Abstract
A copper hexacyanoferrate film was obtained on a modified electrode prepared by self-assembly of 3-mercaptopropyltrimethoxysilane on a gold surface. The film thickness was controlled using a layer-by-layer technique to tune the electrocatalytic properties of the electrode. Two electrodes with different hexacyanoferrate film thicknesses were prepared via three immersions (AuS/CuHCF3) and six immersions (AuS/CuHCF6) of the film in the precursor solutions. Cyclic voltammetry data were obtained to determine the adequate film thickness. Scanning electron microscopy images showed a roughness increase due to the growth of the film thickness at the electrode surface. Electrochemical impedance spectroscopy showed distinct behavior for the two electrodes prepared; while diffusion and charge transfer processes can be observed in both electrodes, an additional capacitive process at intermediary frequencies was observed for the AuS/CuHCF6 electrode. The charge transfer resistance (R ct) for the AuS/CuHCF3 electrode (19.6 Ω cm2) was lower than for AuS/CuHCF6 (27.9 Ω cm2) due to the hexacyanoferrate film thickness, since the charge transfer process demands the simultaneous diffusion of K+ into the surface. Cyclic voltammetry was used to evaluate the application of the AuS/CuHCF3 electrode as an electrochemical sensor, revealing a linear correlation for hydrazine concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, an increasing interest in producing interfaces with well-defined composition, structure, orientation, thickness, and chemical functionality has been observed. Specific modification of surfaces is a major challenge in the preparation of function-oriented materials. Of the various procedures employed to obtain these types of materials, the layer-by-layer method has been most often used to prepare different types of self-organized systems [1–4].
The literature reports that gold interacts strongly with sulfur, forming a stable bond between the two species; as a consequence, a simple surface modification can be achieved. The alkoxy silane molecular layers have a significant advantage over other types of silanes due to the possibility of acting as a bifunctional group. The formation of self-assembled layers of mercaptopropyltrimethoxysilane on gold increases the electrode-film adhesion via thiol group interaction on both substrate and film. To obtain a tridimensional network on the surface electrode, the sol–gel method starting from a solution where hydrolysis is carried out under optimal conditions and condensation occurs very slowly, is an interesting approach. In these conditions, sulfur bonds with gold at the surface electrode and the self-condensation of Si–OH groups form the network. The gold is covered by the siloxane framework and presents free SH groups at the new electrode surface. The self-assembly of different electroactive layers on a substrate can be used to tune the electrocatalytic properties of the electrode [5–7].
Anionic electroactive complexes, especially Prussian blue analogs, have been extensively studied due their interesting electrocatalytic, electrochromic, ion exchange, ion sensing, and photomagnetic properties. Hexacyanoferrate ion is a chemical species known to have electron mediator properties and can be adsorbed on the surface of a solid by a simple ion exchange reaction to obtain a chemically modified electrode. A wide range of substrates were used to obtain this type of electrode including gold, platinum, glassy carbon, ceramic carbon, and fiber carbon. These compounds are usually prepared by chemical and electrochemical methods [8–11].
Many reports in the literature describe the oxidation process of hydrazine due to high interest in the chemical and pharmaceutical industries. This substance is used in many fields, for example in agriculture, as a pesticide, as a pharmaceutical intermediate, water treatment, corrosion protection, textile dyes, and fuel cells. As a consequence, recent activities have been directed towards the development of sensitive and selective analytical methods for the determination of hydrazine [12–14].
In this work, the experimental procedures for the preparation and characterization of a copper hexacyanoferrate film on a modified electrode prepared by the self-assembly of a 3-mercaptopropyltrimethoxysilane on gold surface are reported. The sol–gel processing method was used to prepare the mercaptopropyl-modified electrode, as described for similar systems [15–18]. The copper hexacyanoferrate (CuHCF) film thickness was carefully controlled using a layer-by-layer technique to achieve an ideal film distribution on the surface and, thus, more efficient mass transport on the electrode [19–21]. The electrochemical response of the copper hexacyanoferrate film and its thickness on the substrate surface were investigated by cyclic voltammetry. The film morphology at the electrode surface was studied by scanning electron microscopy. Electrochemical impedance spectroscopy was used to determine the value of the charge transfer resistance.
