Abstract
Multi-layered electrodes which consist of polyaniline (PANI)/manganese dioxide (MnO2)-multi-walled carbon nanotubes (MWNTs) are prepared as the electrode materials for supercapacitors. MnO2-MWNTs are made by the in situ direct coating method to deposit MnO2 onto MWNTs; the core/shell structure of multi-layered fibrous electrodes can also be obtained by PANI coating onto the MnO2-MWNTs. The effect of PANI coating on the electrochemical performance and cyclic stability of MnO2-MWNTs is investigated. From the cyclic voltammograms, the PANI/MnO2-MWNTs show remarkably enhanced specific capacitance and cycle stability compared to MnO2-MWNTs, where the highest specific capacitance (350 F/g) is obtained at a current density of 0.2 A/g for the PANI/MnO2-MWNTs as compared to 92 F/g for pristine MWNTs and 306 F/g for MnO2-MWNTs. This indicates that the improved electrochemical performance of PANI/MnO2-MWNTs is due to the enhanced electrical properties by nano-scale-coated MnO2 onto MWNTs and the PANI coating that leads to the increased cycle stability by delaying the dissolution of MnO2 during charge/discharge tests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A supercapacitor, which is also known as an electrochemical capacitor, is a charge storage device between the traditional electrostatic capacitor and the rechargeable battery. It has been used as an energy storage system for portable electronic devices, backup power sources, and hybrid electric vehicles due to its high power, energy density, and long cycle life [1–3]. As the electrode for the supercapacitor, carbon materials, transition metal oxides (TMs), and a combination of conducting polymers (CPs) [4] and hybrid systems, consisting of carbon/TMs [5], carbon/CPs [6], TMs/CPs [7], or all three phases, i.e., carbon/TMs/CPs [8], are commonly utilized and hence have been studied for their energy storage capacities to be improved using their synergistic effect.
Recently, much research has been done to design new electrode materials for supercapacitors to enhance their electrochemical behaviors. Ruthenium oxide has a specific capacitance as high as 720 F/g and exhibits excellent cycle life stability in aqueous electrolytes. However, its high cost limits potential applications [9]. Therefore, many research interests have been prompted to focus on other transition metal oxides and conducting polymers. In this regard, manganese oxide is one of the most attractive candidates for electrochemical capacitor electrode materials due to its environmental friendliness and low cost [10, 11], whereas one of the major challenges with manganese oxide is its low electrical conductivity. Indeed thin MnO2 films exhibited an electrical conductivity of about ~700 F/g. However, the specific capacitance gradually decreases with increasing film thickness, and the reported values are usually in the range from 100 to 250 F/g [12]. Thus, many approaches have been taken to overcome this disadvantage through synthesizing specific nanostructures [13] or composites with conducting materials, such as carbon nanotubes and conducting polymers [14, 15]. Of these, conducting polymers have also been widely utilized as electrode materials for supercapacitors owing to their good electrical conductivity and large pseudocapacitance that are comparable to those of transition metals. Among the conductive polymers, polyaniline (PANI) is considered to be the most promising electrode material for supercapacitors due to its excellent capacity for energy storage, good processability, high conductivity, low cost, and environmental stability [16–18].
Many studies have recently been carried out on the electrochemical performance of manganese oxide/carbon materials to improve their electrical properties. However, there has been a limited amount of work published on the electrochemical performance of multi-phase materials prepared from conducting polymer/transition metal/carbon materials for supercapacitor electrodes.
Therefore, in the present study, multi-layered electrodes in the form of conducting polymer/transition metal/carbon nanotubes were prepared in two steps: (1) manganese oxide was coated in nano-scaled thickness onto multi-walled carbon nanotubes (MWNTs) by a direct coating method by the reduction of KMnO4 and (2) PANI was further deposited onto manganese oxide-coated MWNTs (MnO2-MWNTs) using oxidation polymerization. The synergistic effect, resulting from the multi-layered structures and pseudocapacitive reaction, on the electrochemical properties of the PANI/MnO2-MWNTs is discussed.
