Abstract
A method of chemical template synthesis is described for producing composites based on a perfluorinated matrix with polyaniline chains implanted. It has been shown that the choice of experimental and conditioning techniques is relevant for the composites’ investigation. The conductivity, diffusion permeability, selectivity, and electroosmotic permeability of the composites have been investigated in comparison with the same properties of the initial MF-4SC membrane. A model describing the transport behavior of the composites in the doped state as a fibrous-cluster system is proposed. A set of transport and structural parameters of the composites in a H2SO4 solution has been calculated and an analysis of the results observed has been carried out. The set of electrotransport properties is explained by the morphological features of the composites, taking into account the redox heterogeneity of polyaniline. The contribution of electron conductivity to the mixed conductivity of composites with a certain saturation degree by polyaniline has been estimated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production of composite materials based on ion-exchange polymer matrix and electron-conducting polymers (polypyrrole, polyaniline, polythiophene, and others) and the investigation of their functional properties is a new branch in membrane electrochemistry and chemistry of high-molecule compounds, which has attracted increasing attention from researchers in the past 15 years. Nowadays, composite materials are used in electrochemistry for modifying electrode surfaces, in fuel energetics, electrodialysis, in bioelectrochemistry, polymer microelectronics, and for selective gas separation [1–10]. Mixed ion and electron conducting composites have applications as materials for sensors, electrocatalysts, and electrochromic displays [2, 11–15].
A literature analysis shows that much attention is paid to the synthesis of polymer films (polyaniline and polypyrrole) and the study of their electrochemical and structural properties at the electrode/electrolyte interface [2, 6, 11, 16–22]. As a rule, the electrochemical polymerization of monomers is carried out on the surface of platinum, gold, graphite, glassy carbon, and tin-oxide electrodes under electric current. In this case, a hybrid electrode material modified by electroactive polymer film is obtained. The chemical polymerization of electron-conducting components in the matrix of a “host” polymer permits the production of composite films in a “free-standing” state [7, 10, 13, 14, 23–28]. As a base polymer, matrix perfluorinated sulfocationic (Nafion) [8, 13, 23–26, 29], sulfopolystyrene (Neosepta) [7, 10, 15, 30], polyethylene [9, 14, 27], and other films are used. The following redox systems have been used as oxidants: Fe3+/Fe2+ [7, 9, 24, 28]; S2O8 2−/SO4 2− [10, 23]; and titanocene [31], which permits the polymerization of aniline, pyrrole, and other monomers in the basic polymer matrix. The synthesis of such materials is frequently referred to as a “template” synthesis, as it occurs in small volumes of a solid phase and includes the formation of a template phase of a conducting polymer [25, 28, 32]. Both electrochemical and chemical methods of synthesis yield composite materials possessing mixed ion–electron conductivity [23, 33]. The ion and electron conduction ability of a polymer material favors faster kinetics of redox exchange, owing to the proximity of redox sites and counter ions.
At present, composite films obtained by the template synthesis of polyaniline in Nafion matrix are an object of intensive investigations, which is due to the high conductivity of polyaniline, good redox reversibility, good stability, and quick change of color with potential of these films. Results reported on the mechanical, structural, chemical, and transport properties of Nafion membranes imply that their special properties create the basis for the preparation of different composite materials with promising application fields. There are a number of works concerning the preparation, structure, and conductivity of “pure” polyaniline [34–41]. The electrochemical behavior of polyaniline films on an electrode have been studied by optical [10, 19, 22, 38, 39, 42], cyclic voltammetry [20, 21, 32], and impedance spectroscopy techniques [25, 26].
The first publications concerning composites PAni/Nafion established that their conductivity and coloring depend essentially on the synthesis conditions and oxidation degree of polyaniline aromatic chains [13, 23, 24, 33]. The average value of Nafion conductivity in the H+-form is in the range from 1 to 14 S/m [10, 33], that of polyaniline is 104 S/m [5]. The increase of Nafion conductivity (up to 7–30 S/m) after modification by polyaniline was discovered by Barthet and Guglielmi [33] with the help of special synthesis conditions. Therefore, it is necessary to study the synthesis conditions, morphology, and transport properties of composites more thoroughly. However, nowadays, there are only a limited number of papers devoted to the investigation of both transport properties and structural changes of the basic matrix after polyaniline incorporation. Diffusion properties of polyethylene modified by polypyrrole and polyaniline were investigated by Tishchenko et al. and Elyashevich et al. [9, 14, 27] for the preparation of gas separation materials. The detailed investigation of structural and electrochemical properties of composites PAni/Nafion and PAni/Neosepta has been carried out to develop the charge selectivity of electrodialysis membranes [10, 15]. It is well known that the distribution of water between structural fragments in Nafion is the key factor that determines the morphology and performance of these polymer films [8, 43–45]. However, information on the role of water in polymer composites and their electroosmotic permeability is poor.
The aim of this work is to investigate systematically the chemical template synthesis conditions for composite membranes PAni/MF-4SC in a “free-standing” state and their electrotransport properties, as well as the equilibrium properties of the initial matrix. The electrotransport properties represent a set of physicochemical characteristics of ion-exchange polymer membranes—conductivity, selectivity, diffusion, and electroosmotic permeability—which depend on the electrochemical potential gradient, concentration, and type of electrolyte solution. It would be interesting to compare properties of composite films and initial membranes measured under identical experimental conditions.
