Abstract
A stable quercetin–thioglycolic acid-modified gold electrode (Qu–TCA/Au) was prepared as a self-assembled monolayer (SAM) and its electrochemical behavior was investigated by electrochemical methods. In 0.05-M phosphate buffer solution (pH 7.0) quercetin exhibits quasi-reversible signals at the Qu–TCA/Au electrode. The stability of the quercetin-modified gold electrode is very good. The quercetin self-assembled monolayer is an effective mediator for the oxidation of dopamine, which was investigated by cyclic voltammetry and differential pulse voltammetry. Ascorbic acid does not interfere with determination of dopamine at an electrode modified with a mixture of quercetin–thioglycolic acid and quercetin–11-mercaptoundecanoic acid. This modification allows dopamine to be determined in the presence of ascorbic acid in the range from 3×10−5 to 3×10−4 M. The detection limit is 1×10−6 M. Scanning electrochemical microscopy (SECM) was employed to study the electrochemical performances of the modified gold electrode indicating different feedback modes at differently modified surfaces.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dopamine (DA) is a ubiquitous neurotransmitter in mammalian brain tissues and it frequently coexists with ascorbic acid (AA). DA plays a very important role in the functioning of the central nervous, renal, hormonal and cardiovascular systems [1, 2]. Dysfunction of the dopaminergic system in the central nervous system (CNS) is related to neurological disorders such as schizophrenia, Parkinson’s disease and to HIV infection. Therefore, currently great interest of neuroscientists and chemists is focused on the study of dopamine. DA is an electrochemically active compound and can be determined by various electrochemical methods. However, it is known that at a bare electrode the oxidation peaks of AA and DA are situated at the same potential [3]. In the presence of DA, a homogeneous catalytic oxidation of AA occurs by oxidized DA. This leads to incorrect determinations of DA. Therefore, it is essential to develop rapid, simple and selective electrochemical methods for the determination of DA. To improve the selectivity for DA many different strategies have been used to modify the electrode surface. These include modifications by iodide [4], AA oxidase [5], polymer films [6, 7, 8, 9, 10], and self-assembled monolayers of mercaptoalkanes [11, 12, 13, 14], electrochemical pretreatment [15], and covalent modification [16].
Quercetin, a derivative of benzo-γ-pyrone, is a bioflavonoid. Bioflavonoids are a large family of naturally occurring organic compounds widely distributed in plants. Bioflavonoids are highly interesting because they may exert a wide range of beneficial effects on human health and have broad pharmacological activities, including prevention of cardiovascular diseases and different forms of cancer. Further they possess antiviral, anti-allergic, anti-platelet, anti-inflammatory and anti-tumor activities, and possibly even protective effects against chronic diseases [17, 18, 19]. This is the reason for their subsequent use in a great variety of health products [20]. They also can help absorption of AA in the human body [21]. Some workers [21] have claimed better results for the combined use of bioflavonoid and AA than for bioflavonoids alone. DA and AA have similar structures and pharmacological activities. Hence, bioflavonoids might be highly active electron transfer mediators for the electrocatalytic oxidation of DA. Interactions of DA with bioflavonoids have essential significance for their pharmacological activities.
In the present work, we described the electrochemical behavior of a quercetin-SAM-modified gold electrode and its electrocatalytic properties to oxidize DA. The quercetin-modified electrode can be used to determine DA in the presence of AA at physiological pH.
Experimental
Reagents
Dopamine hydrochloride (DA) was purchased from Fluka. Thioglycolic acid (TCA) was obtained from the Chinese Medicament Combine Co. (China). 11-Mercaptoundecanoic acid (MUA) and ascorbic acid (AA) were purchased from Aldrich and Beijing Chemical Laboratory (China), respectively, and quercetin (Qu) was obtained from the Institute of Chemical Physics, Chinese Academy of Sciences (China). 1-Ethyl-3-(3-dimethylamino-propyl) carbodiimide (EDAC) was purchased from Sigma. Working solutions of AA and DA were prepared daily. Other reagents used in this investigation were of analytical reagent grade. Phosphate buffer solution (0.05 M, pH 7.0 and 7.3) was prepared using Na2HPO4 and NaH2PO4 and they contained 0.1 M KCl. All reagents were used as received without further purification unless otherwise noted. All solutions were prepared with doubly distilled water.