Materials and methods
Materials
MWNTs produced by chemical vapor deposition process were obtained from Nanosolution Co. (Korea). The properties of the MWNTs may be summarized as follows: purity >95% (C, 96 %), diameter 10 to 25 nm, and length 25 to 50 μm. MWNTs were used after conventional acid treatment using a sulfuric acid and nitric acid (3:1, v/v) mixture. Aniline and ammonium persulfate (APS) were obtained from Aldrich. Potassium permanganate (KMnO4) was supplied by Sam Chun Chem. (Korea). The citric acid and all other organic solvents used in this study were of analytical grade and were used without further purification.
Synthesis of MnO2-MWNTs
First, 1 g of MWNTs was acid-treated with 200 ml of a sulfuric acid and nitric acid mixture (3:1, v/v) under sonication for 2 h and was then reacted for 6 h at 80 °C under stirring. Acid-treated MWNTs (A-MWNTs) were washed with water until a pH level of 7.0 was attained, after which the washed sample was filtered and dried at 100 °C.
Then, 0.5 g of A-MWNTs was mixed with 100 ml of 0.2 M KMnO4 solution in a flask. The solution mixture was then refluxed at 140 °C with stirring for 12 h. After the reaction, the mixture was filtered and washed with distilled water several times to remove the residual KMnO4. Subsequently, the filter cake was redispersed in 200 ml of deionized water followed by the addition of 10 ml of 1 M citric acid and then maintained at 160 °C for 12 h under vigorous agitation using a magnetic stirrer during the whole course. A condenser was fitted to the reactor to prevent liquid loss by evaporation. Finally, the composite products were obtained through filtering, purifying with water, and drying processes. Consequently, the resulting samples were MnO2-MWNTs.
Synthesis of PANI/MnO2-MWNTs
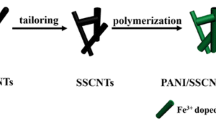
First, 0.1 g of MnO2-MWNTs was dispersed in 100 ml of 1 M HCl solution with the assistance of ultrasonication for 1 h. Then, 5 ml of the aniline monomer was added to the MnO2-MWNTs solution with constant stirring. After that, 100 ml of 0.1 M APS solution was added dropwise to the above solution for 30 min to initiate oxidation polymerization. The reaction was continued for 24 h at 0 to 5 °C. Then, the final product was washed with water and acetone. The remaining powder was dried in a vacuum oven at 80 °C for 24 h. The scheme for PANI/MnO2-MWNTs preparation is presented in Fig. 1.
Measurements
The morphologies of MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs were observed by scanning electron microscopy (SEM, S-4200, Hitachi).
The thermal properties of MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs were measured using thermogravimetric analyses (TGA, Du-Pont TGA-2950 analyzer) from 30 to 750 °C at a heating rate of 10 °C/min in a nitrogen atmosphere.
The structural characteristics of MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs were confirmed by X-ray diffraction (XRD, Rigaku D/Max 2200 V) at 40 kV and 40 mA using Cu Kα radiation. The XRD patterns were obtained in 2θ range between 5° and 70° at a scanning rate of 2°/min.
Surface characterization of MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs was carried out using X-ray photoelectron spectroscopy (XPS, K-Alpha) using a VG Scientific ESCALAB MK-II spectrometer equipped with MgKα (1,253.6 eV) X-ray source and a high-performance multichannel detector operated at 200 W.
Electrochemical performances of MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs were characterized using a three-electrode electrochemical cell. The three-electrode cell consists of a Pt wire as a counter electrode, an Ag/AgCl reference electrode, and SUS mesh coated with the sample as the working electrode. For a working electrode, each sample, carbon black, and PVDF (70:20:10, w/w) were mixed and dispersed in NMP. The slurry mixture was then coated onto SUS mesh, which was dried at 100 °C for 12 h. Cyclic voltammetry measurements were carried out on an IviumStat instrument in 0.5 M Na2SO4 solution at a scan rate of 2 to 50 mV/s in a voltage range of −0.2 to 1.0 V. Galvanostatic charge/discharge curves were measured at a current density of 0.2 A/g.