Experimental
Materials
A perfluorinated sulfocationic membrane MF-4SC (Nafion type) produced by “Plastpolymer” (St.-Petersburg, Russia) was used as a template matrix for the preparation of composites [44, 45]. These composites are considered to be a result of polyaniline intercalation into the initial (nonmodified) membrane. Figure 1 shows the chemical structure of the template matrix (that is, perfluorinated sulfocationic membrane) and of polyaniline in the emeraldine form. The properties of MF-4SC membrane after oxidative-thermal conditioning are as follows: l=0.008–0.032 cm, Q=8.7·10−4 mol/gsw, W w=18.0%, and n m=11.5 mol H2O/mol–SO3 −. The oxidative-thermal conditioning technique includes the successive boiling of films in 5% HNO3 (recovers the transparency of membranes and converts them into the H+-form), 10% H2O2 (oxidizes the products of destruction in the membranes), and distilled water for 3 h in each solution [46]. It has been shown by Berezina et al. [46] that the corresponding properties of a Nafion-117 membrane, determined after the same procedures, are close to those of MF-4SC being investigated. The following chemicals were used in this work: twice-distilled aniline, sulfuric acid of an ultra-high purity grade, FeCl3, NaCl, HCl, NaOH, K2S2O8, and (NH4)2S2O8 of a reagent grade.
Template synthesis of composites PAni/MF-4SC
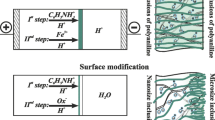
The method of chemical template synthesis is used for producing composites according to Fabrizio et al. [24]. In version 1, a vertically fixed membrane is positioned between solutions of 0.01 M FeCl3 in 0.5 M H2SO4 (initiator of the polymerization process) and 0.01 M aniline in 0.5 M H2SO4. The polymerization is carried out in a two-chamber cell by the counter diffusion method (Fig. 2). During the template synthesis, we measured the resistance of solutions in both chambers (R 1, R 2) by using measurement platinized platinum electrodes. Values of R 1 and R 2 remained virtually invariant due to a high concentration of the supporting sulfuric acid solution.
In version 2, the composite membranes were obtained with the help of the successive diffusion of the working solutions 0.5 M H2SO4, 0.01 M aniline in 0.5 M H2SO4, and 0.01 M FeCl3 in 0.5 M H2SO4 on one side of the membrane, with water on the other side. The processes were carried out in the cell shown in Fig. 2 with registration of solution resistances in both chambers. In this case we observed changes of the resistance at all stages (diffusion steps) of transition of the membrane from the H+-form to the anilinium form and then to the formation of composite. At each stage a fresh portion of distilled water and corresponding solution were used. After the synthesis the green composite film was equilibrated in a 0.5-M H2SO4 solution. In these experiments, the thicknesses of samples varied from 0.008 to 0.032 cm (in the swollen state).
In version 3, samples of membranes in the H+-form are placed in a Petri dish filled with a mixture of the monomer and oxidant. In this case we used different initiators of polymerization, such as FeCl3, K2S2O8, and (NH4)2S2O8, either in water or in a 0.5-M H2SO4 supporting solution. Afterwards, depending on the sample pretreatment and synthesis time, the membrane would turn blue and then, rapidly, emerald-green. The increase of polymerization time up to 24 h leads to obtaining of black nontransparent films. As a result, we could prepare samples with different color intensities by varying the exposure in the working solutions.
The main stages of the synthesis are shown in Fig. 3. In the acidic medium, aniline exists in solution in the protonated form as anilinium cations An+, which are counterions for MF-4SC. The synthesis involves three stages: First, the sorption of aniline counterions by the ion-exchange mechanism with self-organization of the monomer in the vicinity of charged centers of the perfluorinated matrix (effect of the “recognition” of fixed –SO3 − ions by An+ counterions) and the nonexchange sorption in the vicinity of oxygen groups at the side fragments of the matrix takes place. Second is the initiation of aniline polymerization by the electrons of redox system Fe3+/Fe2+ or S2O8 2−/SO4 2−. Finally, aniline polymerization with the formation of polyaniline chains in the template matrix and the formation of a polyaniline redox system capable of reversible doping and undoping effects takes place. So, as a result of the oxidative polycondensation of aniline, we have obtained poly-n-phenylamineimine [2, 34].
Characterization
A set of experimental techniques for membrane materials characterization is elaborated and certified in the Laboratory of Membrane Materials Science of the Department of Physical Chemistry of the Kuban State University (Krasnodar, Russia). The choice of experimental and conditioning techniques was relevant for the composites’ investigation. The static exchange capacity (Q) of membranes was measured shifting the equilibrium by adding a titrant excess. The membranes were first converted completely to the H+-form. After that, the samples were washed with distilled water to remove any adsorbed electrolytes. The membranes were then placed in a 0.1-M NaOH solution, which contained 5% NaCl. After the ion exchange process was finished, the ion exchange capacity could be determined by titration of the excessive sodium hydroxide with a 0.1-M HCl [47].