Experimental
Preparation of mercaptopropyl modified gold electrode
The Au working electrode was polished with alumina slurry and sonicated in water/ethanol (1:1 v/v) for 15 min. The polished Au electrode was then electrochemically cleaned by cycling the potential between 0.2 and 1.5 V in 0.05 mol L−1 H2SO4 at a scan rate of 0.1 V s−1 for 10 min to obtain a cyclic voltammogram characteristic of a clean Au electrode.
The sol–gel network was prepared using a 20 mmol L−1 3-mercaptopropyltrimethoxysilane (Acros Organics) ethanolic solution at a pH adjusted to 3.0 with HCl. The cleaned Au electrode was modified by immersion in this solution for 30 min. The resulting modified Au electrode was then thoroughly washed with water to remove any residue.
Preparation of copper hexacyanoferrate film on modified gold electrode
To control the thickness of the copper hexacyanoferrate film, the obtained 3-mercaptopropyltrimethoxysilane modified gold electrode (AuS) was immersed in a 20 mmol L−1 copper chloride aqueous solution and then washed with water to remove the physically adsorbed metal ions. The electrode was then put into contact with a 20 mmol L−1 potassium hexacyanoferrate aqueous solution to form a film of copper hexacyanoferrate complex (K 4-2n Cu n [Fe(CN)6]) on the surface, hereafter designated as AuS/CuHFC. The effect of the number of immersions in copper and hexacyanoferrate ion were studied from one to six immersions. For the preparations with three and six immersions, the contact time of the electrode with the copper and iron solutions was varied from 5, 10, 20, and 30 min. With the preparation conditions established, the prepared electrodes with three and six immersions for 20 min each were used for the electrochemical studies and, hereafter, designated as AuS/CuHCF3 and AuS/CuHCF6, respectively.
Scanning electron microscopy
The AuS/CuHCF3 and AuS/CuHCF6 modified electrodes prepared on gold wires were fixed onto an aluminum support with double-sided tape and coated with a layer of Au (ca. 15 nm) by a BalTec SCD 050 Sputter Coater apparatus (60 mA current for 60 s). Scanning electron microscopy (SEM) analyses were carried out on a JSM 5900LV low-vacuum microscope operating at an accelerating voltage of 25 kV while utilizing the secondary electron detector.
Electrochemical study
Electrochemical studies were carried out using cyclic voltammetry and electrochemical impedance spectroscopy (EIS) techniques. A three-electrode system consisting of the modified gold working electrode, a saturated calomel reference electrode, and a platinum wire counter electrode was utilized. Impedance experiments were carried out with a frequency range between 100 kHz and 10 mHz and a 10 mV amplitude sinusoidal voltage. The supporting electrolyte solution for the experiments was 0.10 mol L−1 KNO3. All measurements were taken with an Autolab PGSTAT 30 potentiostat–galvanostat apparatus.
Results and discussion
Electrochemical electrode characterization
The modified gold electrode coating was prepared via the self-assembly and condensation of a (CH3O)3Si(CH2)3SH precursor in the presence of water and hydrochloric acid as the condensation catalysts. The copper hexacyanoferrate film was prepared with consecutive immersions in the solutions of the complex components. Solid metal hexacyanometalates are very useful as ion-selective materials, as the redox reactions of lattice metal ions are accompanied by a reversible exchange of cations between the electrolyte solution and interstitial holes of the solid compound. Figure 1 shows a schematic representation of the idealized cubic structure for the first layer [22]. The hexacyanoferrate (II) ion is attached to the electrode surface by a well described covalent interaction between the metal and the free SH groups at the siloxane network [1, 5, 10].
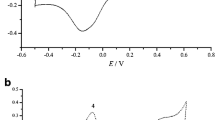
Figure 2 shows the cyclic voltammograms of the AuS/CuHCF modified gold electrode in 0.10 mol L−1 KNO3 solution at a scan rate of 50 mV s−1. The results showed the iron oxidation peak at approximately 0.67 V and the respective reduction peak at 0.62 V. The current density reveals a direct relation with the number of immersions as expected, indicating an increase of the copper hexacyanoferrate film thickness after each immersion. The redox process at 0.90 V can be attributed to the reduction of free hexacyanoferrate ion, which remains at the surface after washing the electrode [23].