Instrumentation
Electrochemical experiments were carried out using a CHI-832 electrochemical analyzer (CH Instruments Inc., USA) with a bare gold or a modified gold electrode as the working electrode, a platinum wire as a counter electrode, and a saturated calomel reference electrode (SCE). Scanning electrochemical microscopy experiments were carried out with a CHI-900 electrochemical workstation (CH Instrument Inc., USA). A 10-µm diameter gold electrode was fabricated as described in [22] and served as the SECM tip. A Pt wire with a diameter of 0.5 mm and an Ag | AgCl (KCl saturated) electrode were used as counter and reference electrodes, respectively. Before each experiment, the tip was polished with 0.05-µm alumina, and then rinsed with water. A Branson 200 Ultrasonic cleaner (USA) was used to clean the working electrode. Experiments were carried out at laboratory temperature. The following instrumental parameters were used to perform differential pulse voltammetry: Potential increment E: 4 mV; amplitude: 50 mV; pulse width: 0.06 s; pulse period: 0.2 s; quiet time: 2 s.
Preparation of modified gold electrode
Before modification, the gold electrode was polished with 1-µm, followed by 0.3-µm alumina slurry on a polishing pad, and then rinsed with distilled water and acetone, ultrasonicated in a water bath for 2 min and in acetone for 5 min. The gold electrode was then electropolished by potential cycling scan (+1.4V to −0.2 V) in 0.5 M H2SO4 with a scan rate of 100 mV s−1 until the CV characteristic for a clean Au electrode was obtained. After being rinsed with twice with distilled water and ethanol and dried in nitrogen gas, a clean bare gold electrode was obtained. The resulting gold electrode was immersed in an ethanol solution containing 2 mM TCA or MUA, or mixtures of TCA and MUA, respectively, for 8 h, then removed and washed copiously with the solvent. Then the modified gold electrode was activated by inserting in an ethanol solution containing 0.1 g L−1 1-ethyl-3-(3-dimethylamino-propyl) carbodiimide as coupling agent for 1 h, 0.1 mg quercetin was added and the reaction was allowed to proceed for 24 h to obtained Qu–TCA/Au or Qu–MUA/Au or Qu–TCA–MUA/Au modified electrodes. This was followed by rinsing sufficiently with the solvent and drying with a stream of pure nitrogen before performing the voltammetric experiments.
Results and discussion
Characterization of the Qu-SAM-modified gold electrode by cyclic voltammetry and SECM
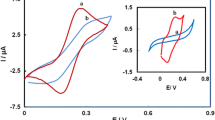
Figure 1 shows the cyclic voltammograms of 5 mM potassium ferricyanide solution containing 0.1 M NaCl supporting electrolyte at a bare gold electrode (curve a), at a Qu–TCA/Au electrode (curve b) and at a Qu–MUA/Au electrode (curve c). From Fig. 1 it is apparent that a pair of redox peaks for Fe(CN)63−/4− appear at the bare gold electrode. The peak separation (ΔE) of 87 mV shows an almost reversible one-electron transfer process. However, at the Qu–TCA/Au electrode the redox peaks of Fe(CN)63−/4− decrease and they disappear at the Qu–MUA/Au electrode. Scheme 1 depicts the gold electrode modified with a quercetin TCA self-assembly monolayer. The scheme makes clear that the electron transfer must take place through a compact monolayer of alkyl chains and therefore the electron transfer kinetics may exhibit an exponential increase with decreasing alkyl chain length. Many references indicate that the long-chain monolayer has no measurable pinholes [23, 24, 25]. Substantial energetic barriers of the long alkyl chain monolayers on Qu–MUA/Au electrode prevent the electron transfer between the gold electrode and the Fe(CN)63−/4− ions. However, in the literature [23, 24, 25] it is indicated that thioglycolic acid with a 3-C chain provides a weaker barrier for electron transfer than an 11-C chain. Accordingly, the redox peak current of Fe(CN)63−/4− at a Qu–TCA/Au electrode is higher than that at a Qu–MUA/Au electrode.
