Abstract
Thin films of biodegradable corn starch-based biopolymer electrolytes were prepared by solution casting technique. Lithium hexafluorophosphate (LiPF6) and 1-butyl-3-methylimidazolium trifluoromethanesulfonate (BmImTf) were employed as lithium salt and ionic liquid, respectively. With reference to the temperature dependence study, Arrhenius relationship was observed. The highest ionic conductivity of (6.00 ± 0.01) × 10−4 S cm−1 was obtained at 80 °C. Based on x-ray diffraction (XRD) result, the peaks became broader with doping of ionic liquid revealing the higher amorphous region of the biopolymer electrolytes. Ionic liquid-based biopolymer electrolytes exhibited lower glass transition temperature (T g).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
From a green viewpoint, many researchers have a keen interest on ionic liquid-based biopolymer electrolytes. Ionic liquid-based polymer electrolytes have a great tendency to replace conventional polymer electrolytes in electrochemical, electronic and power storage devices as ionic liquid provides potential environmental advantages relative to conventional solvents. Room temperature ionic liquids (RTILs) are molten organic salt consisting of bulky organic cations with delocalized charges and organic or inorganic anions [1]. RTILs are promising candidates because of their inherent physical and chemical characteristics such as nonvolatility, nonflammability, negligible vapor pressure, low melting point, low toxicity, high conductivity and excellent thermal and electrochemical stabilities as well as a wide electrochemical window [1–3].
In order to develop environmentally friendly polymer electrolytes, biodegradable polymer has been widely investigated to alternate conventional nondegradable or incompletely degrading synthetic biopolymers. Starch is an abundant, renewable and biodegradable raw material with low price [4]. Other unique properties are excellent thermal and steel adhesion properties, superior biocompatibility, good solubility, high recrystallization stability of the amorphous phase and great ability to form films [5–7]. In this present work, lithium hexafluorophosphate (LiPF6) was chosen because of its good environmental impacts, high solubility and high conductivity in various solvents [8]. It possesses many advantages, for example, excellent electrical stability, low moisture absorption and is less dangerous and poisonous [9, 10]. In addition, it could form suitable solid electrolyte interface (SEI) membrane in electrodes, especially in cathode. It also implements the passivation for anode current collectors to prevent their dissolution [9]. Ionic liquid, 1-butyl-3-methylimidazolium trifluoromethanesulfonate (BmImTf) were embedded in this work. This ionic liquid consists of 1-butyl-3-methylimidazolium cation (BmIm+) and trifluoromethanesulfonate (or known as triflate) anion (Tf−). Among all the cations, 1,3-dialkylimidazolium (Im) is the appealing cation because of its favorable properties and ease to gather abundant and useful information [11]. Other than that, the imidazolium ILs bearing hydrophilic anions are capable of dissolving corn starch [12]. On the other hand, triflate anion was selected due to its bulky structure. This bulky anion favors the delocalization of charge and reduces the interaction between anion and cation in the complex with lower lattice energy, leading to the high degree of dissociation [13].
Experimental
Materials
Ionic liquid-based biopolymer electrolytes were prepared by solution casting technique. Biodegradable corn starch (Sigma-Aldrich), lithium hexafluorophosphate (LiPF6) (Aldrich) and 1-butyl-3-methylimidazolium trifluoromethanesulfonate (BmImTf) (Aldrich) were employed as host polymer, lithium salt and ionic liquid, respectively.
Preparation of thin films
Different stoichiometric quantities of BmImTf were doped in the solution with a fixed ratio of 80 wt.% of corn starch to 20 wt.% of LiPF6. The quantity of materials added is expressed as weight percent (wt.%) and the designation of the samples are as tabulated in Table 1. Appropriate amounts of corn starch, LiPF6 and BmImTf were dissolved in distilled water. The solution was then stirred overnight at 80 °C. The resulting solution was thus cast on a glass Petri dish and dried in oven, forming biopolymer films.
Characterizations
All the samples were subjected to ac-impedance spectroscopy, x-ray diffraction (XRD), scanning electron microscopy (SEM) and differential scanning calorimetry (DSC).
