Abstract
TiO2 thin films were deposited on ITO/Glass substrates by the rf magnetron sputtering in this study. The electrochromic properties of TiO2 films were investigated using cyclic voltammograms (CV), which were carried out on TiO2 films immersed in an electrolyte of 1 M LiClO4 in propylene carbonate (PC). As- deposited TiO2 thin film was amorphous, while the films post-annealed at 300~600°C contained crystallized anatase and rutile. With the increase of the annealing temperature, the surface roughness of film increased from 1.232 nm to 1.950 nm. Experimental results reveal that the processing parameters of TiO2 thin films will influence the electrochromic properties such as transmittance, ion-storage capacity, inserted charge, optical density change, coloration efficiency and insertion coefficient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, titanium oxide films are useful for applications such as catalysis, optical coatings, gas sensors, and smart windows [1–3]. Moreover, TiO2 thin films show reversible coloration upon ion intercalation. The coloration of thin films due to the electrochemical intercalation of small cations, such as H+, Li+, Na+ and K+ is known as electrochromism. Consequently their synthesis has aroused much interest in the world. The electrochromic properties of almost all the transition metal oxides have been investigated [4]. These oxides can be colored anodically (NiOx, IrO2, V2O5) or cathodically (WO3, Nb2O5, TiO2). This cathodic coloration material is reversibly colored by the double injection of electrons and cations, and described as follows [5–7]:
where M+ can be H+ or Li+ . The amount of insertion coefficient, x value, can be saturated up to 1 depending on the microstructure of the deposited thin films.
Many research groups have fabricated TiO2 thin films by various methods, including sputtering, evaporation deposition and sol-gel methods [8–13]. However, the morphology and the coloration/bleaching behavior of the films are strongly related to the deposition conditions. Among the film deposition techniques, the sputtering is widely used in the glass industry and could enhance adhesion and large area deposition. In the present work, TiO2 thin films were deposited by the rf magnetron sputtering of ceramic (TiO2) target to study the correlation between the electrochromic properties and the structures of the films. The influence of the sputtering process on the electrochromic properties was also discussed in this study.
Experimental
TiO2 thin films were deposited by the rf magnetron sputtering technique. Ceramic TiO2 (2 inches in diameter, 99.99%) and ITO-coated glass (15 Ω/sq.) were used as the target and the substrate, respectively. The distance between the target and the substrate was 5 cm. The vacuum chamber is capable of achieving a base pressure of 6.5×10−4 Pa and the working pressure for sputtering was 1×10−1 Pa. The target was pre-sputtered at the working pressure for 5 min. The duration of deposition varied from 5 min to 30 min and rf power varied from 60 W to 120 W. The depositions were performed in oxygen concentration (O2/Ar+O2) from 0% to 30% range. After deposition, TiO2 films were post-annealed at different temperature between 300°C and 600°C for 0.5 h.
Thickness of TiO2 film was determined by a surface profiler and the deposition rate is approximately 16 nm/min. Crystal structures of TiO2 thin films were analyzed by means of X-ray diffraction (XRD, Rigaku D/Max). The surface root mean squares (rms) of thin films were observed using atomic force microscope (AFM, NanoMan NS4+D3100). The electrochemical behaviors of TiO2 films were investigated using cyclic voltammograms (CV, CH Workstation 6330), which were carried out on TiO2/ITO/Glass immersed in an electrolyte of 1 M LiClO4 in propylene carbonate (PC). A standard three-electrode cell was adopted with TiO2/ITO/Glass as the working electrode (WE), Pt as a counter electrode (CE), and Ag/AgCl as a reference electrode (RE). The cyclic voltammograms in the range of +1.0 V ~ −3.2 V were obtained by potential sweeps of 50 mV/s. Ultraviolet-visible (UV-Vis) spectrum were taken as for transmittance spectra of the deposited specimen, colored at −3.0 V and bleached at +3.0 V, by using a spectrometer (HP Agilent 8453) operated from 200 to 1,000 nm. The optical density change (Δ OD) is given by [14].
where T Bleaching and T Coloring represent the transmittance at wavelength of 550 nm for the coloring and bleaching states. To calculate the inserted charge (Q) for the coloration states and bleaching states of TiO2 films we use the formula (3) for integrating between the starting and ending time of each period.
