Abstract
Single-walled carbon nanotube (SWNT)/Polyaniline (PANI) composite film with good dispersion was prepared by electropolymerization of aniline containing well-dissolved SWNTs. Platinum (Pt) particles were electrodeposited on the SWNT/PANI composite film subsequently. The presence of SWNTs and platinum in the composite film was confirmed by XRD analysis. Four-point probe investigation exhibits that the electrical conductivity of SWNT/PANI composite film is significantly higher than that of pure PANI film. Cyclic voltammogram and Chronoamperogram show that Pt-modified SWNT/PANI electrode performs higher electrocatalytic activity than Pt-modified pure PANI electrode toward formic acid oxidation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Formic acid can be activated to decompose to smaller fragments, protons, electrons and CO2 at high efficiency. Its applicability as a fuel has already been tested in a PEM fuel cell [1, 2]. More recent data shows that formic acid fuel cells are attractive alternatives for small portable fuel cell applications [2].
For formic acid electro-oxidation, the commonly used catalyst is platinum (Pt) and Pt based alloy [3]. Today, to reduce costs and enhance the oxidation efficiency, novel electrode materials are prepared. Conducting polymers with porous structure and high surface area are usually used as matrix to incorporate noble metal catalyst [4]. Many literature surveys indicate that Pt micro-particles dispersed in Polyaniline (PANI) matrices perform high activity toward formic acid electro-oxidation [3, 5–10].
Since the discovery of carbon nanotubes (CNTs), extensive research in the fields of applied physics, chemistry and materials science and engineering has rapidly emerged [11–13]. As a result, CNT-based nanostructures and functional materials have become popular subjects of study. Many studies report that the introduction of CNTs into a polymer matrix improves the electrical conductivity as well as the mechanical properties of the original polymer matrix [14–16]. Many efforts have focused on the design and preparation of CNT/polymer composites and a large number of composites based on CNTs have been prepared.
Polyaniline is a unique and promising candidate for practical applications due to its good process ability, environmental stability, and reversible control of electrical properties by charge-transfer doping and protonation [17, 18]. To date, several studies concerning CNT/PANI composites have been reported [19–21]. The dispersion of CNTs into PANI matrix for the fabrication of CNT/PANI composite devices has naturally stimulated significant interest among researchers. For instance, Zengin et al. [22] mixed multiwalled carbon nanotubes (MWNTs) with aniline via stirring, followed by in situ polymerization. They reported that the MWNT/PANI composites exhibit drastic increase in electrical conductivity. Deng et al. [20] used a similar method to prepare CNT/PANI hybrid materials, and studied the conductivity in these composites. They proposed that even 2‰ of CNTs could improve the conductivity of the CNT/PANI composite more than three times than that of PANI.
However, the development of such composites has been impeded by the inability to disperse CNTs in the polymer matrix due to the lack of chemical compatibility between the polymers and the CNTs [23–26]. Recent studies have shown that SWNTs can be dissolved in aniline via formation of donor–acceptor complex [27]. The solubility of SWNTs in aniline is up to 8 mg/ml.
In this work, we fabricated SWNT/PANI composite film by electropolymerization of aniline with SWNTs dissolved in. Platinum particles were electrodeposited on the SWNT/PANI composite film subsequently. The aim of this work is to study the electro catalytic oxidation efficiency of formic acid at Pt-modified SWNT/PANI electrode. To the knowledge of the present authors, little work has been done to study the electrocatalytic oxidation efficiency of formic acid at Pt-modified SWNT/PANI electrode.
Experimental
Preparation of SWNT-aniline solution
SWNTs were produced by a chemical vapor deposition method. The process of their preparation and purification was described in detail elsewhere [28, 29]. In this work, SWNTs were synthesized by using methane as carbon reactant and MgO-supported Fe nanoparticles as catalyst, and the reaction was carried out at 850 °C under Ar atmosphere for 30 min. The produced SWNTs are rope-like and the diameter is about 1–2 nm [30].
Aniline was distilled under reduced pressure and stored under nitrogen gas. All other reagents were of analytical grade and were used as received. All the solutions were prepared with twice distilled water.
Single-walled carbon nanotubes were added to 50 ml aniline with content of 0, 2, 4, 8 wt% (weight percent with respect to aniline monomer). The mixture was heated at reflux for 3 h in the dark. After being cooled to room temperature, SWNTs-aniline solution were obtained by filtration through Ø 0.1 μm Super Membrane disc filters(German) under vacuum [31].
Preparation of Pt-modified SWNT/PANI electrode and Pt-modified pure PANI electrode
The electrochemical experiments were performed on CHI660A electrochemical working station (Covarda) in a three-electrode system and controlled by CH instrument electrochemical software. The substrate-working electrode was a platinum sheet (geometric surface area=1 cm2). Another platinum sheet and a saturated calomel electrode (SCE) were used as counter and reference electrode, respectively. The platinum electrodes were polished mechanically using emery paper (grade 1200) to a mirror surface and then cleaned by potential cycling between 0.2 V and 1.2 V at 50 mV/s in 0.5 M H2SO4 until a stable cyclic voltammogram was obtained.