Temperature dependence–ionic conductivity studies
The prepared samples were subjected to ac-impedance spectroscopy. The thickness of the samples was measured by using a micrometer screw gauge. The ionic conductivities of the samples were determined by using HIOKI 3532-50 LCR HiTESTER over a frequency range between 50 Hz and 5 MHz. The ionic conductivity was measured from ambient temperature to 80 °C. Samples were mounted on the holder with stainless steel (SS) blocking electrodes under spring pressure with the configuration SS/SPE/SS. The bulk ionic conductivity is determined by using the equation below.
where l is the thickness (cm), R b is bulk resistance (Ω) and A is the known surface area (cm2) of polymer electrolyte film. The semicircle fitting was accomplished to obtain R b value. As shown in Fig. 1, R b of the thin electrolyte films is calculated from interception of the semicircle and the spike.
X-ray diffraction (XRD) studies
The amorphous nature of biopolymer electrolytes was investigated via XRD study. The XRD patterns were recorded on a Siemens D 5000 diffractometer with Cu-Kα radiation (λ = 1.54060 Å) over the range of 2θ = 5–80° at ambient temperature.
Differential scanning calorimetry (DSC)
DSC analysis was evaluated under nitrogen flow rate of 10 ml min−1 by the means of TA Instrument Universal Analyzer 2000 which comprised of DSC Standard Cell FC as the main unit and Universal V4.7A software. Samples weighing 4–5 mg hermetically sealed in the aluminum T zero pans and an empty hermetically sealed aluminum pan was used as the reference cell. As a preliminary step, the samples were heated from 25 °C to 105 °C to remove any trace amount of solvent and water at a heating rate of 30 °C min−1 and were maintained at 105 °C for 5 min to ensure complete evaporation. Subsequently, the samples were rapidly cooled to −40 °C and reheated to 70 °C at the preset heating rate. The final heating scan was used to evaluate T g.
Results and discussion
Ac–impedance studies
A typical Cole–Cole plot of complex impedance of Tf 0 is depicted in Fig. 1. As can been seen, the complex impedance plot is composed of two well-defined regions: a slanted spike at low frequency and a semicircle at high frequency. The semicircle represents the combination of bulk resistance and bulk capacitance of polymer matrix in parallel arrangement. The resistance reveals the ionic transportation through the voids within the polymer matrix which can be detected by a resistor. On the other hand, the capacitance implies the polarization of immobile polymer chain in the alternating field which is represented by the capacitor. A slanted spike is observed instead of a vertical spike. This discloses the nonideal mode of capacitance behavior in the polymer electrolytes and ultimately leads to the formation of electrical double layer at the electrode–electrolyte boundary as a result of the polarization effect when the electrical potential alternates between the positive and negative electrodes in the alternate current field. The configuration of this constant phase element (CPE) further entails non-Debye properties of the polymer electrolytes. On the other hand, the degree and slope of the inclination of the peak corresponds to the relaxation time distribution where the charge carriers are accumulated at the electrode–electrolyte interface before the electric field changes the direction.
However, upon inclusion of ionic liquid, the semicircle becomes incomplete as illustrated in Figs. 2 and 3. It suggests that the plasticizing effect of ionic liquid weakens the interactive coordination bonds within the polymer system. Hence, it reduces the polarization of the dipole in the polymer chain and hinders the charge accumulation at the electrode–electrolyte interface, giving rise to the imperfection of capacitance behavior. The semicircle disappears with impregnation of 80 wt.% of BmImTf. It is due to the random dipole orientation in the side chains of the polymer matrix and induction to the noncapacitance properties of the polymer electrolytes. Therefore, only the resistive components in the polymer electrolytes prevail at this moment and, thus, construct a local effective conducting pathway for ionic conduction mechanism. As a result, it promotes the ionic migration. It can be concluded that current charge carriers are ions which responsible for the ionic transportation with the disappearance of the semicircle region.
Another observation is also obtained from Figs. 1, 2 and 3. The R b values decrease with BmImTf mass loadings. In other words, the ionic conductivity increases with BmImTf concentration. It is mainly attributed to the strong plasticizing effect of ionic liquid. The plasticizing effect would soften the polymer backbone, improve the flexibility of polymer matrix and then promote the ion dissociation from the transient coordinative bonds. As a result, it initiates the ionic migration within the polymer matrix and yields the ionic conduction, resulting in higher ionic conductivity.