Scanning electrochemical microscopy was employed to study the electrochemical performances of the modified gold electrodes. The bare gold electrode, the Qu–TCA/Au electrode, and the Qu–MUA/Au electrode were used as SECM substrates. The feedback mode is the main quantitative operation mode of SECM. A positive feedback means that the substrate is conductive, whereas a negative feedback is observed when the substrate is insulating. Figure 2 shows the approach curves of ferricyanide at pH 7.0. The results indicate that the electron transfer (ET) of Fe(CN)63−/4− on the Qu–MUA/Au electrode could be fully blocked and also that there was no blocking effect for both the bare gold electrode and the Qu–TCA/Au electrode. A reasonable explanation is that the substantial barriers of the long alkyls block the diffusion of Fe(CN)63−/4− onto the substrate surface. A sufficiently negative potential was applied to the tip so that the reduction of Fe(CN)63− to Fe(CN)64− was controlled by the diffusion of Fe(CN)63−, and then Fe(CN)64− diffused to the surface of the substrate. While the tip approaches the substrate, the tip current response to distance depends only on the kb value of the reverse reaction on the substrate at a certain pH value. The effective heterogeneous rate constant kb for the ET reaction can be extracted from fitting the experimental SECM current (IT)–distance (d) curve (or approach curve) to the theoretical value [26]. Figure 2 shows that kb (0.00778 cm s−1) obtained from fitting the curve for the bare gold electrode is larger than kb (0.00477 cm s−1) obtained from fitting the curve for the Qu–TCA/Au electrode. These results prove that the barrier of the short alkyl chains slows down the electron transfer rate, whereas the substantial barrier of the long alkyl chains fully blocks the electron transfer.
Normalized tip current–distance curves of 5 mM Fe(CN)63− containing 0.1 M KCl supporting electrolyte solution for the tip approaching the bare gold electrode (open circles); the Qu–TCA/Au electrode (open triangles); the Qu–MUA/Au electrode (open squares) fitted with theoretical curves (line). The dotted line, solid line and dashed line represent the theoretical positive feedback curves of Fe(CN)63− at the bare gold electrode and at the Qu–TCA/Au electrode, and negative feedback curves of Fe(CN)63− at the Qu–MUA/Au electrode respectively
Electrochemical behavior of the Qu-SAM-modified gold electrode
A typical cyclic voltammogram, obtained in phosphate buffer solution (pH 7.0) after soaking a Qu–TCA/Au electrode (curve a) or a Qu–MUA/Au electrode (curve b), is shown in Fig. 3. The cyclic voltammogram exhibited a pair of peaks of quercetin at the Qu–TCA/Au electrode and no peaks at the Qu–MUA/Au electrode. The reason is that the barrier provided by the thioglycolic acid with a 3-C chain is weaker than that provided by the 11-mercaptoundecanoic acids with an 11-C chain.
The cyclic voltammetry exhibited a quasi-reversible wave of quercetin at the Qu–TCA/Au electrode. Hendrickson et al. [27, 28] reported that this pair of peaks can be ascribed to the 3’,4’-dihydroxy groups on the B-ring of quercetin and the oxidation of the 3’,4’-dihydroxy groups on the B-ring of quercetin is a 2e−–2H+ reversible process. The peak separation of quercetin at the Qu–TCA/Au electrode is greater than that at the bare gold electrode because the alky chain monolayer provided a barrier that blocks electron transfer. The influence of the scan rates on the anodic peak current of quercetin was investigated at the Qu–TCA/Au electrode. The anodic peak current increased with increasing scan rates. The result is such that the anodic peak current is directly proportional to the scan rates, as respected for some characters of the surface wave.