The water content (W w) in membranes was determined by air-heat drying of a membrane sample in the H+-form. The water was evaporated for 5 h at 105 °C and results were obtained by gravimetry as the water mass/swollen sample mass ratio. The membrane water capacity (n m) characterizing the H2O mole amount per 1 mol of the functional groups was calculated from the data on the water content exchange capacity of the samples [45]. Studying the colored composite membranes was carried out by a spectrophotometer Specord-M40.
The specific conductivity (κ m) was calculated by measuring electrical resistance as the active part of the sample impedance using the mercury contact method at an alternating current frequency of 200 kHz. The κ m values of membranes were calculated from the following relationship
Here, R is the resistance measured with a TESLA BM-507 impedance meter (ohm), and S=0.785 cm2 is the membrane surface area being in contact with mercury. The thickness of samples, l, was measured using an MK-type micrometer with an error of no more than 3%. Membranes of type MF-4SC are electrochemically homogeneous materials having the structural inhomogeneity parameter f 2≤0.1 in accordance with the microheterogeneous model of conductivity [45, 48, 49]. According to the relationship between the conductivity of ion exchange membranes measured by using direct and alternating current, the difference between these values for the samples studied can be neglected [48, 50]. The conductivity was determined for samples in the salt, acid, and anilinium forms equilibrated with NaCl and H2SO4 solutions in a wide concentration range. Processing these concentration dependences of conductivity within the frames of the two-phase conductivity model of an ion-exchange membrane allows us to calculate the volume fraction of a free solution in the membrane phase (f 2) and to determine the conductivity value at the isoconductivity point (κ iso) [49].
To characterize the diffusion of electrolytes through membranes, we used the differential membrane permeability coefficient (P*), which was calculated by taking into consideration the experimental results obtained in a periodic-action nonflow cell. In those experiments, we investigated the diffusion of different electrolytes into a distilled water-filled compartment of the cell. The electrolyte buildup rate in water (dC/dt) was controlled by the conductivity measuring technique and used for calculating the diffusion flux of electrolyte (j) and the differential membrane permeability coefficient (P*):
where S is the membrane area (cm2); dC/dt is the rate of electrolyte concentration increase in the compartment of volume V (cm3) with water initially filled (in diffusion cell); dlnj/dlnC is found as a slope from dependences j-C in bilogarithmical coordinates (parameter β), which characterizes the shape of the concentration profile in a membrane; l is the membrane thickness; while C and C 0 are the solution concentrations (mol/l) at time t and an initial instant, respectively. A detailed description of the results processing procedure is given by Gnusin et al. and Berezina and Kubaisy [49, 51].
The electroosmotic permeability coefficient of membranes or the water transport number (t w), which is equal to the number of moles of water transported by 1 F of electricity, were found by the volumetric method in a two-chamber cell with reversible silver chloride electrodes and measurement capillaries [45, 49]. This method is based on the change of liquid volume as a function of time when passing a certain current level through the membrane.
where V is water volume in the capillary tubes (cm3), F=26.8 A·h/mol is the Faraday constant, υ m=18 cm3/mol is the water molar volume, S is membrane area (cm2), i is current density (A/cm2), and t is time (h). These techniques allow us to measure κ m, P*, and t w values with a relative systematic error of 5–7% under isothermal conditions at 25 °C.
The ion transport numbers of membrane (t i) were calculated by using the conductivity and diffusion permeability data in NaCl and H2SO4 solutions [51, 52]. The obtained experimental data were used to calculate transport and structural parameters (TSPs) by applying model approach and computer software [49, 52, 53].
Results and discussion
Set of electrotransport properties for composites PAni/MF-4SC
Polymerization time dependences
It was established that an induction period is needed for the accumulation of aniline ions in cluster zones of the template matrix. This effect is connected with a self-assembling phenomenon, which precedes the formation of polyaniline chains. The anilinium ions sorption by the membrane was investigated with the help of conductivity measurements by the impedance method, by radiotracers [20, 21, 54], and by spectroscopy techniques.
The change of synthesis conditions, pH values, and use of phototreatment permits us to observe electrochromic effects (from blue to black and brown) in the composite film [10, 28]. A visual study of the effect of polymerization time on the film color showed that increasing the time of contact with working solutions makes the samples more intensively colored. Figure 4 shows spectra for colored samples. The peaks at 700–800 nm are known to correspond to emeraldine during the aniline polymerization. The peak at 400 nm is referred to the radical-cation form of polyaniline [22]. The emeraldine form in the doped-by-acid state has electron (polaron) conductivity.
In Fig. 5, photographs of the composites’ surfaces with different polymerization times are presented. As can be seen, the surface has a grainy character, which is identical to that of the surface images obtained by Neves and Polo Fonseca, Sari et al., and Lockshin et al. [26, 39, 55] by SEM. The photos are taken with a magnification factor of 90. The real size of the grains is approximately 10–30 μm. It has been shown that the fractal dimension of polyaniline particles on the surface of composites is equal to 2.4, which is close to the spherical geometry of the surface particles [26].
It was interesting to investigate the electrotransport characteristics of these new materials using experimental and model approaches to their description. It is well known that, during real use, a lot of different transport phenomena occur in membrane systems. The basic properties of polymer materials are conductivity (κ m), ionic selectivity (t i), diffusion (P*), and electroosmotic permeability (t w). The fluxes of ions and water transferred across the membrane are a result of acting of different forces: chemical potential gradients, electric potential, temperature, and pressure (Fig. 6). What role does polyaniline play in the matrix of a MF-4SC membrane?