Results and discussion
Characterization of PANI/MnO2-MWNTs
Figure 2 shows the TGA thermogram of MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs. It can be seen that MWNTs exhibit higher thermal stability (weight loss, about 5%) of up to 750 °C with partial weight loss. However, the thermogram of MnO2-MWNTs is visibly different. MnO2-MWNTs exhibit a gradual weight loss (14%) up to melting point (535 °C), and then the total weight loss with second degradation is about 20%. The thermal degradation of PANI/MnO2-MWNTs is a three-step weight loss process. The first weight loss at approximately 100 °C is attributed to the loss of residual solvents and the weight loss from 220 to 310 °C results from the elimination of HCl as a dopant. The third weight loss, from approximately 400 to 600 °C, corresponds to the thermal decomposition of the PANI and MnO2 [19, 20].
The structural features of MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs are determined using XRD (shown in Fig. 3). The pristine MWNTs revealed reflections corresponding to d(002) and d(100) planes of the crystalline graphite-like materials at 2θ = 26° and 43°, respectively [6], whereas five peaks in MnO2-MWNTs are detected at 2θ = 18°, 36°, 38°, 44°, 56°, and 64°, corresponding to d(131), d(230), d(300), d(160), and d(242) of MnO2 coated onto MWNTs (Joint Committee on Powder Diffraction Standards card no. 14-0644). These results indicate that nano-scaled MnO2 was successfully deposited on the MWNTs. In the PANI/MnO2-MWNTs, two broad peaks can be observed at 2θ = 20° and 25°, resulting from the periodicity parallel and perpendicular to the polymer chain [21]. However, the main peaks of MWNTs and MnO2 overlapped with each other and therefore disappeared after PANI coating.
Recently, the composites between electrical materials, such as carbons and conductive polymers, and MnO2 have been studied by many researchers to improve the electrical properties of neat MnO2 [22, 23]. Figure 4 shows SEM images of the pristine MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs. The pristine MWNTs (Fig. 4a) are in an aggregated form and have a smooth surface where the diameters are approximately 20 to 40 nm and the lengths are of a few micrometers. Compared to pristine MWNTs, MnO2-MWNTs exhibit a rougher surface and a thicker diameter due to the MnO2 coating. The diameter of the MnO2-MWNTs is approximately 30 to 50 nm, indicating that the MnO2 layer is about 10 nm, although the diameter is locally decreased by the strong oxidation effect. To improve the electric and electrochemical properties of MnO2, MWNTs were used as the substrate for the nano-scaled MnO2 layer, and nano-scale coating of MnO2 onto MWNTs leads to improved electrical properties and an increase in the specific surface area of MnO2. In addition, it can also provide a synergistic effect not found in either MnO2 or MWNTs alone. In general, the pseudocapacitive reaction of manganese dioxide occurs at its surface, presenting a nano-scale layer of MnO2 onto MWNTs that can achieve high specific capacity and good high-rate capability. Furthermore, in the PANI/MnO2-MWNTs, the thickness of MnO2-MWNTs is also increased with the coating of PANI. As the diameter of the PANI/MnO2-MWNTs is about 60 to 80 nm, this means that the PANI layer is about 30 nm.
XPS analysis of PANI/MnO2-MWNTs
The elemental composition of MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs was confirmed by XPS analysis (shown in Fig. 5 and Table 1). The survey scans, in the 200- to 800-eV binding energy range, confirm that acid-treated MWNTs mainly consist of carbon (285 eV) and oxygen (530 eV). Compared to MWNTs, with the incorporation of MnO2, MnO2-MWNTs exhibit peaks for MnO2 (approximately 640 to 650 eV) as well as for carbon and oxygen groups, where the MnO2 content is about 13.1 % (shown in Table 1). After further PANI coating, as expected, a nitrogen group (about 400 eV; content, 11 %) was newly formed on the PANI/MnO2-MWNTs surface, whereas only a very small amount of MnO2 was detectable [over the depth (> 10 nm) of analysis]. Consequently, the thickness of the PANI layer is estimated to be over 10 nm, as the SEM images suggest (Fig. 4). From the XPS results, the nano-scale-coated MnO2 layer and the presence of high nitrogen content in the multi-layered composites could influence the electrochemical performance of the PANI/MnO2-MWNTs by a pseudocapcitance reaction and subsequently increase the wettability of the electrolyte.
