is calculated to be 0.808 nm, corresponding to the given value in the JCDS 43–1003 card [39].
a TEM image of GNS, b TEM image of Co3O4 nanoplates obtained from the composites after agitation for 1 h; c, d TEM image of Co3O4/7.0 % GNS composite; e HRTEM image of the Co3O4 nanoparticle surface viewed from the broad plane. The insets in c and d show the corresponding SAED pattern and fast Fourier transform pattern
For Raman spectroscopy, the G band at around 1,590 cm−1 represents the E2g phonons of C sp2 atom, while the D band at around 1,330 cm−1 is breathing modes of rings or K-point phonons of A1g symmetry. In general, the I D/I G intensity ratio reflects the disorder density of carbon materials [40]. Figure 6 shows the Raman spectra of GO, GNS, and Co3O4/7.0 % GNS composite in the range from 400 to 2,000 cm−1. The intensity ratio (I D/I G) of GO is 0.962. Compared with GO, the I D/I G of GNS is increased to 1.236 due to the unrepaired defects after the removal of oxygen-containing functional groups, which leads to destruction of sp2 network [41]. For Co3O4/7.0 % GNS composite, the I D/I G is 1.247 and the additive peaks were detected at around 191, 476, 518, and 680 cm−1, which can be attributed to Eg, E 12g , F 22g , and A1g modes of Co3O4 [42].
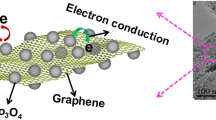
According to the results presented earlier, the formation processes of Co3O4 nanoplates/GNS composite is proposed in Fig. 7. Previous studies have indicated that the oxygen functional groups such as epoxy and hydroxyl decorates on GO sheets, and the carbonyl and carboxyl groups are presumably located at the edges. After the treatment in TMAOH aqueous solution, the GO sheets are exfoliated by the intercalation of TMA+ into the interlayer of GO and negatively charged through the ion exchange of OH− to H+ from carboxyl [43]. The metal ions (Co2+) are adsorbed onto the surface of GO sheets due to the electrostatic force, and cobalt hydroxide was grown on the surface accompanied with the reduction of GO to graphene under a hydrothermal treatment. Because of the removal of water molecules during calcination, the cobalt oxide nanoplates become porous, which is beneficial for permeating of electrolyte. Co3O4 nanoplates are firmly attached to the nanosheets of graphene, which could effectively prevent the restacking of graphene nanosheets. Meanwhile, the volume expansion of Co3O4 could also be restrained, suggesting that the Co3O4 nanoplates/GNS composite may show good electrochemical performance.
Electrochemical properties
Figure 8a displays the CV curves of Co3O4/7.0 % GNS composite at various scan rates of 5, 10, 20, and 50 mV s−1. The CV curves are different from the ideal rectangular shape of the typical electric double-layer capacitance. There are a couple of redox peaks at about 0.21 and 0.37 V (vs SCE) similar to that of pure Co3O4, which suggest that the capacitance is mainly from the pseudocapacitance of Co3O4. The peaks are related to the reactions between different oxidation states of Co according to the following equations [44, 45]:
With the increasing rates, the anodic peaks shift toward positive potential and the cathodic peaks shift toward negative potential. The potential difference between anodic and cathodic peak materials is around 0.16 V at a scan rate of 5 mV s−1. The coulombic efficiency of electrode calculated from the ratio of discharge time to charge time is 92.8 % at the specific current of 1.25 A g−1. These two factors indicate the quasi-reversible feature of redox couples [38]. Furthermore, the specific current increases from 5 to 50 mV s−1, which indicates fast redox reaction. For comparison, CV curves for Co3O4/7.0 % GNS composite, pure Co3O4 nanoplates, and GNS at 10 mV s−1 are shown in Fig. 8b. The internal area of CV curve for Co3O4/7.0 % GNS composite is apparently larger than that of pure Co3O4 nanoplates, implying that the specific capacitance of composite is higher than that of pure Co3O4 nanoplates. In addition, the CV curve of GNS is almost ideally rectangular, exhibiting the electric double-layer capacitance of GNS [33].