Figure 7a–c demonstrates the dependence of conductivity, diffusion coefficient, and thickness of composite film upon the polymerization time (for the same sample). It is necessary to note that the composite films were prepared by synthesis version 3, which leads to more isotropic distribution of polyaniline. The conductivity and diffusion permeability values for composites in a 0.25-mol/l H2SO4 solution depending on the synthesis time show an opposite character: after 5 h of synthesis, the mixed conductivity increases by 15% but the diffusion permeability decreases by about 40% compared to the H+-form of the basic film. After 9 h, the conductivity values became close to those of the initial membrane, but the diffusion permeability began to increase (Fig. 7a,b).This character of the transport properties is explained by structural reorganization of the aromatic fibrils of polyaniline during polymerization. The penetration of polyaniline into structural cavities of the template matrix leads to the interruption of percolation ways of electrolyte transport across the composite film [9]. So, these results confirm the morphological changes in the structure of polyaniline in the template matrix, which have a dynamic character in the synthesis process. The measurements of composite thickness show an increase of this parameter due to the transition from the compact to the expanded state of the composite (Fig. 7c). A different character of the diffusion permeability dependences for ion-exchange membranes and polyethylene films on the pyrrole polymerization time has been observed by Sata et al. [7] for dry composites. Investigation of the diffusion permeability of polyethylene films, which depends on the time of polypyrrole synthesis, permitted the discovery of an increase and subsequent decrease in diffusion permeability. These effects were explained by the formation of polypyrrole layers on the surface of pores with their subsequent complete blocking [9].
Concentration dependences of electrotransport properties
The measurements of electrotransport properties were performed by using composite samples with a certain saturation degree by polyaniline, after 5 h of polymerization. This polymerization time corresponds to a saturation degree of 25–30%, determined by the ion-exchange capacity of membranes. Conductivity and diffusion permeability in NaCl and H2SO4 solutions (concentration range 0.005–0.5 mol/l) were determined for composites in the “free-standing” state. Figure 8 demonstrates the variations of conductivity (a) and diffusion permeability (b) in dependence upon the NaCl and H2SO4 concentration. We can see that these dependences have typical shapes for both initial sample and composite in a wide range of concentrations. To compare the conducting properties of membranes, a similar dependence for a Nafion-117 membrane (curve 1) is shown in Fig. 8a [44]. The obtained results imply that after oxidative-thermal conditioning the conductivity of MF-4SC virtually coincides with the values of conductivity obtained for the Nafion-117 sample [46]. As can be seen from Fig. 8a, the difference between the conductivity of the original and modified membranes is insignificant (curves 2, 3 and 4, 5). Probably due to the nanostructure of polyaniline aromatic chains, the changes of conductivity are not too big. The average conductivity value of composites is equal to 4.5 S/m in H2SO4 solutions, which is 3.5 times as high as that in NaCl solutions. This effect is explained by the hopping mechanism of the proton transfer through ionic conductors [51]. The high activation energy of the elementary act, which is due to the higher energy of cleavage of hydrogen bonds between the hydrate water molecules in the sulfo-group —counterion ion-dipole associates of the latter membrane, is one of the reasons for the protons to slow down in the cluster zones of the membrane. The hydrogen-bond cleavage energy in these systems is ten times as much as the electrostatic energy of bond cleavage in an ion pair [56]. Concerning the role of polyaniline chains in the overall conductivity of composites, it should be noted that similar conductivity values of original and composite membranes (Fig. 8a) can be explained by the dominating contribution of ionic transport for NaCl solution (Na+-transport) both in the cluster zones and in inner nanosized inclusions of the salt solution. In this case, polyaniline is in the undoped state. However, in the case of acid solutions, the membranes demonstrate close values of conductivity due to the coinfluence of two factors: First, the high rate of H+-ions movement across the cluster zones and inner solution zones plays the main role. Second, polyaniline is presented in the doped state in the template matrix, which leads to the appearance of electron transfer in a system of polyconjugated bonds. So, polyaniline incorporated in a MF-4SC matrix does not decrease the overall conductivity but stabilizes its values.
Studying diffusion properties of membranes before and after the template synthesis of polyaniline showed that the diffusion permeability of modified membranes was lower by 10–12% for all samples with a 25% saturation degree. The diffusion permeability of the H+-form composite films is equal to 2.1·10−11 m2/s, which, unlike in the previous case, is three times as low as that in salt solutions. We can see a drastic increase in conductivity with the change of electrolyte from NaCl to H2SO4, but the change of diffusion permeability has an opposite character. These features are explained by different mechanisms of transfer across the polymer film in the case of conductivity and diffusion (transport of counterions and transport of coions, respectively) [51].
The estimation of transport numbers of H+-ions was carried out on the basis of the analysis of concentration dependences of conductivity and diffusion permeability of composites PAni/MF-4SC in a wide range of NaCl and H2SO4 concentrations. It was shown that composite films had high selectivity (close to one), which confirms the domination of the ion conductivity mechanism.