MnO2-MWNTs are further examined to determine the oxygen anion content and the oxidation state of the Mn cations in manganese dioxide coated onto MWNTs. Mn2P and O1S spectra form MnO2-MWNTs are shown in Fig. 6, and the valence of Mn cations is investigated using the Mn2P spectrum. In Fig. 6a, the Mn2P3/2 and Mn2P1/2 binding energy peaks are located at 642.1 and 653.9 eV, respectively. The peak width between Mn2P3/2 and Mn2P1/2 is 11.8 eV, indicating an Mn valence of 4+ and/or 3+. The O1S spectrum consists of two major components, namely, MnO2 (Mn4+) and MnOOH oxide (Mn3+), and one minor component, the residual water. Of the two major components, the amount of MnO2 is greater than that of MnOOH. These results provide useful information regarding the state of oxygen bonding, hydration of the oxide nanocrystals, and the valence of manganese cations, which can help to understand the capacitance mechanism as a fraction of the reaction sites accessible to the faradic process [24].
Electrochemical performance of PANI/MnO2-MWNTs
One-dimensional (1D) nanostructured materials provide short transport/diffusion path lengths for both ions and electrons, leading to faster kinetics and larger specific surface areas, resulting in high charge/discharge capacities [25]. In this work, cyclic voltammetry measurements were carried out to determine the electrochemical performance of MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs at 5 mV/s in a 0.5-M Na2SO4 electrolyte solution. The CV curves of MWNT-, MnO2-MWNT-, and PANI/MnO2-MWNT-based electrodes are presented in Fig. 7a. It is clear that the current density of pristine MWNTs is lower than those of the multi-layered composite samples. However, it can be significantly improved with the incorporation of nano-scale-coated MnO2, giving a larger current density. Furthermore, the current density of MnO2-MWNTs is also increased by the application of PANI layers. The improved electrochemical performance of PANI/MnO2-MWNTs is related to the formation of nano-scaled MnO2 layers onto MWNTs, which provides a high specific surface area and enhanced electrical property of MnO2 compared to the general particles, leading to easy and rapid penetration of electrolyte ions. Furthermore, the presence of polar nitrogen groups by PANI coating enhances the wettability between the electrode and electrolyte during charge–discharge, and the highly pseudocapacitive reaction is caused by the MnO2 and nitrogen groups [26].
The rate capability for the supercapacitor is an important factor. To determine the rate capability of the electrode, CV measurements of PANI/MnO2-MWNTs were performed in the range from 2 to 50 mV/s as shown in Fig. 7b. When the scan rate was increased up to 10 mV/s, the shape of the CV curve remained stable, and the current density was significantly increased without deformation of the CV curves, indicating a good rate capability of PANI/MnO2-MWNTs. However, the CV curves are deformed at a high scan rate of over 10 mV/s due to the resistance of the electrodes.
It is well known that MnO2 is one of the most promising electrode materials for supercapacitors due to its excellent capacitive performance in the aqueous electrolytes. The charging mechanism of MnO2 is described by the following reaction [27]:
where B+ = Li+, Na+, K+, H+. Equation 1 indicates that a large surface area and high ionic and electronic conductivity of the electrode material are necessary in order to utilize the high theoretical specific capacitance (theoretic value, 1,380 F/g) of MnO2 [28].