To control the copper hexacyanoferrate film thickness, the immersion time was studied with two different numbers of immersions at the same conditions. The results are presented in Fig. 3. Current density shows a proportional increase with immersion time. Considering the maximum density current at the iron oxidation peak, the hexacyanoferrate film thickness was calculated for the conditions used in the experiment [15, 24], and the results are presented in Table 1. For the AuS/CuHCF3 electrode, the thickness increase occurs mainly at 20 minutes of immersion time, while for the AuS/CuHCF6 electrode the increase occurs at 10 min of immersion time. The time of 20 minutes was chosen because the intention is to achieve the best condition between a lower thickness and good surface recovery. In addition, the thickness difference between the AuS/CuHCF3 and AuS/CuHCF6 at this time is higher for 30 min, which allows a better situation to study the thickness influence on diffusion process and electron transfer. The electrode which was immersed three times presented an adequate thickness, as there was no saturation of the electrode surface, allowing for a more efficient mass transport into the film. The prepared electrodes had long-term stability, as there was no change in the current density after ten to 50 cycles.
Cyclic voltammograms using different supporting electrolytes were obtained to determine the formation of the copper hexacyanoferrate film at the electrode surface. The literature reports on the solution cation effect on the electrochemical behavior of electrodes with hexacyanoferrate films [15, 25]. The cation diffuses into the film to maintain system electroneutrality. When a cation with a high hydrated radius is present in the solution, the voltammogram presents broader iron oxidation-reduction peaks. This can be explained by a lower diffusion velocity of the supporting electrolyte cation into the CuHCF film and the incorporation of cation into the hexacyanoferrate film, resulting in a mixture of complex with different stoichiometric. This leads to different iron oxidation-reduction potentials and the subsequent overlapping of the reactions. The redox reaction at the electrode surface is complex and involve the mixture of two compounds, Cu2[Fe(CN)6] / K2Cu[Fe(CN)6], which can be represented by K2Cu3[FeII(CN)6]2. An overall representation can be written as follows [26]:
The cyclic voltammograms for AuS/CuHCF3 and AuS/CuHCF6 obtained with different supporting electrolytes in the solution are presented in Fig. 4. The result shows characteristic electrochemical behavior as described for similar systems, with a broader oxidation-reduction peak and a change of the peak potentials for the electrode with a thicker hexacyanoferrate film (AuS/CuHCF6) [12].
Copper hexacyanoferrate film on AuS/CuHCF electrode surface
Images obtained by scanning electron microscopy of the electrode surface are presented in Fig. 5. A roughness increase at the electrode surface owing to surface modification of the gold electrode with the mercaptopropyl sol–gel network can be observed (Figs. 5a and b). Figure 5c presents a different morphology at the surface when compared with 5b; this can be attributed to copper hexacyanoferrate film formation. With the magnification used, a surface roughness increase is observed when comparing AuS/CuHCF3 (Fig. 5c) with AuS/CuHCF6 (Fig. 5d), owing to the copper hexacyanoferrate film growth at the electrode surface [15].
Electrochemical studies
Figure 6 shows the cyclic voltammetry curves for AuS/CuHCF3 (taken as a representative sample) sweeping the potential between 0.0 and 1.0 V with a scan rate between 20 and 500 mV s−1. A well-defined redox couple with mid-point potential E m = 0.64 V (E m = (E pa + E pc)/2, where, E pa and E pc are the anodic and cathodic peak potentials, respectively) was observed at 20 mV s−1 in 0.1 mol L−1 KNO3 solution as the supporting electrolyte. With the scan rate increment, the difference potential between the oxidation and reduction pick increased, indicating a quasi-reversible system. A linear relationship between the current density and the square root of the scan rate was observed for AuS/CuHCF3, indicating a high dependence on counter-ion diffusion at the surface electrode. The electrochemical behavior presented is typical for electrodes with Prussian blue analogs, and the E m observed is very similar to those found for copper hexacyanoferrate immobilized on different substrate surfaces [27, 28]. The cyclic voltammograms with an increase of the potential scan rate for the AuS/CuHCF6 electrode presented the same behavior described for AuS/CuHCF3.