The coloration efficiency (η) is defined as below:
Results and discussion
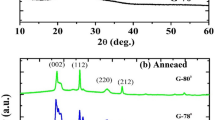
The XRD patterns of TiO2 thin films deposited at rf power of 80 W and post-annealed at different temperature are shown in Fig. 1. It can be seen that the as-deposited film was amorphous. However, the polycrystalline TiO2 films can be obtained at annealing temperature higher than 300°C. The crystallization of TiO2 thin films is reported to be in the temperature range [15]. Figure 1 also shows that the predominant phase is anatase, but there is evidence of rutile phase R(110) in the TiO2 films annealed above 300°C. Furthermore, the degree of crystallinity increased with increasing annealed temperature. Figure 2 shows the AFM image of as-deposited and post-annealed TiO2 films. As can be seen, with an increase of the annealing temperature, the root mean square (rms) of surface roughness of TiO2 film increased from 1.232 nm to 1.950 nm.
The optical transmittance spectra of TiO2 films deposited at room temperature and rf power of 80 W with various deposition times are shown in Fig. 3. The insertion of Li+ ions changes the transmissivity from near UV up to the near-infrared range and the reversible color of the film from transparent to blue [16]. The maximum change in transmittance (at 550 nm) is varying from about 82% to 60% for a 164-nm thick TiO2 film with deposition time of 10 min. Figs. 4, 5 and 6 show the transmittance of TiO2 films obtained by various rf power, oxygen concentration and annealing temperature, respectively. It was found that the electrochromic properties of TiO2 films depend on the deposition and post-annealing process. The as-deposited TiO2 films obtained from deposition time of 10 min, rf power of 80 W and 0% oxygen concentration exhibit good electrochromic properties between colored and bleached in the whole spectral range. The results also show that as-deposited TiO2 films have large charge capacity. It can be explained that the annealing temperature is one of the key parameters which control the microstructure of the films as shown in Fig. 2. Post-annealing can improve the crystallinity of TiO2 film, however, its Li+ ion capacity is affected, probably due to the microstructure change of the film. The influence of post-annealing on the microstructure of TiO2 film is consistent with that reported by Wang and Hu [17]. It is known that the charge injected into the crystalline film is much less than into the amorphous film, and both the Li+ intercalation/deintercalation reaction and\({\text{Ti}}^{{{\text{4 + }}}} \rightleftarrows {\text{Ti}}^{{{\text{3 + }}}} \) redox reaction lead to film coloration/decoloration process [18, 19].
The cyclic voltammogram and current density of the TiO2 film deposited at deposition time of 10 min and rf power of 80 W are shown in Fig. 7. In Fig. 7a, the TiO2 film shows a cathodic peak at −1.54 V (−0.45 mA/cm2) and an anodic peak at −2.34 V (0.09 mA/cm2). To calculate the inserted charge for the bleached and colored stable states, we use the formula (3) for integrating between the initial and final inserted charge from 80 s to 100 s. Fig. 7b shows the amount of charge inserted during colouration is 28.21 mC/cm2 and extracted during bleaching is 2.95 mC/cm2 . Figure 8 shows the dependence of optical density change (Δ OD) and coloration efficiency (η) of TiO2 thin films on various deposition time (rf power of 80 W). It can be seen that Δ OD increases significantly with an increase of deposition time, however, it decreases as the deposition time exceeds 10 min. The result shows the TiO2 film has a maximum Δ OD of 0.13 at wavelength of 550 nm. Apparently, the result also shows the transmittance variation is minimal at the deposition time between 15 min and 30 min. This result is the same as shown in Fig. 3. A similar behavior is observed for the η of the TiO2 films. The thickness of TiO2 film which exhibits the maximum η value (4.5 cm2/C) is 164 nm. Figure 9 shows the dependence of Δ OD and η of TiO2 thin films on various rf power (deposition time of 10 min). It can be seen that Δ OD increases with an increase of rf power, however, it decreases as the rf power exceeds 80 W. TiO2 thin films exhibit the maximum Δ OD and η can be obtained at the deposition time of 10 min and the rf power of 80 W.