SWNT/PANI composite film was electropolymerized on the substrate-working electrode by the CV technique at 50 mV/s between −0.1 V and 0.9 V for 13 cycles at 20 °C in 1 M H2SO4 solution containing 0.1 M aniline with 8 wt% SWNTs dissolved in. The thickness of the composite film, varied by changing the number of potential cycles and calculated by the height of the first peak in the redox process [32], reaches 0.39 μm [33]. Platinum particles were electrodeposited on SWNT/PANI film in the solution of 1 mM H2PtCl6 + 0.5 M H2SO4 under constant potential of −0.13 V. Prior to the dispersion of the platinum, the SWNT/PANI coated electrode was soaked in 1 mM H2PtCl6 + 0.5 M H2SO4 solution for 15 min. The magnitude of platinum deposited, calculated from the integral of cathodic charge during deposited process, is 102 μg/cm2 [34].
For comparison, Pt-modified pure PANI electrode free of SWNTs was also fabricated on the same substrate-working electrode with Pt-modified SWNT/PANI electrode. The fabrication procedure, thickness of PANI film and platinum loading is the same with Pt-modified SWNT/PANI electrode too.
Measurements
X-ray diffraction (XRD) data of the samples were collected using a Rigaku D/MAX 24000 diffract meter with Cu-Kα radiation. Scan electron microcopy (SEM) studies were performed using a JSM-6700F (JEOL) instrument. The conductivity measurements were carried out by four-point probe method.
Results and discussion
XRD analysis
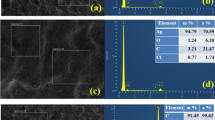
The presence of SWNT and platinum in Pt/SWNT/PANI composite film was confirmed by XRD. Figure 1 shows the XRD spectrum of Pt/SWNT/PANI composite film. The peaks at 39.7°, 46.2°, 67.4°, and 81.2° can be assigned to Pt(111), Pt(200), Pt(220) and Pt(311) crystalline plane diffraction peaks, respectively. These peaks can be indexed as the Pt face-centered cubic (fcc) phase based on the data of the JCPDS file [35]. The peak at 26.42° is assigned to SWNT(002) [36, 37].
SEM analysis
Figure 2 shows the SEM of Pt-modified SWNT/PANI electrode. The white spots in the micrograph can be attributed to platinum micro-particles. For the porous structure and high surface of SWMT/PANI composite [30], the platinum atoms are readily grown and formed small clusters which favors it to achieve a high degree of dispersion and large surface area of the platinum particles and increased the electrocatalytic oxidation activity for formic acid, consequently.
Electrical conductivity of SWNT/PANI composite film
Figure 3 shows the electrical conductivity of SWNT/PANI composite film obtained by CV polymerization in 0.5 M H2SO4 containing 0.1 M aniline with 0, 2, 4, 8 wt% SWNTs dissolved in, respectively. The potential limits were between −0.1 V and 0.9 V and the scan rate was 50 mV/s. The cycle number was 15 cycles. The electrical conductivity of the SWNT/PANI film containing 8 wt% SWNT attaches 12.6 (S.m−1 ×10−3), 14 times as high as pure PANI. Even though the SWNT content is 1 (wt%), the electrical conductivity of SWNT/PANI can attach nearly two times as high as pure PANI.
Electrocatalytic activity towards formic acid
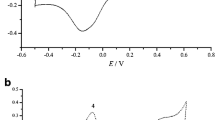
Figure 4 shows the cyclic voltammogram of formic acid oxidation at (a) Pt-modified pure PANI electrode and (b) Pt-modified SWNT/PANI electrode in the solution of 0.5 M HClO4 + 0.5 M HCOOH. It is clearly observed that the Pt-modified SWNT/PANI electrode performs significantly higher catalytic activity than Pt-modified pure PANI electrode. Comparing the two electrodes, since the substrate-working electrode is the same one, and the thickness of SWNT/PANI and PANI film, the magnitude of platinum loading are approximately equal. So the higher electrocatalytic activity of Pt-modified SWNT/PANI electrode should be attributed to the SWNTs incorporated in PANI film. It assumes a very important role in increasing the electrical conductivity of SWNT/PANI composite film and facilitates electrons shuttling through composite film, between the substrate and dispersed platinum particles where the electrocatalytic reaction occurs. Thus, higher electrocatalytic activity is achieved.
The generally accepted mechanism for electrochemical oxidation of formic acid has been reported in many literatures [2, 38, 39]. As shown in Fig. 4, during the positive scan, the peak at 0.21 V is attributed to oxidation of weakly bounded species and the one at 0.68 V to oxidation of the strongly chemisorbed species. During the negative scan, the peak at 0.50 V is attributed to direct oxidation of formic acid to form CO2 without forming a carbon monoxide intermediate. Fig. 5 shows chronoamperometric curve for formic acid oxidation at (a) 0.21 V, (b) 0.50 V, (c) 0.68 V at Pt-modified pure PANI electrode and Pt-modified SWNT/PANI electrode, respectively. It is clearly observed that the Pt particles incorporated in SWNT/PANI matrix exhibits higher catalytic activity toward formic acid oxidation. After 200 s electrolysis, the current density on these two electrodes reached the stability state and the current density on Pt-modified SWNT/PANI electrode is well above that of the Pt-modified PANI electrode.