According to the formula given by Laviron [29]:
If values of nΔEp>200 mV can be obtained experimentally, α and Ks can be easily determined by using Eqs. (1) and (2). A graph of Ep=f(log v) yields two straight lines with a slope equal to –2.3RT/αnF for the cathodic peak, and 2.3RT/(1−α)nF for the anodic peak. α can be determined from the slope of the straight lines. Ks can be calculated with the help of the equation
From the voltammograms with various potential scan rates, a linear regression equation Epa=0.5409+0.03767 lg v, with a correlation coefficient (r=0.9720), was obtained (Fig. 4). The estimated coefficient of electrons transferred of quercetin in the Qu–TCA film is 0.2, and the standard rate constant is 20 s−1 at high scan rate.
The stability of the Qu–TCA/Au electrode was examined. The peak currents of quercetin did not change apparently for four repetitive scans at scan rate of 50 mV s−1. The peak currents increased a little after the electrode was stored at 4 °C for 3 days.
Oxidation of DA at the Qu-SAM-modified gold electrode.
Figure 5 shows the cyclic voltammogram of 5×10−4 M DA at a bare gold electrode (curve a) and at a Qu–TCA/Au electrode (curve b) in phosphate buffer solution (pH 7.0). There is a quasi-reversible wave of DA at the bare electrode. The peak current is linearly proportional to the scan rates in the range from 30 mV s−1 to 250 mV s−1, indicating that the process is adsorption controlled. The anodic peak increased markedly and the cathodic peak did not change at the Qu–TCA/Au electrode. The reductive peak of DA shifted negatively from 0.396 V to 0.363 V and the overpotential decreased to 33 mV. These observations indicated that quercetin is an effective mediator for the oxidation of DA. The peak separation (ΔE=145 mV) of DA at a Qu–TCA/Au electrode was greater than that (ΔE=54 mV) at a bare gold electrode because a quercetin self-assembled monolayer slows down electron transfer. At pH 7.0, DA exists as a cation with a positively charged amino group (pKa 8.9) [30, 31]. The mechanism of DA interaction with quercetin can be explained by assuming that quercetin at the electrode surface is oxidized, and then DA is attracted to the electrode surface and subsequently reacts with the oxidized form of quercetin. At the same time DA is oxidized and quercetin is deoxidized (Scheme 1).
The reductive peak current of DA increased with an increase of the scan rate at the Qu–TCA/Au electrode which exhibited a linear relation to the scan rate in the scan rate range from 30 mV s−1 to 250 mV s−1, the linear regression equation ip=1.30216×10−6+8.46964×10−6v and a correlation coefficient of 0.9936, indicating a fast electrocatalytic reaction was controlled by the adsorption of redox species [32].
Analytical application of dopamine at Qu–SAM/Au electrode
AA often simultaneously exists with DA in a biological sample and it disturbs the determination of DA. However, at a mixture-modified electrode of quercetin–thioglycolic acid and quercetin–11- mercaptoundecanoic acid (Qu–TCA–MUA/Au) a fine peak shape of DA is obtained and the oxidation of AA is prevented. So DA can be detected in the presence of AA. When the ratio of thioglycolic acid and 11-mercaptoundecanoic acids is 2:1 the peak shape of DA is quite fine and AA is not oxidized.
The determination of DA was performed by differential pulse voltammetry in phosphate buffer solution (pH 7.3) containing 0.1 M KCl (Fig. 6). The oxidative peak current of DA was selected as the analytical signal. The results showed that the oxidative peak current was proportional to the concentration of DA in the range from 3×10−5 M to 3×10−4 M. The detection limit was 1×10−6 M. Under the same conditions the peak currents of DA did not change when the concentration of AA ranged from 5×10−5 M to 3×10−4 M. So DA can be detected in the presence of AA.