The calculation of water transport numbers from electroosmotic experiments permits the establishment of the difference between water cotransport with counterions H+ and Na+ in the composite films. It was found that this characteristic for the H+-form of the composite was 2.5 times as low as the Na+-form of the membrane. It was established that the implantation of the aromatic chains of polyaniline into the perfluorinated template matrix caused the reorganization of water molecules on the boundaries between the base polymer and the electron-conducting chains of polyaniline.
Table 1 shows the above-mentioned properties of composites in NaCl and acid solutions. As can be seen from the table, composites obtained in the emeraldine form are new polymer materials, whose protonic form has an optimized set of properties: good mixed conductivity, high selectivity, low diffusion, and electroosmotic permeability. The application of these materials in separation processes will permit us to reach high values of electric current efficiency.
Model approach to the description of the transport properties
Theoretical analysis of all these phenomena, which lies in the base of measured electrotransport properties, permits the development of thermodynamic and model approaches to a more detailed characterization of polymer membranes and composites. As known from the research of professor Gnusin and his coworkers and Zabolotsky and Nikonenko [45, 48, 49, 52, 57], concentration dependences of conductivity and diffusion permeability are the fundamental properties of ion-exchange membranes, which can be used for the calculation of other transport properties and model parameters.
The two-phase model of the composite film structure, which includes four structural fragments, was proposed on the basis of the theory of generalized conductivity. Fibrous inclusions of polyaniline are united with the cluster zones and nonconductive, hydrophobic polymer chains of perfluorinated matrix forming the first “pseudophase.” Inclusions of the inner electrolyte solution in the structural cavities of the composite form the second “pseudophase” (Fig. 6). We use the term “pseudophase” in this model because all structural fragments have no visible phase boundaries. So, the model of the composite membrane is based on taking into account the conductivity mechanism: pseudophase 1 has mixed conductivity, pseudophase 2 has a bipolar mechanism of charge transfer equal to external equilibrium solution conductivity.
This model allows calculating a set of TSPs for composites in electrolyte solutions (Table 2). In this paper, the approach was used to estimate TSP for the H+-form of membranes for the first time. We propose a TSP system that contains structural parameters α and f 2 and transport parameters β, κ iso, and G, and characterizes the ion transport in pseudophase 1 of the membrane material. α is a structural parameter reflecting the space orientation of phases in the material. This parameter has two extreme cases: parallel (α=+1) and serial (α=−1) arrangement of the phases with respect to the transport direction. It is clear that when α is close to 0, the conducting phases are randomly orientated. Parameter f 2 is a solution volume fraction in the membrane. The β parameter is a kinetic parameter of the diffusion flux change with the change of concentration. The κ iso parameter is determined as the intersection point of the same experimental dependences “membrane and electrolyte solution specific conductivity—solution concentration.” A special parameter, G, characterizes the diffusion properties of pseudophase 1.
Parameter G is proportional to \(\overline{D} _{\_}\) and reflects the contribution of coions to the diffusion transport across pseudophase 1. Note that the G parameter also contains both the thermodynamic characteristics of a membrane sample (Q and k D) and \(\overline{D} _{\_},\) which is not easy to determine experimentally. We observed that the aromatic chains of polyaniline decreased the diffusion transfer of coions compared to the initial membrane: parameter G decreased by 1.45 times. The electron (polaron) conductivity of protonized polyaniline stabilizes the resulting conductivity values of the composites. A comparison of the Na+-form of perfluorinated membranes Nafion-117 and MF-4SC shows that they exhibit TSPs of the same order of magnitude. Moreover, the conductivity of pseudophase 1 in its protonic form (κ iso) was four times as high as that of the Na+-form within the limits of the two-phase model. No substantial changes in κ iso, f 2, α, and β were observed for the composite and original membranes in the H+-form (Table 2). It can be assumed that the effects of the electron conductivity of polyaniline chains compensate the decrease in the pseudophase 1 conductivity associated with the proton content drop.
The set of the electrotransport properties is explained by the morphological features of the composites. Polyaniline chains constitute nanosized fibrous inclusions in the water clusters system of the template matrix forming a specific interpolymer complex [1, 35, 38]. So, composite films have a cluster-fibrous morphology. The appearance of a new structural element with electron conductivity in the initial membrane supports quite high overall conductivity of the composite.
Effects of electron conductivity of composites PAni/MF-4SC
A possibility to estimate the electron conductivity contribution to the overall conductivity of the composites has been investigated in this work. When measuring the conductivity at different stages of the synthesis, it was shown that conducting properties of the composites increased in comparison with the same parameters of the membrane in the anilinium-ion form. As can be seen in Fig. 9, we observe two opposite phenomena:
-
1.
Decrease in the ion conductivity of the protonic form of the initial membrane during sorption and ion exchange of aniline counterions. These processes lead to small conductivity values. The conductivity value obtained is close to the same value of an MF-4SC membrane in the case of tetraalcylammonium sorption being seven times as low as the initial sample [44, 58]. Note that changes of the aniline concentration in Fig. 9 take place in a 0.5-M H2SO4 solution. The decrease in membrane ion conductivity could be explained by both ion exchange between protons and anilinium ions and overequivalent sorption of anilinium ions, as reported by Ogumi et al. [59]. Those authors have established that the partition coefficient of anilinium cation for Nafion was found to be 1.04·103.
