Abstract
Carbon nanotubes (CNTs), including multi-walled CNTs (MWCNTs) and single-walled CNTs (SWCNTs), are employed as conductive additives in lithium ion batteries. The effects of MWCNTs’ carbon precursors, diameter, and weight fraction on the electrochemical behavior of MWCNTs/LiCoO2 composite cathode are investigated. Meanwhile, a comparison is made between SWCNTs /LiCoO2 and MWCNTs/LiCoO2. Among the three kinds of carbon precursors: CH4, natural gas, and C2H2, MWCNTs prepared from CH4 are very fit for acting as conductive additives due to their better crystallinity and lower electrical resistance. MWCNTs with smaller diameter favor improving the electrochemical behavior of MWCNTs/LiCoO2 composite cathode at higher charge/discharge rate owing to their advantage in primary particle number in unit mass. To make full use of LiCoO2 at higher rate, it is necessary to add at least 5 wt.% of MWCNTs with a diameter 10~30 nm. However, SWCNTs are not expected to be added into LiCoO2 composite cathode since they tend to form bundles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium ions intercalation/deintercalation reaction rates depend on electronic conducting because the process of lithium ion insertion/deinsertion is simultaneously accompanied by electron removal [1, 2]. In lithium ion batteries, the layered compounds LiCoO2, LiNiO2 and spinel compound LiMn2O4 have served as very effective cathode active materials. Their high electrical resistance easily results in a serious polarization and poor utilization of active materials. Therefore, carbon materials [1–11], owing to their high electrical conductivity and chemical inertness, are often employed as conductive additives for improving the utilization of active materials of the cathode. As one of carbon materials, carbon nanotubes’ (CNTs’) high electrical conductivity and one-dimensional structure make them very qualified for acting as conductive additives. Some studies [12–17] have demonstrated that carbon nanotubes are more effective for decreasing the internal resistance and improving electrochemical behavior of the composite cathode when compared with acetylene black and chemical vapor deposit carbon fibers.
It is well-known that CNTs’ properties vary with their carbon precursors, their diameter, etc. At present, carbon precursors, used in chemical vapor deposition in our laboratory, involve CH4, natural gas (NG) and C2H2, and CNTs’ average diameter specification ranges from 10~30, 30~50, 80~100 to 100~200 nm. So far, we cannot obtain any information about the effect of these parameters on the electrochemical performance of LiCoO2 cathode from relevant literature. This work will focus how the CNTs’ carbon precursors, diameter, and weight fraction etc. influence the electrochemical behavior of CNTs/LiCoO2 composite cathode.
Experimental
The working electrode consists of 94 wt.% of active material LiCoO2, 3 wt.% of conductive additives, and 3 wt.% of binder LA132 (whose chemical composition is polyacrylonitrile). The conductive additives used in the experiments involve multi-walled CNTs (MWCNTs) and single-walled CNTs (SWCNTs).
All of the CNTs were provided by Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences. MWCNTs with different diameter were prepared through chemical vapor catalytic decomposition of carbon over Ni/La2O3 with a various mol ratio 0.3, 0.5, 0.7, and 0.9 at 973.15 K for 30 min in a fixed-bed reactor, and SWCNTs were prepared over Co/MgO with a mol ratio 0.3. To remove the metal particles attaching to CNTs, these CNTs samples were additionally treated in dilute nitric acid at 60 °C for 3 h. LiCoO2 with a theoretical capacity 156 mAh g−1 was provided by BTR Energy Materials Limited Co., Ltd, Shenzhen, China, and the binder LA132 was obtained from Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences.
The powder XRD patterns of CNTs were obtained with a Philips PW 1730 diffractometer using CuKα radiation. TEM images and SEM images were obtained from JEOL JEM-100CX and JEOL JSM-35, respectively. The electrical resistance of the additives and corresponding composite cathodes was measured by the four-point probe [18] equipment D41-11C/ZM621.21C made by Beijing Sevenstar Electronics Co., Ltd, Beijing, China.