Conclusions
The electrocatalytic activity of Pt-modified SWNT/PANI electrode is significantly higher than that of Pt-modified pure PANI electrode toward formic acid oxidation. SWNT/PANI can be exploited as a promising replacement for pure PANI to incorporate noble metal catalyst to improve the oxidation efficiency of formic acid.
References
Weber M, Wang JT, Wasmus S, Savinell RF (1996) J Electrochem Soc 143:1158
Rice C, Ha S, Masel RI, Waszczuk P, Wieckowski A, Barnard T (2003) J Power Sources 115:229
Markovic NM, Ross PN Jr (2002) Surf Sci Rep 45:117
Thackeray JW, Wrighton MS (1986) J Phys Chem 90:6674
Gholamian M, Contractor AQ (1990) J Electroanal Chem 289:69
Gholamian M, Sundaram J, Contractor AQ (1987) Langmuir 3:741
Parsons R, Vandnoot T (1988) J Electroanal Chem 257:9
Malinauskas A (1999) Synthetic Met 107:75
Kazarinov VE, Andreev VN, Spitsyn MA, Mayorov AP(1990) Electrochim Acta 35:1459
Napporn WT, Laborde H, Léger J-M, Lamy C (1996) J Electroanal Chem 404:153
Iijima S, Ichihashi T (1993) Nature 363:603
Wong EW, Sheehan PE, Lieber CM (1997) Science 277:1971
Dresselhaus MS, Dresselhaus G, Avouris Ph (2001) Top Appl Phys 80:1
Schadler LS, Giannaris SC, Ajayan PM (1998) Appl Phys Lett 73:3842
Wagner HD, Lourie O, Feldman Y, Tenne R (1998) Appl Phys Lett 72:188
Qian D, Dickey EC, Andrews R, Rantell T (2000) Appl Phys Lett 76:2868
Skotheim TA, Elsenbaumer RL, Reynolds JR (1997) Handbook of conducting polymers. Marcel Dekker, New York
Premamoy G, Samir KS, Amit C (1999) Eur Polym J 35:699
Hassanien A, Gao M, Tokumoto M, Dai L (2001) Chem Phys Lett 342:479
Deng J, Ding X, Zhang W, Peng Y, Wang J, Long X, Li P (2002) Eur Polym J 38:2497
Maser WK, Benito AM, Callejas MA, Seeger T, Martinez MT, Schreiber J, Muszynski J, Chauvet O, Osváth Z, Koós AA, Biró LP (2003) Mater Sci Eng C 23:87
Zengin H, Zhou WS, Jin JY et al (2002) Adv Mater 14:1480
Hadjiev VG, Iliev MN, Arepalli S, Nikolaev P, Files BS (2001) Appl Phys Lett 78:3193
Peigney A, Flahaut E, Laurent C, Chastel F, Rousset A (2002) Chem Phys Lett 352:20
Bower C, Rosen R, Zhou O (1999) Appl Phys Lett 74:3317
Haggenmueller R, Gommans HH, Rinzler AG, Fischer J E, Winey KI (2000) Chem Phys Lett 30:219
Sun Y, Wilson SR, Schuster DI (2001) J Am Chem Soc 123:5348
Li QW, Yan H, Cheng Y, Zhang J, Liu ZF (2002) J Mater Chem 12:1179
Li XH, Zhang J, Li QW, Li HL, Liu ZF (2003) Carbon 41:598
Zhou YK, He BL, Zhou WJ, Li HL (2004) J Electrochem Soc 151A:1052
Guo DJ, Li HL (2004) J Solid State Electrochem (in press)
Stilwell DE, Park SM (1988) J Electrochem Soc 135:2491
Niu L, Qiuhong L, Fenghua W, Xiao C, Hao W (2003) Synthetic Met 139:271
Laborde H, Léger J-M, Lamy C (1994) J Appl Electrochem 24:219
Join Committee on Power Diffraction Standards (1991) Diffraction data file: JCPDS International Center for Diffraction Data. Swarthmore PA
Terrones M, Hsu WK, Schilder A, Terrons H, Grobert N, Hare JP (1998) Appl Phys A 66:307
Feng W, Bai XD, Lian YQ, Liang J, Wang XG, Yoshino K (2003) Carbon 41: 1551
Capon A, Parsons R (1973) J Electroanal Chem 45:205
Wieckowski A, Sobkowski J (1975) J Electroanal Chem 63:365
Acknowledgements
We are grateful for the financial supports from National Natural Science Foundation of China (NSFC 6989022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, J., Guo, Dj., Wang, Z. et al. Electrocatalytic oxidation of formic acid on platinum particles dispersed in SWNT/PANI composite film. J Solid State Electrochem 9, 634–638 (2005). https://doi.org/10.1007/s10008-004-0624-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-004-0624-8