The specific capacitance (SC), based on galvanostatic charge/discharge measurement, can be calculated from the following equation:
where I is the discharge current (A), t is the discharge time (s), m is the mass of the electrode materials (g), and V is the discharging potential range.
As shown in Fig. 9a, the discharge curves consist of a sudden potential drop from 0.4 to 0.3 V and a slow potential decay from 0.3 to 0.2 V. It is in good agreement with results corresponding to CV curves. The charge–discharge curve of GNS is presented in Fig. 9b. The voltage decreases linearly along the discharge time, which shows ideal double-layer capacitive behavior. According to Eq. (3), the specific capacitance of Co3O4/7.0 % GNS composite is calculated to be 703.4, 667.9, 472.5, 412.5, and 385.1 F g−1 at the current density of 0.625, 1.25, 2.5, 5.0, and 12.5 A g−1, respectively. Compared to 334.2 F g−1 for pure Co3O4 nanoplates and 224.2 F g−1 for GNS at 0.5 A g−1, the capacitance of the composite is remarkably enhanced, which is also higher than Co3O4/graphene materials prepared by microwave-assisted method (243.3 F g−1) [28] and surfactant-assisted synthesized Co3O4/reduced graphene oxide (163.8 F g−1) [46]. The remarkable enhancement in specific capacitance is attributed to the unique properties of the Co3O4/GNS composite, shown as follows: (1) the graphene nanosheets provide a large surface for well-anchored Co3O4 nanoplates and a highly conductive network for electron transport; (2) the Co3O4 nanoplates inhibit the restacking of graphene, improving the absorption of electrolyte on the surface of electrode; and (3) the channel between graphene and Co3O4 nanoplates facilitates ion transportation [47].
a Galvanostatic discharge curves of pure Co3O4 nanoplates and Co3O4/7.0 % GNS composite electrodes in 2 M KOH solution at different current densities. b Galvanostatic charge–discharge curves of GNS electrode in 6 M KOH solution at a current density of 0.5 A g−1. c Galvanostatic discharge curves of Co3O4/GNS composites with the graphene content of 4.1, 7.0 and 12.3 % in 2 M KOH solution at a current density of 1.25 A g−1
To further understand the effect of graphene mass ratio on specific capacitance, the galvanostatic charge/discharge curves of Co3O4/GNS composites with the graphene content of 4.1, 7.0, and 12.3 % at 1.25 A g−1 are presented in Fig. 9c. The Co3O4/7.0 % GNS composite shows maximum capacitance, which is attributed to a unique morphology that can be observed from the SEM. When the graphene loading is less than 7.0 %, the graphen does not effectively improve the conductivity of composite and it would result to the low utilization of Co3O4 nanoplates. When the graphene content is more than 7.0 %, the graphene nanosheets agglomerate with each other, which could lead to the decrease of specific surface and electroactive site [48].
To investigate the long cycling performance of Co3O4/7.0 % GNS composite and pure Co3O4 nanoplates, the charge–discharge tests were performed at 2.5 A g−1 for 1,000 cycles (Fig. 10). The special capacitance of Co3O4/GNS composite is increased by 25.2 % after 450 cycles. In the following 550 cycles, only slight capacitance loss could be observed. After 1,000 cycles, the capacitance still increases to 18.7 % of the initial capacitance. During cycling, flexible GNS can inhibit the volume change and detachment of Co3O4 nanoplates [28]. It is noted that the specific capacitance of the composite is gradually increased. This phenomenon could be attributed to the reasons as follows: At the initial cycle, the nanostructures wrapped by graphene have not been fully activated. After continual charge/discharge cycling, the electrochemical active sites will be fully exploited to the electrolyte [15, 49]. For pure Co3O4 nanoplates, the capacitance fading is 15.2 % after 1,000 cycles.