Temperature dependence–ionic conductivity studies
Figure 4 portrays temperature dependence–ionic conductivity of Tf 0, Tf 2, Tf 3, Tf 5, Tf 7, Tf 8 and Tf 9 from ambient temperature to 80 °C. There is no abrupt change in the value of conductivity with respect to temperature, inferring no phase transitions within the temperature regime for all the complexes [14]. As can been seen, the ionic conductivity increases with temperature. High internal vibrational mode of polymer complex is produced as temperature increases. This high kinetic energy of molecules in the polymer membrane would improve the bond rotation and help in destroying the weak interactive bonds between the oxygen atom of corn starch and lithium cations (Li+). Thus, the ions are more easily to be decoupled from the polymer backbone which assists the ionic migration within the polymer matrix and increases the ionic conductivity at a high temperature. As determined in Fig. 1, the increase of ionic conductivity is in this order: Tf 0 < Tf 2 < Tf 3 < Tf 5 < Tf 7 < Tf 9 < Tf 8. This is in good agreement with the previous study. Tf 8 achieves the highest ionic conductivity of (6.00 ± 0.01) × 10−4 S cm−1 at 80 °C. However, the ionic conductivity is decreased by adding 10 wt.% more of BmImTf (or denoted as Tf 9). It is suggestive of the ion association which leads to the formation of ion pairs and ion aggregates. Eventually, it impedes the ionic transportation within polymer matrix by blocking the conducting pathway.
As shown in Fig. 1, the regression values of all the samples are close to unity. Thus, it declares that the samples follow the Arrhenius behavior and further implies the ionic hopping mechanism within the polymer complexes. In this thermally activated phenomenon, the conductivity is expressed as follow:
where A is a constant which is proportional to the amount of charge carriers, E a is activation energy, k is Boltzmann constant and T is the absolute temperature in Kelvin. As aforementioned, the charge carriers are dissociated from corn starch polymer backbone. Arrhenius theory states that this ionic decoupling would create the vacancy or voids in the polymer electrolytes. So, the neighboring ions from adjacent sites will occupy the vacant site, recoordinate with the polymer chain and, eventually, generate the ionic hopping process. It can be concluded that more vacancies and ionic transportation are formed as the charge carriers are more easily detached from the polymer chain at an elevated temperature.
In order to probe the ion dynamic of polymer electrolytes further, activation energy, E a is determined by fitting it in the Arrhenius equation. E a is defined as the required energy to overcome the reorganization and reformation of the interactive bonds between polymer chain and Li+ [14, 15]. As shown in Eq. 1, E a is inversely proportional to ionic conductivity. The E a values are tabulated in Table 1. Among the samples studied, the most ionic conducting sample, Tf 8, illustrates the lowest E a value which indicates that ionic conduction is the most favorable. The E a value is greatly decreased with increasing BmImTf mass fraction. It is primarily related to the plasticizing effect of the ionic liquid with predominant amorphous region. Less energy is required to break the physical and chemical bonds as the ionic liquid assists in weakening the interaction within the polymer chains. On comparison between Tf 2, Tf 3, Tf 5, Tf 7 and Tf 8, Tf 8 shows the lowest E a values because of its strong plasticizing effect. This would weaken the coordinative bonds and, therefore, it requires lesser energy to disrupt the linkages of polymer chains. Theoretically, Tf 9 should display lower activation energy due to the plasticizing effect of ionic liquid. However, an opposite result has been observed. Again, it reveals the formation of ion pairs and ion aggregates which acquires more energy to break the interactive bonds between the ion pairs and ion aggregates with a restricted polymer segmental mobility.
X-ray diffraction XRD studies
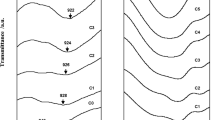
Figure 5 exemplifies the XRD patterns of pure corn starch, pure LiPF6 and Tf 0, whereas Fig. 6 demonstrates the x-ray diffractograms of Tf 0, Tf 3, Tf 5 and Tf 8. As shown in Fig. 5b, the sharp intense peaks at 2θ = 8°, 21.7°, 25.3°, 28.3°, 35.3°, 37°, 42.8°, 49.3°, 55.9°, 60.6°, 61° and 68.4° disclose the crystalline character of LiPF6. On the contrary, pure corn starch shows a broad peak at 16° and a medium sharp peak at 23.3° and further divulges its semicrystalline behavior. Upon inclusion of lithium salt, these diffraction peaks have been shifted to 17° and 22.2°, asserting the change in crystallographic organization [15]. These peaks become broader compared to neat pure corn starch. The relative intensity of the peaks also decreases significantly by doping lithium salt. This signifies that the addition of lithium salt disorders the ordered arrangement within the polymer membrane and reduces the crystallinity degree. All the crystalline peaks of LiPF6 are absent in TF 0. This signifies that LiPF6 is well dissociated to form the complexation between corn starch and LiPF6 [16].