The LiCoO2 composite electrode was fabricated as follows: First, a pre-dispersed suspension of CNTs (MWCNTs or SWCNTs) was prepared by ultrasonicating in ethanol with polyvinylpyrrolidone as dispersing agent, and then the active material LiCoO2 was homogeneously mixed with the pre-dispersed CNTs suspension and the binder LA132 in an agate mortar. After that, the mixed slurry was spread onto a 10-μm-thick Al foil to form an electrode. Next, the electrode was dried under vacuum at 100 °C for 24 h and finally was pressed with a roll to enhance the contact of the particles. The electrode thickness was about 60 μm. The coin cells were assembled in a glove box under an Ar atmosphere with the humidity less than 5 ppm. A porous polypropylene separator (Celgard #2400) was packed into the cells.
The working electrodes were evaluated in a two-electrode cell in which metal Li sheet acted as the counter electrode. Electrochemical measurements of the charge–discharge of LiCoO2 composite electrode were conducted in an electrolyte solvent EC/DEC (1:1 in volume, EC and DEC mean ethylene carbonate and diethyl carbonate, respectively) containing 1 M LiClO4 (Mitsubishi Chemical Corporation) at room temperature. The cells were cycled between 4.3 and 3.0 V versus Li/Li+. The rate capacity was measured after five cycles at 0.2 C. The current density corresponding to 1.0 C is 0.56 mA cm−2.
Results and discussion
Effect of carbon precursors of MWCNTs
SEM, XRD, and electrical resistance
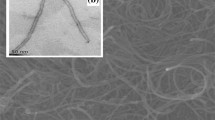
Figure 1 displays the TEM images of MWCNTs prepared from the decomposition of various carbon precursors. Every kind of MWCNTs has an almost uniform diameter 30~50 nm. From the appearance, we can see the MWCNTs prepared from CH4 almost do not show any defects. After treated in dilute nitric acid, the MWCNTs from the three kinds of carbon precursors are very clear, and no metal particles and amorphous carbon are observed to attach to the surface of MWCNTs.
Figure 2 shows the XRD patterns of MWCNTs prepared from various carbon precursors. The lattice spacing d (002) can be used to characterize the degree of graphitization. For the MWCNTs prepared from CH4, NG, and C2H2, the corresponding d (002) values are 0.3402, 0.3422, and 0.3458 nm, respectively. Apparently, the d (002) value of MWCNTs prepared from CH4 is the closest to that of graphite (0.3354 nm). This fact suggests MWCNTs prepared from CH4 have higher crystallinity than those from the other two precursors. Meanwhile, Raman spectra in Fig. 3 offer similar evidence for this. Obviously, the spectrum of MWCNTs prepared from CH4 exhibits the smallest D-band and a distinct G-band. Its peak intensity ratio of the D-band and the G-band equals 0.214, smaller than the values of MWCNTs prepared from NG and C2H2, 0.252, and 0.348. Combining Figs. 2 and 3 with Table 1, we naturally draw a conclusion that electrical resistances of MWCNTs and the corresponding composite cathodes depend on the MWCNTs’ crystallinity. In other words, the higher the crystallinity of MWCNTs is, the lower the electrical resistance is.
Effect of carbon precursors on electrochemical behavior of MWCNTs/LiCoO2 composite cathodes
As shown in Fig. 4, at lower current rate, the three kinds of MWCNTs/LiCoO2 composite cathodes have approximately equivalent capacity. However, with the increase of the current rate, their difference in discharge capacity tends to increase. Particularly, the difference of specific capacity between CH4/LiCoO2 and C2H2/LiCoO2 reaches up to about 15 mAh g−1 at the current rate 3.0 C. Among the three kinds of MWCNTs/LiCoO2 composite cathodes, the cathode, in which MWCNTs are prepared from CH4, exhibits the best electrochemical performance. This phenomenon is attributed to the internal resistance of the composite cathode. The lower internal resistance results in the lower polarization potential and then the higher discharge potential. Therefore, as shown in Fig. 5, the velocity of ending discharge slows down, and the higher discharge specific capacity is obtained.
Effect of MWCNTs’ diameter on electrochemical behavior of MWCNTs/LiCoO2 composite cathode
Owing to the best electrochemical performance of the composite cathode in which MWCNTs are prepared from CH4, this kind of MWCNTs is used in the following experiments. MWCNTs’ weight fraction still remains at 3 wt.% in the cathode.