Figure 8 shows the charge/discharge curves of the MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs. As shown in Fig. 8, at a current density of 0.2 A/g, MWNTs are in an ideal triangular shape with a small ohmic drop at the beginning of the discharge, and this reveals that MWNTs possess excellent capacitive behaviors [29]. However, MnO2-MWNTs and PANI/MnO2-MWNTs exhibit a large ohmic drop compared to that of MWNTs, presenting the high equivalent series resistance (ESR) in the interface of multi-layered electrodes. Although the ESR of the multi-layered electrodes is high, their charge/discharge duration is significantly increased with the incorporation of MnO2 and further PANI layers. From the charge–discharge curve, the specific capacitance (C spec) for the MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs can be calculated as:
where I is the discharge current, m is the electrode mass, t is the discharge time, and ΔV is the voltage range. The C spec (92 F/g) of MWNTs is largely enhanced with the MnO2 and PANI layer since the C spec values of the MnO2-MWNTs and PANI/MnO2-MWNTs are 306 and 350 F/g, respectively. The enhancement of the C spec is attributed to the redox reaction by MnO2 and nitrogen groups of PANI, and the improved electrochemical performance of the multi-layered electrode system is mainly related to the nano-scale MnO2 coating rather than the PANI coating, resulting from the CV curves and charge/discharge results.
Cycle stability of PANI/MnO2-MWNTs
The long-term cycle stability of each sample, i.e., MWNTs, MnO2-MWNTs, and PANI/MnO2-MWNTs, was also evaluated in this study by cycling the charge/discharge test between −0.2 and 1.0 V (vs. Ag/AgCl) at a current density of 0.2 A/g during 500 times. The cycle stability of each sample is shown in Fig. 9. It is obvious that the pristine MWNT-based electrode shows high cycle stability over the entire test period and after 500 cycles, and the capacitance decreased by approximately 7% of the initial capacitance. However, despite the specific capacitance of MnO2-MWNTs being higher than that of pristine MWNTs, it is gradually decreased with the increasing number of charge–discharge tests, revealing the low cycle stability of about 20%. Previous studies have reported that the reduction of the electrochemical performance of the MnO2 with cycling is closely related to the physicochemical and structural characteristics, such as grain size, surface area, morphology, and crystallinity [30]. In this system, the gradual decrease of the MnO2-MWNTs specific capacitance is attributed to the partial dissolution of MnO2 coated onto MWNTs in the electrolyte during cycling, resulting in an increase of equivalent series resistance at the interface between the electrode materials and current collector [31, 32].
After an additional PANI coating, the PANI/MnO2-MWNTs exhibit more stable cycle features than the MnO2-MWNTs where the capacitance decreases by approximately 16 % of the initial capacitance since the PANI layers can reduce the dissolution rate of MnO2 in the electrolyte solution. It is well known that a combination of MnO2 and other materials, such as conducting polymers, may reduce both the MnO2 dissolution and the mechanical failure and hence lead to excellent electrochemical performance after the number of cycling tests [33]. However, the specific capacitance is likewise decreased during the charge/discharge test to an extent that is even greater than that of pristine MWNTs. The decrease of the specific capacitance of the PANI/MnO2-MWNTs could account for the expansion and shrinkage of the PANI during the long-term charge/discharge processes [34]. Consequently, the nano-scale coated MnO2 on the MWNTs could provide improved capacitance with higher electric conductivity of MnO2 as well as enhanced electrolyte migration. The coating of PANI also further improves the electrochemical performance of MnO2-MWNTs. Therefore, multi-core/shell electrodes are suitable to be manufactured as electrode materials and have superior electrochemical performance when compared to that of conventional manganese oxide/carbon electrodes.
Conclusions
In this work, multi-layered electrodes, i.e., PANI/MnO2-MWNTs, were prepared as electrode materials for a supercapacitor. The effect of PANI coating on the electrochemical performance and cyclic stability of MnO2-MWNTs was determined. From the cyclic voltammograms, PANI/MnO2-MWNTs showed remarkably enhanced specific capacitance and cycle stability compared to pristine MWNTs and MnO2-MWNTs, and the specific capacitance of PANI/MnO2-MWNTs (350 F/g) was higher than those of pristine MWNTs (92 F/g) and MnO2-MWNTs (306 F/g). This indicated that the improved electrochemical performance of PANI/MnO2-MWNTs was due to the synergistic effect among three components, i.e., PANI, MnO2, and MWNTs, and the PANI coating resulted in increased cycle stability by delaying the dissolution of MnO2 during charge/discharge tests.