The crystalline peaks are noted to disappear in ionic liquid-based biopolymer electrolytes. This implies the complete dissolution of LiPF6 in the polymer electrolytes and contributes to the complexation of LiPF6 with corn starch and BmImTf [17]. Upon addition of 30 wt.% of BmImTf, the two diffraction peaks of corn starch are still present. As can be observed, these two characteristic peaks have been shifted to 2θ angles of 15.4° and 24.3°. However, the peaks become broader in comparison with Tf 0. The intensity of a peak is also drastically reduced with inclusion of 30 wt.% of BmImTf. It can be inferred that addition of ionic liquid would weaken the transient bonds within the polymer system, distort the ordered arrangement of the polymer backbone and eventually decrease the degree of crystallinity. In other words, it increases the amorphous region in polymer electrolytes, facilitates the ion mobility and provides a more flexible backbone, which in turn leads to higher ionic conductivity. The amorphous behavior becomes more visible with increasing the ionic liquid loadings. Obviously, the XRD pattern has undergone the change in shape, from two weak peaks to a broad band. Tf 5 is illustrated to show a more intense band than Tf 8 by comparing Fig. 3c with d. This reveals that Tf 5 has lesser amorphous degree which leads to lower ionic conductivity. As shown in Fig. 6, Tf 8 illustrates the lowest peak intensity and indicates the highest amorphous degree in comparison with other samples studied. This highest amorphous behavior of Tf 8 would enhance the ion transportation to a greater extent and thereby increase the ionic conductivity to a maximum.
Differential scanning calorimetry (DSC)
The thermal properties of samples are examined through DSC analysis. The result is shown in Fig. 7. A small decrease in heat, from exothermic to endothermic, is known as glass transition temperature (T g). A T g is defined as a critical temperature at which the amorphous material changes its behavior from glassy state to rubbery state. Pure corn starch demonstrates T g of 56 °C. However, T g exhibits an abrupt decrease upon addition of LiPF6 and a subambient T g of −19 °C is observed. The decrease in T g infers the higher segmental mobility of polymer backbone. This is due to the distortion of the weak interactive hydrogen bonds within the polymer chains by doping lithium salt which eventually forms the complexation. The relative T g value is further decreased with addition of ionic liquid. Strong plasticizing effect is the main attributor for this phenomenon. Ionic liquid weakens the dipole–dipole interactions and transient inter- and intramolecular bonds among starch granules. Thus, it tends to soften the polymer network and improves the flexibility of polymer backbone, enhancing the segmental mobility of the macromolecules which confers higher ionic conductivity.
Upon addition of 30 wt.% of BmImTf, T g of −22 °C is observed. The result is comparable with the ionic liquid-free biopolymer electrolytes because of its deficient plasticizing effect. However, it is also found that plasticizing effect becomes more noticeable with increasing ionic liquid loadings. It causes the drop in T g to −24 °C and −29 °C for Tf 5 and Tf 8, respectively, allowing a conclusion that the most ionic conducting character of Tf 8 is ascribed to the strongest plasticizing effect which produces the most flexible polymer complex. Therefore, it enhances the mobility of polymer segments significantly and then favors the ionic transportation with a highly flexible polymer backbone.
There is an endothermic peak in the thermogram, which is well-known as the melting peak, T m. It corresponds to the melting point of BmImTf as no transition is observed for Tf 0 in the temperature regime [18, 19]. Sharp melting peaks are observed for Tf 3 and Tf 5 at 10 °C and 13 °C, respectively. In contrast, a weaker peak with smaller peak breadth (or termed as heat of fusion) has been observed for Tf 8 at 15 °C. It reflects the lower crystalline fraction in the polymer electrolyte compared to Tf 3 and Tf 5. In other words, inclusion of 80 wt.% of BmImTf would disorder the ordered arrangement among the macromolecules to the greatest extent. Accordingly, it boosts the flexibility of polymer matrix and ultimately accelerates the ionic movement, increasing the ionic conductivity to a maximum level. Furthermore, the melting temperature of the transition peak increases with the mass ratio of ionic liquid. It is assigned to the formation of self cross-linkage as a result of excessive N–O bonding between BmIm cations and the polymer complex. The higher the ionic liquid quantity, the more cross-linkages would form. Hence, more energy is required to deform the crystalline region into melted form. In conclusion, it exhibits higher melting temperature at higher ionic liquid loadings.