In order to evaluate the effect of graphene in electrode materials, EIS measurement was performed at 0.1 V in the frequency range of 0.01 to 105 Hz. The Nyquist impedance plots of three electrodes (Fig. 11) exhibit a semicircle at high frequency and a straight line at low frequency. At very high frequency, the crossover point of the semicircle on the real part is a combinational resistance of the electrolyte resistance, intrinsic resistance of substrate, and contact resistance between the active materials and current collector (R e) [50]. The values of R e are about 0.5 Ω, almost the same for three electrodes. Meanwhile, the semicircle corresponds to a parallel RC element, the charge transfer resistance (R ct), and the constant phase capacitance [49]. With the intercalation of graphene, the R ct decreased from 8.38 to 0.98 Ω obviously. The straight line in low frequency represents the Warburg resistance (W) related to the electrolyte diffusion process and OH− ion diffusion into electrode [51]. The almost vertical shape, indicating fast diffusion of ions in electrolyte during charge/discharge process, is an ideally capacitive behavior [52].
Conclusions
A series of Co3O4 nanoplates/graphene nanosheet composites has been prepared by a facile and green hydrothermal reduction–thermal decomposition method. GNS effectively restrain the volume expansion of Co3O4 nanoplates during cycling and improve the conductivity of electrode, whereas the Co3O4 nanoplates prevent the restacking of GNS. Among the composites, the Co3O4/7.0 % GNS composite exhibits excellent electrochemical performance with the highest specific capacitance and long cycling life. The special capacitance of 703.4 F g−1 is achieved at a current density of 0.625 A g−1. Even at 12.5 A g−1, 385.1 F g−1 is retained with the retention of 63 %. After 1,000 cycles, the increase of capacitance is 18.7 % of the initial value.
References
Conway BE (1999) Electrochemical supercapacitors, scientific fundamentals and technological applications. Kluwer Academic, New York
Simon P, Gogotsi Y (2008) Nat Mater 7:845–854
Hu CC, Chang HK, Lin MC, Wu YT (2006) Nano Lett 6:2690–2695
Yang GW, Xu GL, Li HL (2008) Chem Commum 44:6537–6539
Wang HQ, Li ZS, Yang JH, Li QY, Zhong XX (2009) J Power Sources 194:1218–1221
Wang DW, Li F, Liu M, Lu GQ, Cheng HM (2008) Angew Chem Int Ed 47:373–376
Chen Z, Qin YC, Weng D, Xiao QF, Peng YT, Wang XL, Li HX, Wei F, Lu YF (2009) Adv Funct. Mater 19:3420–3426
Zhang XJ, Shi WH, Zhu JX, Kharistal DJ, Zhao WY, Lalia BS, Hng HH, Yan QY (2011) ACS Nano 5:2013–2019
Lang JW, Yan XB, Xue QJ (2011) J Power Sources 196:7841–7846
Wang Y, Zhang HJ, Lu L, Stubbs LP, Wong CC, Lin JY (2010) ACS Nano 4:4753–4761
Wang JG, Yang Y, Huang ZH, Kang FY (2011) Electrochim Acta 56:9240–9247
Wang HL, Casalongue HS, Liang YY, Dai HJ (2010) J Am Chem Soc 132:7472–7477
Chen S, Zhu JW, Wang X (2010) J Phys Chem C 114:11829–11834
Wang L, Wang DL (2011) Electrochim Acta 56:5010–5015
Gao Z, Wang J, Li ZS, Yang WL, Wang B, Hou MJ, He Y, Liu Q, Mann T, Yang PP, Zhang ML, Liu LH (2011) Chem Mater 23:3509–3516
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Science 306:666–669
Yoo EJ, Kim JD, Hosono E, Zhou HS, Kudo T, Honma I (2008) Nano lett 8:2277–2282