The electrochemical behaviors of MWCNTs/LiCoO2 cathodes containing MWCNTs with different diameter are compared in Fig. 6. At lower current rate, MWCNTs/LiCoO2 cathodes containing MWCNTs with different diameter almost show the same discharge capacity. However, when it comes to higher current rate, the situation is completely different. The cathode containing MWCNTs with relatively smaller diameter begins to exhibit their advantages. For instance, when the composite cathodes are charged/discharged at the rate 3.0 C, the specific capacity of the composite cathode containing MWCNTs with outer diameter 10~30 nm is about 30 mAh g−1 larger than that of the cathode containing MWCNTs with outer diameter 100~200 nm. The trend in Fig. 6 suggests us that MWCNTs with smaller diameter favor improving electrochemical behavior of the MWCNTs/LiCoO2 composite cathode at higher charge/discharge rate. A simplifying model helps us to explain the phenomenon.
As we all know, MWCNTs contain from three or four to 20 or more cylindrical layers with concentric caps formed on the ends. In order to conveniently estimate the relative number of MWCNTs, MWCNTs are firstly simplified as following: (1) MWCNTs are considered to be composed of regular cylinders by neglecting the caps on the two ends (see Fig. 7); (2) MWCNTs’ other physical parameters, such as their length, density, etc., are looked as the same except their diameter. Based on the above assumptions, for two kinds of MWCNTs with outer diameter D 1 and D 2, respectively, when they have the same mass M, we have the following expression.
where d 1 and d 2 represent the inner diameter of MWCNTs whose corresponding outer diameter is D 1 and D 2, respectively. Similarly, n 1 and n 2 refer to the primary particle number of corresponding MWCNTs. ρ means MWCNTs’ density, and L is MWCNTs’ length. According to the above expression, MWCNTs with smaller outer diameter possess a larger number of primary particles in unit mass than those with larger outer diameter. For example, the primary particle number of MWCNTs with D 1 20 nm and d 1 5 nm is 26.4 times as large as those with D 2 100 nm and d 2 10 nm. Thus, in the presence of MWCNTs with smaller outer diameter, more continuous conductive networks form in the electrode and more electron channels are provided to lessen the internal resistance and polarization loss. The result is that more active materials are effectively utilized, especially at higher current rate.
Effect of MWCNTs’ weight fraction on electrochemical behavior of LiCoO2 composite cathodes
On the basis of the above experiments, all the MWCNTs utilized in this part are prepared from CH4, and their diameter is about 10~30 nm. The amount of binder LA132 is fixed at 3 wt.%. As shown in Fig. 8, the specific capacity of LiCoO2 composite cathode increases with the increase of the content of MWCNTs when MWCNTs’ content ranges from 1 to 5 wt.%. But if we continue to increase the MWCNTs’ content from 5 to 7 wt.%, the difference in the specific capacity is neglectful no matter what rate at which the cathodes are discharged. It is worthy to point out that when the content of MWCNTs in the cathode is 1 wt.%, the capacity of LiCoO2 composite cathode dramatically decreases with the increase of charge/discharge rate. Apparently, this is because the electron channels provided by only 1 wt.% of MWCNTs cannot meet the need of the electron transportation in the cathode and guarantee an effective utilization of active material LiCoO2. In order to take full advantage of LiCoO2 at higher rate, it is necessary to add at least 5 wt.% of MWCNTs.
Comparison between SWCNTs/LiCoO2 composite cathode and MWCNTs/LiCoO2 composite cathode
The idea substituting SWCNTs for MWCNTs comes from the fact that MWCNTs with smaller outer diameter favor improving electrochemical behavior of the MWCNTs/LiCoO2 at higher charge/discharge rate. If we take the primary particle number of CNTs in unit mass into account, SWCNTs should be much better for improving electrochemical behavior of the LiCoO2 cathode than MWCNTs because SWCNTs’ outer diameter equals about 1.70 nm. What is more, SWCNTs display some smaller electrical resistivity (0.01 Ω cm) than MWCNTs.