-
2.
After the contact of the membrane with the acidic solution of iron chloride being a polymerization initiator, the membrane becomes green-colored and its conductivity increases fivefold. In this case, the composite conductivity is close to the conductivity of the initial membrane. The film has a uniform green color and remains transparent. However, using K2S2O8 and (NH4)2S2O8 as oxidants does not permit increasing the conductivity. The samples have a nonuniform black-green color, which implies polyaniline formation in the pernigraniline form [60]. When applying the system S2O8 2−/SO4 2− without sulfuric acid media, the conductivity values become lower than the conductivity of films in the anilinium ion form in 0.5 M H2SO4 (Fig. 9). The effects of essential decrease of the Nafion conductivity were described by Tan and Belanger [10], where more concentrated (NH4)2S2O8 solutions (0.1 M, 1 M) were used. The differences in the conductivities of composites prepared with different oxidants are explained by the influence of ion charges. Anions S2O8 2− are coions with respect to cation exchange membrane and do not enter the bulk of the membrane in accordance with the Donnan exclusion law. On the contrary, ions Fe3+ in the system studied are counterions that promote more uniform distribution of aniline being polymerized in the emeraldine form in the bulk of the membrane. It is well known that polyemeraldine has highest conductivity in acidic solutions [2, 39].
Taking into account the saturation degree of the film by emeraldine (25%) and residual conductivity, we can conclude that the contribution of electron conductivity is 70–75%.
The composites obtained with the help of the successive diffusion method (version 2) were equilibrated in a 0.5-M H2SO4 solution. The measurements of resistance were carried out for four samples of different thickness. The thickness of samples varied from 0.008 to 0.032 cm (in the swollen state). The average value of conductivity was 3.3 S/m. This value is lower than that in Figs. 7 and 9 and Table 1 for the protonic form of MF-4SC, because the synthesis was done by diffusing the working solutions in distilled water. Under these conditions we observed the osmotic transfer, which decreases the protonization degree of polianiline obtained. After the synthesis, all samples were washed by water very thoroughly (from adsorbed acid) and the conductivity was measured again. These experiments demonstrate insignificant difference of conductivity for the initial and composite membranes (1.1–1.2 S/m). This effect is apparent because the polymerization of aniline in the template matrix of MF-4SC leads to transition from the protonic mechanism in the basic matrix to the polaronic mechanism of charge transfer in the quinoneimine fragments of polyaniline chains, and finally to a mixed conductivity of composite films.
The effect of the electron conductivity of polyaniline was revealed after vacuum drying (105 °C) of samples MF-4SC and composites PAni/MF-4SC. In this case, the conductivity of the composite films was 100 times as high as that of the original samples. Dry MF-4SC samples became isolators with κ m=10−5 S/m, but dry composites retained a residual conductivity of ∼10−3 S/m. This result confirms the data of Sata and coworkers for dry polypirrole films [7] and results for polyaniline composites of Lockshin et al. [55].
Comments to results
The comparative data concerning the conductivity of different materials are shown in Table 3. We can see that the mixed conductivity of composites lies between the values of conducting properties of pure polyaniline and superionic materials [39, 61, 62]. A literature analysis and our results have shown that the chemical template synthesis of composite films on the base of perfluorinated membranes incorporating polyaniline permits the preparation of new materials with a set of promising transport properties. But we did not observe synergetic effects in conductivity values, as it could be expected from comparison with the proton conductivity of the initial membrane. This fact is explained as follows: SEM data show that the nanostructure of polyaniline is formed by particles with a diameter of 8 nm and high values of conductivity. The particles have an amorphous nonconducting layer with a thickness of 0.8 nm and they are conjugated in branch nets containing 30–50 particles. The electron tunneling between particles occurs across the external amorphous layer [1]. It is also reported by Sari et al. and Zhou et al. [39, 63] that polyaniline has fibrous morphology, and the size of fibrils depends on the synthesis parameters (from 100 up to 500 nm in diameter). The template matrix of the ion-exchange membrane is a special nanoreactor, where the formation of nanosized fibrils of polyaniline takes place [64]. As can be seen from our results, we have not observed essential influence of the nanosized inclusions of polyaniline aromatic chains on the macrokinetic properties of the original membrane (Table 1). But all transport properties of the composites studied have stable character and maintain the optimal level of charge, electrolyte solution, and water transfer. Note that there are a number of morphological factors that do not permit reaching higher conductivity of the composites: some content of aniline oligomers [10] and Fe3+-counterions [9, 15], effects of configuration interactions of the growing polyaniline chains with the side segments of the perfluorinated membrane structure, the block structure of polyemeraldine, and redox heterogeneity of the aromatic chains [65]. The redox heterogeneity of the polymer is provided by different oxidation degrees of polyaniline chains, and this leads to the formation of a block structure, which is due to the alternation of quinoid and amine fragments. Recently, the redox heterogeneity of polyaniline inside another polymer [29] has been discovered and explained by the nonlinear character of transport processes in the polymer matrix during the synthesis. So, the above-mentioned factors help us to understand the results obtained.