To determine the conducting character of the material, i.e., the capacitive and diffusion processes that occur on the surface of the material, EIS measurements were obtained. Figure 7 shows the complex plane impedance plots for AuS/CuHCF6 at (a) OCP potential and (b) iron oxidation potential (0.674 V). The results given in Fig. 7a present a capacitive behavior for all frequency ranges, which is characteristic for systems in chemical equilibrium. Different processes can be observed for measurements obtained at the iron oxidation potential (Fig. 7b). At higher frequency values, electron transfer from iron in the hexacyanoferrate film occurs. A capacitive process can be observed at intermediary frequencies, and the behavior at low frequencies can be attributed to the K+ diffusion process into the film to maintain electroneutrality of the system [8, 29].
Figure 8 presents the complex plane impedance plot for the AuS/CuHCF3 and AuS/CuHCF6 electrodes. The results obtained show a strong dependence of the charge transfer process on the thickness of the hexacyanoferrate film on the electrode surface. For AuS/CuHCF3, only the diffusion and charge transfer processes can be observed; however, for AuS/CuHCF6 an additional capacitive process at intermediary frequencies can be observed. [30]
The electron transfer resistance was determined from the intersection of the semicircle, which can be related to iron oxidation and reduction. The values of electrolyte, polarization and charge transfer resistance obtained for the AuS/CuHCF3 and AuS/CuHCF6 are presented in Table 2.
The small difference in the R s values obtained for the prepared electrodes can be attributed to the electrochemical cell arrangement. The R ct value determined for the AuS/CuHCF6 electrode was higher than that obtained for the AuS/CuHCF3 electrode. This can be attributed to the hexacyanoferrate film thickness on the electrode surface, since the charge transfer process demands the simultaneous diffusion of K+ into the surface. With these results, it can be concluded that the AuS/CuHCF3 electrode presents the best properties for electrocatalytic studies, since the R ct is lower than for the AuS/CuHCF6 electrode and diffusion of counter-ions into the hexacyanoferrate film is faster.
Electrocatalytic oxidation of hydrazine
To evaluate the potential use of the material as an electrochemical sensor, the electrocatalytic oxidation of hydrazine was studied. Figure 9 shows the cyclic voltammograms obtained using (a) Au electrode, (b) AuS electrode, and (c) AuS/CuHCF3 modified electrode in the presence of hydrazine at a concentration of 12.9 × 10−2 mol L−1. The clean Au electrode reveals a peak associated with hydrazine oxidation. There are no electron transfers after the electrode has been modified with the mercaptopropyl film. An increase of the anodic current density of 0.6 mA cm−2 at ~0.7 V was observed for the AuS/CuHCF3 electrode. The cyclic voltammogram (9c) do not show the iron reduction peak because the hydrazine present in solution diffuses toward the electrode and reduces the iron (III) produced electrochemically. An increase of current density on oxidation peak can be observed during the anodic sweep, indicating that the immobilized copper hexacyanoferrate film promotes a higher electrocatalysis rate for hydrazine oxidation at the electrode surface [31].
Figure 10a shows the cyclic voltammograms obtained by cycling the potential between 0.0 and 1.0 V in different concentrations of hydrazine. The peak current density around 0.70 V, which increases with successive hydrazine additions, can be assigned to the hydrazine electrooxidation process at the solid–solution interface. A linear correlation for hydrazine concentrations between 1.48 × 10−4 and 1.29 × 10−3 mol L−1 is observed with a correlation coefficient of 0.9960. The detection limit has been obtained from three times the standard deviation of the blank per the slope of calibration curve [32]. The value was 7.4 × 10−4 mol L−1, a good sensitivity when compared to similar systems [29].
a Cyclic voltammograms of AuS-CuHCFe modified electrode in 0.10 mol L−1 KNO3 solution, with different hydrazine concentrations: a 0 mmol L−1, b 1.48 mmol L−1, c 2.91 mmol L−1, d 4.29 mmol L−1, e 5.63 mmol L−1, f 6.93 mmol L−1, g 8.19 mmol L−1, h 9.42 mmol L−1, i 10.6 mmol L−1, j 11.7 mmol L−1, and l 12.9 mmol L−1. Scan rate from 50 mV s−1. b Plot of the current density versus hydrazine concentration
Conclusion
A simple surface modification was achieved with the self-assembly of silane at the gold electrode surface. The methodology of producing this electrode is very simple and reproducible. The immersion of AuS into the reagent solutions resulted in a well-structured copper hexacyanoferrate film on the electrode surface, as indicated by cyclic voltammetry studies. The complex is not leached from the matrix surface when immersed in electrolyte solutions and submitted to electrochemical studies, probably due to the strong bond between the metal and sulfur.