However, the results about the electrochemical behavior of SWCNTs/LiCoO2 composite cathode do not support our idea. In Fig. 9, at every rate, SWCNTs/LiCoO2 cathode cannot be regarded as in the same category with MWCNTs/LiCoO2 cathode (MWCNTs’ outer diameter is 30~50 nm) in specific capacity, let alone compare with the composite cathode in which MWCNTs’ diameter is 10~30 nm. This phenomenon is attributed to SWCNTs’ property. According to Fig. 10, SWCNTs mainly exist in the form of bundles, whose diameter ranges from tens to hundreds of nanometers. Moreover, bundles may emerge in the shape of rings. Hence, in practice, in comparison with MWCNTs, the effective primary particle number of SWCNTs in the cathode significantly decreases, and the isolated LiCoO2 particles increase. As the result, many LiCoO2 particles do not participate in the electrochemical reaction at all, and the discharge curve of SWCNTs/LiCoO2 composite cathode drops fast as in Fig. 11.
Conclusion
Carbon precursors affect the degree of CNTs’ graphitization and then the electrochemical performance of MWCNTs/LiCoO2 composite electrode. Among the three carbon precursors: CH4, NG, and C2H2, MWCNTs prepared from CH4 are the best one for acting as conductive additives due to their better crystallinity and lower electrical resistance. MWCNTs with smaller diameter display the advantage in primary particle number in unit mass and then go for improving electrochemical behavior of the MWCNTs/LiCoO2 composite cathode at higher charge/discharge rate. As for the weight fraction of MWCNTs with an outer diameter 10~30 nm, to make full use of LiCoO2 material at any rate, it is necessary to add at least 5 wt.% of MWCNTs.
Since SWCNTs tend to form bundles, they are not expected to be added into LiCoO2 composite cathode when compared with MWCNTs.
References
Dominko R, Gaberscek M, Drofenik J, Bele M, Jamnik J (2003) Electrochim Acta 48:3709–3716
Dominko R, Gaberscek M, Drofenik J, Bele M, Pejovnik S, Jamnik J (2003) J Power Sources 119–121:770–773
Dong HJ, Seung MO (1998) Electrochim Acta 43:1023–1029
Qingtang Z, Gongchang P, Guoping W, Meizhen Q, Zuolong Y (2009) Solid State Ion 180:698–702
Junichi S, Masanobu N, Hiromasa I, Yoshiharu U, Masataka W (2004) Electrochem Solid-State Lett 7:A27–A29
Christine AF, Xiaoping S, Chung DDL (1996) J Power Sources 58:41–54
Wang GX, Yang L, Chen Y, Wang JZ, Steve B, Liu HK (2005) Electrochim Acta 43:4649–4654
Momchilov A, Trifonova A, Banov B, Pourecheva B, Kozawa A (1999) J Power Sources 81–82:566–570
Im D, Manthiram A (2003) Solid State Ion 159:249–255
Jin KH, Jong HL, Seung MO (2002) J Power Sources 111:90–96
Shintaro K, Norio T, Mio S, Yuichi S (2003) J Power Sources 119–121:924–928
Wang G, Zhang Q, Yu Z, Qu M (2008) Solid State Ion 179:263–268
Li X, Kang F, Shen W (2006) Carbon 44:1334–1336
Kyuyun S, Young HL, Hong SL (2006) J Power Sources 158:1425–1430
Yan F (2010) Mater Chem Phys 121:302–307
Yunjian L, Xinhai L, Huajun G, Zhixing W, Wenjie P, Yong Y, Rufu L (2008) J Power Sources 184:522–526
Zhang Q, Qu M, Niu H, Yu Z (2007) New Carbon Mater 22:361–364
Christine AF, Xiaoping S, Chung DDL (1996) J Power Sources 58:55–66
Acknowledgment
This research is financed by the project 07C645 from the Education Department of Hunan Province, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, G., Li, H., Zhang, Q. et al. The study of carbon nanotubes as conductive additives of cathode in lithium ion batteries. J Solid State Electrochem 15, 759–764 (2011). https://doi.org/10.1007/s10008-010-1143-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-010-1143-4