Conclusions
A method of chemical template synthesis has been described for producing composites based on a perfluorinated matrix with polyaniline chains implanted. The mechanism of composite template synthesis has been investigated and the features of the multistage process of aniline polymerization have been revealed, taking into account the protonization of aniline in equilibrium solution and self-assembling effects in the membrane phase. The investigation of composite PAni/MF-4SC characteristics in dependence on the polymerization time permits the selection of the synthesis conditions to optimize electrotransport properties.
The conductivity, diffusion permeability, selectivity, and electroosmotic permeability of the composites have been studied in comparison with the same properties of the initial MF-4SC membrane. A model describing the transport behavior of the composites in the doped state as a fibrous-cluster system has been proposed. A set of TSPs of the composites in a H2SO4 solution has been calculated and an analysis of the results observed has been carried out. The set of electrotransport properties is explained by the morphological features of the composites, taking into account the redox heterogeneity of polyaniline. The contribution of electron conductivity to the mixed conductivity of composites with a certain saturation degree by polyaniline has been estimated.
SYMBOLS
- l :
-
Thickness of membrane (cm)
- Q :
-
Ion exchange capacity of membrane (mol/gsw)
- W w :
-
Mass fraction of water (%)
- n m :
-
Membrane water capacity (mol H2O/mol –SO3 −)
- λ :
-
Wave length (nm)
- κ m :
-
Specific AC conductivity of membrane (S/m)
- j :
-
Diffusion flux of electrolyte [mol/(m2·s)]
- P*:
-
System (differential) coefficient of electrolyte diffusion (m2/s)
- t i :
-
Ion transport number of membrane
- t w :
-
Water transport number of membrane (mol H2O/F)
- C :
-
Concentration of an equilibrium solution (mol/l)
- υ m :
-
Water molar volume (cm3/mol)
- i :
-
Current density (A/cm2)
- α :
-
Parameter characterizing the space orientation of phases in a material
- f 2 :
-
Volume fraction of a free solution in membrane phase
- κ iso :
-
AC conductivity of pseudophase 1 at the isoconductivity point (S/m)
- β :
-
Kinetic parameter of the diffusion flux change with the change of concentration
- G :
-
Parameter characterizing the diffusion properties of pseudophase 1 in relation to coions [m5/(mol·s)]
- k D :
-
The Donnan’s constant
- \(\overline{D} _{\_}\) :
-
Ion diffusion coefficient in pseudophase 1 (m2/s)
References
Poole CP, Owens FJ (2003) Introduction to nanotechnology. Wiley-Interscience, New York
Tarasevich MR, Khrushcheva EI (1990) Elektrochimiya polymerov (the electrochemistry of polymers). Nauka, Moscow
Pomogailo AD, Rozenberg AS, Uflyand IE (2000) Nanochasticy metallov v polymerakh (Nanosized metal particles in polymers). Khimiya, Moscow
Pomogailo AD (2002) Russ Chem J 46:64 (in Russian)
Timonov AM, Vasil’eva SV (2000) Soros Educ J 6:33 (in Russian)
Kazarinov VE, Pisarevskaya Å Yu, Ovsyannikova EV, Levi MD, Alpatova NM (1995) Russ J Electrochem 31:879
Sata T, Sata T, Yang W (2002) J Membr Sci 206:31
Heitner-Wirguin C (1996) J Membr Sci 120:1
Tishchenko G, Rosova E, Elyashevich GK, Bleha M (2000) Chem Eng J 79:211
Tan S, Belanger D (2005) J Phys Chem B 109:23480
Nagasubramanian G, Di Stefano S, Moacanin J (1986) J Phys Chem 90:4447
Ali Shah A-ul-H, Holze R (2005) J Solid State Electrochem (in press) (DOI 10.1007/s10008-005-0064-0)
Hsu C-H (1991) Synth Met 41:671
Elyashevich GK, Lavrentyev VK, Kuryndin IS, Yu RE (2001) Synth Met 119:277
Sata T, Funakoshi T, Akai K (1996) Macromolecules 29:4029
Shigehara K, Hara M, Yamada A (1987) Synth Met 18:721
Hirai T, Kuwabata S, Yoneyama H (1988) J Electrochem Soc 135:1132
Orata D, Buttry DA (1988) J Electroanal Chem 257:71
Teragishi Y, Aoki K (1999) J Electroanal Chem 473:132
Andreev VN (2001) Russ J Electrochem 37:605
Andreev VN (2001) Russ J Electrochem 37:612
Ivanov VF, Kucherenko Yu A, Nekrasov AA, Vannikov AV (1992) Russ J Electrochem 28:50 (in Russian)
Aldebert P, Audebert P, Armand M, Bidan G, Pineri M (1986) J Chem Soc, Chem Commun (22):1636
Fabrizio M, Mengoli G, Musiani MM, Paolucci F (1991) J Electroanal Chem 300:23