Conclusion
Corn starch-based biopolymer electrolytes were prepared by solution casting technique with incorporation of dopant salt, LiPF6 and ionic liquid, BmImTf. The highest ionic conductivity of (6.00 ± 0.01) × 10−4 S cm−1 was achieved at 80 °C. Temperature dependence–ionic conductivity study verified that the samples exhibit Arrhenius behavior and indicated the ion hopping mechanism in the polymer matrices. Inclusion of ionic liquid helps in demolishing the crystalline phase of the polymer electrolytes, as proven in the XRD analysis. Lower T g was determined for ionic liquid-based biopolymer electrolytes. This discloses the higher flexibility of polymer electrolytes due to the plasticizing effect of the ionic liquid.
References
Shamsipur M, Beigi AAM, Teymouri M, Pourmortazavi SM, Irandoust M (2010) J Mol Liq 157:43–50
Liang R, Yang M, Xuan X (2010) Chin J Chem Eng 18:736–741
Ramesh S, Liew C-W, Ramesh K (2011) J Non-Cryst Solids 357:2132–2138
Xiong H, Tang S, Tang H, Zou P (2008) Carbohydr Polym 71:263–268
Pawlicka A, Sabadini AC, Raphael E, Dragunski DC (2008) Mol Cryst Liq Cryst 485:804–816
Marcondes RFMS, D'Agostini PS, Ferreira J, Girotto EM, Pawlicka A, Dragunski DC (2010) Solid State Ionics 181:586–591
Ma X, Yu J, He K, Wang N (2007) Macromol Mater Eng 292:503–510
Magistris A, Mustarelli P, Quartarone E, Tomasi C (2000) Solid State Ionics 136–137:1241–1247
Liu J-W, Wang Z-X, Guo H-J, Peng W-J, Zhang Y-H, Hu Q-Y (2010) Trans Nonferrous Met Soc China 20:344–348
Zinigrad E, Larush-Asraf L, Gnanaraj JS, Sprecher M, Aurbach D (2005) Thermochim Acta 438:184–191
Kim KS, Park SY, Choi S, Lee H (2006) J Power Sources 155:385–390
Sankri A, Arhaliass A, Dez I, Gaumont AC, Grohens Y, Lourdin D, Pillin I, R-Sabate A, Leroy E (2010) Carbohydr Polym 82:256–263
Aravindan V, Vickraman P, Krishnaraj K (2009) Curr Appl Phys 9:1474–1479
Ramesh S, Yin TS, Liew C-W (2011) Effect of dibutyl phthalate as plasticizer on high-molecular weight poly(vinyl chloride)-lithium tetraborate-based solid polymer electrolytes. Ionics. doi:10.1007/s11581-011-0568-9
Kumara R, Subramania A, Sundaram NTK, Kumar GV, Baskaran I (2007) J Membr Sci 300:104–110
Ramesh S, Liew C-W, Morris E, Durairaj R (2010) Thermochim Acta 511:140–146
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2006) Solid State Ionics 177:2679–2682
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD (2001) Green Chemistry 3:156–164
Xu W, Wang LM, Nieman RA, Angell CA (2003) J Phys Chem B 107:11749–11756
Acknowledgment
This work was supported by the Fundamental Research Grant Scheme (FRGS) from Ministry of Higher Education, Malaysia (FP009/2010B). One of the authors, Chiam-Wen Liew, gratefully acknowledges the “Skim Bright Sparks Universiti Malaya (SBSUM)” for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liew, CW., Ramesh, S., Ramesh, K. et al. Preparation and characterization of lithium ion conducting ionic liquid-based biodegradable corn starch polymer electrolytes. J Solid State Electrochem 16, 1869–1875 (2012). https://doi.org/10.1007/s10008-012-1651-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1651-5