Conclusion
In the present article, a stable quercetin–thioglycolic acid-modified gold electrode (Qu–TCA/Au) was prepared. Quercetin exhibits quasi-reversible signals at a Qu–TCA/Au electrode. The stability of the quercetin-modified gold electrode is very good. The anodic peak current of DA at the Qu–TCA/Au electrode is higher than that at a bare gold electrode. The overpotential decreased by 33 mV at a Qu–TCA/Au electrode. These results indicated that quercetin is an effective mediator in electrocatalytic oxidation of DA. At a Qu–TCA–MUA/Au mixture-modified gold electrode the catalyzing current is linearly proportional to the concentration of DA in the range from 3×10−5 M to 3×10−4 M in the presence of AA. Interaction of DA with quercetin will improve our understanding of the pharmacology of quercetin and DA.
References
Wightman RM, May LJ, Michael AC (1988) Anal Chem 60:769A
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) Brain 122:1437
Dayton MA, Ewing AG, Wightman RM (1980) Anal Chem 52:2392
Lane RF, Hubbard AT, Fukanaga K, Blanchard RJ (1976) Brain Res 114:346
Nagy G, Rice ME, Adams RN (1982) Life Sci 31:2611
Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN (1984) Brain Res 290:390
Saraceno RA, Pack JG, Ewing AG (1986) J Electroanal Chem 197:265
Ciszewski A, Milczarek G (1999) Anal Chem 71:1055
Ekinci E, Erdogdu G, Karagozler AE (2001) J Appl Polym Sci 79:327
Rubianes MD, Rivas GA (2001) Anal Chim Acta 440:99
Malem F, Mandler D (1993) Anal Chem 65:37
Giz MJ, Duong B, Tao NJ (1999) J Electroanal Chem 465:72
Dalmia A, Liu CC, Savinell RF (1997) J Electroanal Chem 430:205
Raj CR, Tokuda K, Ohsaka T (2001) Bioelectrochemisty 53:183
Gonon F, Buda M, Cespuglio R, Jouvet J, Pujol JF (1981) Brain Res 223:69
Downsard AJ, Roddick AD, Bond AM (1995) Anal Chim Acta 317:303
Chantal CLM, France VM, Muriel T, Helene SM, Jacques M, Marc SW (1996) Toxicology 114:19
Hollman PCH, Katan MB (1999) Health Effects and Bioavailability, Food and Chemical Toxicology 37:937
Polissero C, Lenczowski MJP, Chinzl D, Cuisset BD, Sumpter JP, Fostier A (1996) J Steroid Biochem Molec Biol 57:215
Volikakis GJ, Efstathiou CE (2000) Talanta 51:775
Tang JL, Wu ZY, Wang JG, Wang EK (2001) Electroanalysis 13:1315
Bard AJ, Fan F, Mirkin MV, (1993) in: Bard AJ (ed), Electroanalytical chemistry, vol 18. Marcel Decker, New York, pp 243
Ptoter MD, Bright TB, Allara DL, Chidsey CED (1987) J Am Chem Soc 109:3559
Munakata H, Kuwabata S, Ohko Y, Yoneyama H (2001) J Electroanal Chem 496:29
Viana AS, Jones AH, Abrantes LM, Kalaji M (2001) J Electroanal Chem 500:290
Ye JY, Liu JY, Zhang ZQ, Hu JM, Dong SJ (2001) J Electroanal Chem 508:123
Hendrickson HP, Kaufman AD, Lunte CE (1994) J Pharm Biomed Anal 12:325
Roy PR, Okajima T, Ohsaka T (2003) Bioelectrochemistry 59:11
Laviron E (1979) J Electroanal Chem 101:19
Zen JM, Chen PJ (1998) Electroanalysis 10:12
Chen M, Li HL (1998) Electroanalysis 27:477
Zhang XH, Wang SF (2003) Sensors 3:61
Acknowledgements
The present work was supported by a grant from the National Natural Science foundation of China (No. 20275031) and KJCXGC-01 from Northwest Normal University. The authors were also grateful to the State Key Laboratory of Electroanalytical Chemistry (SKLEAC), Chinese Academy of Sciences for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, J., Zhuo, L., Lu, X. et al. Electrochemical behavior of dopamine at a quercetin-SAM-modified gold electrode and analytical application. J Solid State Electrochem 9, 114–120 (2005). https://doi.org/10.1007/s10008-004-0571-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-004-0571-4