The EIS results showed that the copper hexacyanoferrate film thickness was successfully tuned to obtain an optimized condition for electron transfer, since they indicated that the oxidation/reduction reaction process in the AuS/CuHCF3 system occurred with a lower charge transfer resistance than for the AuS/CuHCF6 electrode. Considering the high stability of the material, the system may be useful in the electroanalytical determination of hydrazine.
References
Wang F, Wang J, Chen H, Dong S (2007) Assembly process of CuHCF/MPA multilayers on gold nanoparticles modified electrode and characterization by electrochemical SPR. J Electroanal Chem 600:265–274
Bilewicz R, Witomski J, Van der Heyden A, Tagu D, Palin B, Rogalska E (2001) Modification of electrodes with self-assembled hydrophobin layers. J Phys Chem B 105:9772–9777
Ricci F, Palleschi G (2005) Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens Bioelectron 21:389–407
Bharathi S, Nogami M, Ikeda S (2001) Layer by layer self-assembly of thin films of metal hexacyanoferrate multilayers. Langmuir 17:7468–7471
Shen Y, Wu T, Zhang Y, Li J (2005) Comparison of two-typed (3-mercaptopropyl)trimethoxysilane-based networks on Au substrates. Talanta 65:481–488
Piwonski I, Grobelny J, Cichomski M, Celichowski G, Rogowski J (2005) Investigation of 3-mercaptopropyltrimethoxysilane self-assembled monolayers on Au(111) surface. Appl Surf Sci 242:147–153
Sibottier E, Sayen S, Gaboriaud F, Walcarius A (2006) Factors affecting the preparation and properties of electrodeposited silica thin films functionalized with amine or thiol groups. Langmuir 22:8366–8373
Yang G, Shen Y, Wang M, Chen H, Liu B, Dong S (2006) Copper hexacyanoferrate multilayer films on glassy carbon electrode modified with 4-aminobenzoic acid in aqueous solution. Talanta 68:741–747
de Tacconi NR, Rajeshwar K, Lezna RO (2003) Metal Hexacyanoferrates: electrosynthesis, in situ characterization, and applications. Chem Mater 15:3046–3062
Cheng WL, Dong SJ, Wang EK (2003) Site-selective self-assembly of MPA-bridged CuHCF multilayers on APTMS-supported gold colloid electrodes. Chem Mater 15:2495–2501
Pauliukaite R, Hocevar SB, Hutton EA, Ogorevc B (2008) Novel electrochemical microsensor for hydrogen peroxide based on iron-ruthenium hexacyanoferrate modified carbon fiber electrode. Electroanalysis 20:47–53
Prabakar SJR, Narayanan SS (2008) Amperometric determination of hydrazine using a surface modified nickel hexacyanoferrate graphite electrode fabricated following a new approach. J Electroanal Chem 617:111–120
Salimi A, Miranzadeh L, Hallaj R (2008) Amperometric and voltammetric detection of hydrazine using glassy carbon electrodes modified with carbon nanotubes and catechol derivatives. Talanta 75:147–156
Chen SM, Wu MH, Thangamuthu R (2008) Preparation, characterization, and electrocatalytic properties of cobalt oxide and cobalt hexacyanoferrate hybrid films. Electroanalysis 20:178–184
Marafon E, Lucho AMS, Francisco MSP, Landers R, Gushikem Y (2006) Thin film of copper hexacyanoferrate dispersed on the surface of a conducting carbon ceramic material, SiO2/ZrO2/C-graphite: Characteristics and electrochemical studies. J Braz Chem Soc 17:1605–1611
Pissetti FL, Francisco MSP, Landers R, Gushikem Y (2007) Phosphoric acid adsorbed on silica-ceria matrix obtained by sol-gel method: Studies of local structure, texture and acid property. J Braz Chem Soc 18:976–983
Lucho AMS, Pissetti FL, Gushikem Y (2004) Al2O3-coated 3-N-propylpyridinium chloride silsesquioxane polymer film: preparation and electrochemical property study of adsorbed cobalt tetrasulfophthalocyanine. J Colloid Interface Sci 275:251–256