Qu LT, Liu Y, Beak JB, Dai LM (2010) ACS Nano 4:1321–1326
Wang X, Zhi LJ, Müllen K (2008) Nano lett 8:323–327
Liu CG, Yu ZN, Neff D, Zhamu A, Jang BZ (2010) Nano Lett 10:4863–4868
Zhu YW, Murali S, Stoller MD, Ganesh KJ, Cai WW, Ferreira PJ, Pirkle A, Wallace DM, Cychosz KA, Thommes M, Su D (2011) stach EA, Ruoff RS. Science 332:1537–1541
Stoller MD, Park SJ, Zhu YW, An JH, Ruoff RS (2008) Nano lett 8:3498–3502
Wang B, Park JS, Wang CY, Ahn HJ, Wang GX (2010) Electrochim Acta 55:6812–6817
Yan J, Fan ZJ, Wei T, Qian WZ, Zhang ML, Wei F (2010) Carbon 48:3825–3833
Zhang JT, Jiang JW, Zhao XS (2011) J Phys Chem C 115:6448–6454
Chen YL, Hu ZA, Chang YQ, Wang HW, Zhang ZY, Yang YY, Wu HY (2011) J Phys Chem C 115:2563–2571
Lu T, Zhang Y, Li H, Pan L, Li Y, Sun Z (2010) Electrochim Acta 55:4170–4173
Yan J, Wei T, Qiao WM, Shao B, Zhao QK, Zhang LJ, Fan ZJ (2010) Electrochim Acta 55:6973–6978
Deng MJ, Huang FL, Sun IW, Tsai WT, Chang JK (2010) Nanotechnology 20:175602–175607
Kim HY, Seo DH, Kim SW, Kim JS, Kang KS (2011) Carbon 49:326–332
Li BJ, Cao HQ, Shao J, Li GQ, Qu MZ, Yin G (2011) Inorg Chem 50:1628–1632
Hummers WS, Offman RE (1958) J Am Chem Soc 80:1339
Xu YX, Sheng KX, Li C, Shi GQ (2010) ACS Nano 4:4324–4330
Liu ZH, Wang ZM, Yang X, Ooi K (2002) Langmuir 18:4926–4932
Wu ZS, Ren WC, Wen L, Gao LB, Zhao JP, Chen ZP, Zhou GM, Li F, Cheng HM (2010) ACS Nano 4:3187–3194
Zhang WN, He W, Jing XL (2010) J Phys Chem B 114:10368–10373
Dong Z, Fu YY, Han Q, Xu YY, Zhang H (2007) J Phys Chem B 111(50):18475–18478
Kong LB, Lang JW, Liu M, Luo YC, Kang L (2009) J Power Sources 194:1194–1201
He T, Chen DR, Jiao XL, Wang YL (2006) Adv Mater 18:1078–1082
Kudin KN, Ozbas B, Schnlepp HC (2008) Prud’homme RK, Aksay IA, Car R. Nano Lett 8:36–41
Gao W, Alemany LB, Ci L, Ajayan PM (2009) Nat Chem 1:403–408
Liu HC, Yen SK (2007) J Power Source 166:478–484
Li HJ, Zhu G, Liu ZH, Yang ZP, Wang ZL (2010) Carbon 48:4391–4396
Svegl F, Orel B, Mutchins MG, Kalcher K (1996) J Electrochem Soc 143:1532–1539
Lin C, Ritter JA, Popov BN (1998) J Electrochem Soc 145:4097–4103
Zhou WW, Liu JP, Chen T, Tan KS, Jia XT, Liu ZQ, Cong CX, Yang HP, Li CM, Yu T (2011) Phys Chem Chem Phys 13:14462–14465
Zhou GM, Wang DW, Li F, Zhang LL, Li N, Wu ZS, Wen L, Lu GQ, Cheng HM (2010) Chem Mater 22:5306–5313
Zhang LJ, Zhang XG, Shen LF, Gao B, Hao L, Lu XJ, Zhang F, Ding B, Yuan CZ (2012) J Power Sources 199:395–401
Wang HW, Hu ZA, Chang YQ, Chen YL, Wu HY, Zhang ZY, Yang YY (2011) J Mater Chem 21:10504–10511
Wu MS, Hsieh HH (2008) Eletrochim Acta 53:3427–3435
Li YH, Huang KL, Liu SQ, Yao ZF, Zhuang SX (2011) J Solid State Electrochem 15:587–592
Di Fabio A, Giogori A, Mastragostino M, Soavi F (2001) J Electrochem Soc 148:A845–A850
Acknowledgments
This work was supported by National Natural Science Foundation of China (no. 50972165) and Nonferrous Metal Research Fund of Hunan Province Nonferrous Group (no. Y2008-01-007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Liu, S., Wang, H. et al. Facile and green synthesis of Co3O4 nanoplates/graphene nanosheets composite for supercapacitor. J Solid State Electrochem 16, 3593–3602 (2012). https://doi.org/10.1007/s10008-012-1744-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1744-1