Neves S, Polo Fonseca C, Zoppi RA, Cordoba de Torresi SI (2001) J Solid State Electrochem 5:412
Neves S, Polo Fonseca C (2001) Electrochem Commun 3:36
Elyashevich GK, Terlemezyan L, Kuryndin IS, Lavrentyev VK, Mokreva P, Rosova E, Sazanov N (2001) Thermochim Acta 374:23
Berezina NP, Kubaisy AA-R, Alpatova NM, Andreev VN, Griga EI (2004) Russ J Electrochem 40:325
Lai EKW, Beattie PD, Orfino FP, Simon E, Holdcroft S (1999) Electrochim Acta 44:2559
Sata T (1993) J Membr Sci 82:247
Vorotyncev MA, Graczyk M, Lisowska-Oleksiak A (2005) Abstracts of VIII International Frumkin Symposium. Moscow, p 247
Alpatova NM, Andreev VN, Danilov AI, Molodkina EB, Polukarov Yu M, Berezina NP, Timofeev SV, Bobrova LP, Belova NN (2002) Russ J Electrochem 38:913
Barthet C, Guglielmi M (1995) J Electroanal Chem 388:35
Chiang J-C, Macdiarmid AG (1986) Synth Met 13:193
Volfkovich Yu M, Sergeev AG, Zolotova TK, Afanasiev SD, Efimov ON, Krinichnaya EP (1999) Electrochim Acta 44:1543
Lei W, Kocherginsky NM (2000) React Funct Polym 45:65
Nekrasov AA, Ivanov VF, Vannikov AV (2001) Electrochim Acta 46:3301
Ivanov VF, Cheberjako KV, Nekrasov AA, Vannikov AV (2001) Synth Met 119:375
Sari B, Talu M, Jildirim F (2002) Russ J Electrochem 38:707
Roman LS, Mello RMQ, Cunha F, Hümmelgen IA (2004) J Solid State Electrochem 8:118
Zhou H, Chen H, Luo S, Lu G, Wei W, Kuang Y (2005) J Solid State Electrochem 9:574
Malinauskas A, Holze R (1999) J Solid State Electrochem 3:429
Zawodzinskiy Th A, Springer TE, Uribe F, Gottesfeld S (1993) Solid State Ionics 60:199
Berezina NP, Timofeev SV, Rollet AL, Fedorovich NV, Dyuran-Vidal’ S (2002) Russ J Electrochem 38:903
Berezina N, Gnusin N, Dyomina O, Timofeev S (1994) J Membr Sci 86:207
Berezina NP, Timofeev SV, Kononenko NA (2002) J Membr Sci 209:509
Kraaijeveld G, Sumberova V, Kuindersma S, Wesselingh H (1995) Chem Eng J 57:163
Zabolotsky V, Nikonenko V (1993) J Membr Sci 79:181
Gnusin NP, Berezina NP, Kononenko NA, Dyomina OA (2004) J Membr Sci 243:301
Gnusin NP, Dyomina OA, Meshechkov AI, Turyan I Ya (1985) Russ J Electrochem 21:1525 (in Russian)
Berezina NP, Kubaisy AA-R (2006) Russ J Electrochem 42:91
Berezina NP, Kononenko NA, Demina OA, Gnusin NP (2004) Polym Sci A 46:672
Berezina NP, Komkova EN (2003) Colloid J 65:1
Kazarinov VE, Andreev VN (1984) In: Yeager E, Bockris JO’M, Conway BE, Sarangapani S (eds) Comprehensive treatise of electrochemistry, vol 9. Plenum, New York, p 393
Lockshin NA, Sergeev VG, Zezin AB, Kabanov VA (2003) Abstracts of X Russian Conference “Structure and dynamics of molecular systems”. Yalchik Lake, p 162
Butyrskaya EV, Shaposhnik VA (2002) Opt Spectrosc 92:370
Zabolotsky VI, Nikonenko VV (1996) Perenos ionov v membranach (Ion transport in membranes). Nauka, Moscow
Kononenko NA, Berezina NP, Loza NV (2004) Colloids Surf A Physicochem Eng Asp 239:59
Ogumi Z, Toyama K, Takehara Z-I, Katakura K, Inuta S (1992) J Membr Sci 65:205
Karyakin AA, Maltsev IA, Lukachova LV (1996) J Electroanal Chem 402:217
Duic L, Mandic Z, Kovacicek F (1994) Polym Sci A 32:105
Gurevich Yu Ya, Kharkats Yu J (1992) Superionic conductors. Nauka, Moscow
Zhou HH, Jiao SQ, Chen JH, Wei WZ, Kuang YF (2004) Thin Solid Films 450:233
Suzdalev IP (2006) Nanotechnologiya: fiziko-himiya nanoklasterov, nanostruktur i nanomaterialov (Nanotechnology: physic-chemistry of nanoclusters, nanostructures and nanomaterials). KomKniga, Moscow
Ivanov VF, Gribkova OL, Novikov SV, Nekrasov AA, Isaakova AA, Vannikov AV, Meshkov GB, Yaminskiy IV (2005) Synth Met 152:153
Acknowledgements
The authors are grateful to the Russian Foundation for Basic Research and Krasnodar Region Administration for the financial support of this work. The authors express their gratitude to Dr. Jaroslav Sytchev (University of Miskolc, Hungary) for his valuable help with the work on this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berezina, N.P., Kubaisy, A.A., Timofeev, S.V. et al. Template synthesis and electrotransport behavior of polymer composites based on perfluorinated membranes incorporating polyaniline. J Solid State Electrochem 11, 378–389 (2007). https://doi.org/10.1007/s10008-006-0159-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-006-0159-2