Fujiwara ST, Pessoa CA, Gushikem Y (2003) Hexacyanoferrate ion adsorbed on propylpyridiniumsilsesquioxane polymer film-coated SiO2/Al2O3: use in an electrochemical oxidation study of cysteine. Electrochim Acta 48:3625–3631
Baioni AP, Vidotti M, Fiorito PA, Ponzio EA, de Torresi SIC (2007) Synthesis and characterization of copper hexacyanoferrate nanoparticles for building up long-term stability electrochromic electrodes. Langmuir 23:6796–6800
Wu BY, Hou SH, Yu M, Qin X, Li S, Chen Q (2009) Layer-by-layer assemblies of chitosan/multi-wall carbon nanotubes and glucose oxidase for amperometric glucose biosensor applications. Mater Sci Eng C-Biomim Supramol Syst 29:346–349
Ou CF, Chen SH, Yuan R, Chai YQ, Zhong X (2008) Layer-by-layer self-assembled multilayer films of multi-walled carbon nanotubes and platinum-Prussian blue hybrid nanoparticles for the fabrication of amperometric immunosensor. J Electroanal Chem 624:287–292
Kahlert H, Retter U, Lohse H, Siegler K, Scholz F (1998) On the determination of the diffusion coefficients of electrons and of potassium ions in copper(II) hexacyanoferrate(II) composite electrodes. J Phys Chem B 102:8757–8765
Karyakin AA (2001) Prussian Blue and its analogues: electrochemistry and analytical applications. Electroanalysis 13:813–819
Siperko LM, Kuwana T (1983) Electrochemical and spectroscopic studies of metal hexacyanometalate films. 1. Cupric hexacyanoferrate. J Electrochem Soc 130:396–402
Prabakar SJR, Narayanan SS (2007) Amperometric determination of paracetomol by a surface modified cobalt hexacyanoferrate graphite wax composite electrode. Talanta 72:1818–1827
Rutkowska IA, Stroka J, Galus Z (2008) Electrochemical properties of modified copper–thallium hexacyanoferrate electrode in the presence of different univalent cations. Electrochim Acta 53:3870–3878
Chen SM, Chan CM (2003) Preparation, characterization, and electrocatalytic properties of copper hexacyanoferrate film and bilayer film modified electrodes. J Electroanal Chem 543:161–173
Makowski O, Stroka J, Kulesza PJ, Malik MA, Galus Z (2002) Electrochemical identity of copper hexacyanoferrate in the solid-state: evidence for the presence and redox activity of both iron and copper ionic sites. J Electroanal Chem 532:157–164
Siperko LM, Kuwana T (1987) Electrochemical and spectroscopic studies of metal hexacyanometalate films. 3. Equilibrium and kinetic-studies of cupric hexacyanoferrate. Electrochim Acta 32:765–771
de Tacconi NR, Rajeshwar K, Lezna RO (2006) Electrochemical impedance spectroscopy and UV-vis reflectance spectroelectrochemistry of cobalt hexacyanoferrate films. J Electroanal Chem 587:42–55
Abbaspour A, Kamyabi MA (2005) Electrocatalytic oxidation of hydrazine on a carbon paste electrode modified by hybrid hexacyanoferrates of copper and cobalt films. J Electroanal Chem 576:73–83
Analytical Methods Committee (1987) Recommendations for the definition, estimation and use of the detection limit. Analyst 112:199–204
Acknowledgments
F. L. P. thanks FAPEMIG for post-doctoral fellowship (CEX 00202/08). A.M.S.L. is indebted to FAPEMIG (CEX APQ-3716-5.02/07) and Y.G. to FAPESP for financial support. The authors are also indebted to Prof. Claudia Torres for manuscript revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Morais, A., Pissetti, F.L., Lucho, A.M.S. et al. Influence of copper hexacyanoferrate film thickness on the electrochemical properties of self-assembled 3-mercaptopropyl gold electrode and application as a hydrazine sensor. J Solid State Electrochem 14, 1383–1390 (2010). https://doi.org/10.1007/s10008-009-0965-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-009-0965-4