The transmittance of the colored state of TiO2 film is dependent on the charge of Li+ intercalation, expressed by the insertion coefficient x in the LixTiO2 compound [15]. The dependence of x in LixTiO2 on deposition time and rf power are shown in Fig. 10a and 10b, respectively. It is obvious that both injection and extraction curves appear as a maximum value at the deposition time of 10 min and the rf power of 80 W. The maximum x value is 0.18 for colored state and 0.039 for bleached state, respectively.
Conclusions
TiO2 thin films were prepared by the rf magnetron sputtering and their electrochromic properties were investigated in this paper. The following was concluded :
-
1.
The deposition rate of TiO2 thin film is approximately 16 nm/min. With an increase of the annealed temperature, the rms of surface roughness increases from 1.232 nm to 1.950 nm.
-
2.
It was found that the transmittance of electrochromic TiO2 films depend on the deposition and post-annealing process. A good reversible blue color upon Li+ insertion and bleached process can be obtained. However, performance of oxygen concentration and annealed temperature were found to depress the colored phenomenon. Therefore, all as-deposited TiO2 films are confirmed to be in the amorphous state.
-
3.
The optimal sputtering parameters for TiO2 film on electrochemical properties were the deposition time of 10 min (thickness of 164 nm) and the rf power of 80 W. In addition, the oxygen concentration and the annealing process could not be performed. The best experimental results were obtained as follows:
-
(a)
Transmittance: 60% (colored state)
-
(b)
Inserted charge: 28.21 mC/cm2 (colored state)
-
(c)
Maximum optical density change (Δ OD): 0.13
-
(d)
Coloration efficiency (η): 4.5 cm2/C
-
(e)
Insertion coefficient (x in Li x ClO4): 0.18 for injection (colored state) and 0.039 for extraction (bleached state).
-
(a)
The possibility of TiO2 thin films to change their color from transparent to blue under the applied potential can be very interesting for future application. We hope rf magnetron sputtering TiO2 coatings would find a practical use for the smart windows in future studies, for instance in association with conducting polymers in all-solid-state electrochromic devices.
References
Weinberger R, Garber RB (1995) Appl Phys Lett 66:2409
Manno D, Micocci G, Rella R, Serra A, Taurino A, Tepore A (1997) J Appl Phys 82:54
Granqvist CG (1991) Appl Phys 52:83
Granqvist CG (1995) Handbook of inorganic electrochromic materials. Elsevier, Amsterdam
Ohtsuka T, Masuda M, Sato N (1987) J Electrochem Soc 134:2406
Natarajan C, Nogami (1996) J Electrochem Soc 143:1547
Krol RVD, Goossens A, Meulenkamp EA (1999) J Electrochem Soc 146:3150
Lourenco A, Masetti E, Decker F (2001) Electrochim Acta 46:2257
Jin P, Xu G, Tazawa M, Yoshimura K (2003) Appl Phys A 77:455
Natarajan C, Nogami G (1996) J Electrochem Soc 143:1547
Ozer N, Sabuncu S, Cronin J (1999) Thin Solid Films 338:201
Wang Z, Hu X (2001) Electrochim Acta 46:1951
Avellaneda CO, Bueno PR, Faria RC, Bulhoes LOS (2001) Electrochim Acta 46:1977
Bange K, Gambke T (1990) Adv Mater 2:10
Yakovlev VV, Scarel G, Aita CR, Mochizuki S (2000) Appl Phys Lett 76:1107
Ozer N (1992) Thin Solid Films 214:17
Wang Z, Hu X (1999) Thin Solid Films 352:62
Gutarra A, Azens A, Stierna B, Granqvist CG (1994) Appl Phys Lett 64:1604
Cao F, Oskam G, Searson PC, Stipkala JM, Heimer TA, Farzad F, Meyer GJ (1995) J Phys Chem 99:11974
Acknowledgements
This study is supported by the National Science Council, R.O.C., under contract no. NSC 93-2216-E-230-004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C.M., Lin, S.Y. Electrochromic properties of sputtered TiO2 thin films. J Solid State Electrochem 10, 255–259 (2006). https://doi.org/10.1007/s10008-005-0690-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-005-0690-6