References
Conway BE (1999) Electrochemical Supercapacitors. Kluwer Academic, New York
Kisacikoglu MC, Uzunoglu M, Alam MS (2009) Int J Hydrogen Energy 34:1497–1507
Lim JW, Jeong E, Jung MJ, Lee SI, Lee YS (2012) J Ind Eng Chem 18:116-122
Zhang X, Ji L, Zhang S, Yang W (2007) J Power Sources 173:1017–1023
Subramanian V, Zhu H, Wei B (2006) Electrochem Commun 8:827–832
Kim KS, Park SJ (2011) Electrochim Acta 56:1629–1635
Sen P, De A (2010) Electrochim Acta 55:4677–4684
Konyushenko EN, Kazantseva NE, Stejskal J, Trchová M, Kovářová J, Sapurina I, Tomishko MM, Demicheva OV, Prokeš J (2008) J Magnetism Magnetic Mater 320:231–240
Ma SB, Nam KW, Yoon WS, Yang XQ, Ahn KY, Oh KH, Kim KB (2008) J Power Sources 178:483–489
Reddy RN, Reddy RG (2003) J Power Sources 124:330–337
Wang Y, Liu H, Sun X, Zhitomirsky I (2009) Scripta Mater 61:1079–1082
Long JW, Young AL, Rolison DR (2003) J Electrochem Soc 150:A1161–A1165
Fischer AE, Saunders MP, Pettigrew KA, Rolison DR, Long JW (2008) J Electrochem Soc 155:A246–A252
Zhang S, Peng C, Ng KC, Chen GZ (2010) Electrochim Acta 55:7447–7453
Razak SIA, Ahmad AL, Zeina SHS, Boccaccini AR (2009) Scripta Mater 61:592–595
Li D, Kaner RB (2006) J Am Soc Chem 128:968–975
Li H, Wang J, Chu Q, Wang Z, Zhang F, Wang S (2009) J Power Sources 190:578–586
Kim YY, Yun J, Lee YS, Kim HI (2011) Carbon Lett 12:48-52
Lee D, Char K (2002) Polym Degrad Stabil 75:555–560
Xie X, Gao L (2007) Carbon 45:2365–2373
Chiou NR, Epstein AJ (2005) Adv Mater 17:1679–1683
Zheng H, Kang W, Fengming Z, Tang F, Rufford TE, Wang L, Ma C (2010) Solid State Ionics 181:1690–1696
Zou W, Wang W, He B, Sun M, Yin Y (2010) J Power Sources 195:7489–7493
Djurfors B, Broughton JN, Brett MJ, Ivey DG (2005) Acta Mater 53:957–965
Hu CC, Chang KH, Lin MC, Wu YT (2006) Nano Lett 6:2690–2695
Sharma RK, Rastogi AC, Desu SB (2008) Electrochim Acta 53:7690–7695
Khomenko V, Raymundo-Piñero E, Béguin F (2006) J Power Sources 153:183–190
Toupin M, Brousse T, Belanger D (2004) Chem Mater 16:3184–3190
Kim S, Park SJ (2008) Anal Chim Acta 619:43–48
Brousse T, Toupin M, Dugas R, Athouel L (2006) J Electrochem Soc 153:2171–2180
Comaba S, Ogata A, Tsuchikawa T (2008) Electrochem Commun 10:1435–1437
Belanger D, Brousse T, Long JW (2008) Electrochem Soc Interf 17:49–52
Babakhani B, Ivey DG (2010) J Power Sources 195:2110–2117
Khomenko V, Frackowiak E, Beguin F (2005) Electrochim Acta 50:2499–2506
Acknowledgments
This work was supported by the IT Industrial Source Technology Development Business of the Ministry of Knowledge Economy, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KS., Park, SJ. Synthesis and high electrochemical performance of polyaniline/MnO2-coated multi-walled carbon nanotube-based hybrid electrodes. J Solid State Electrochem 16, 2751–2758 (2012). https://doi.org/10.1007/s10008-012-1694-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1694-7